Advances in Animal and Veterinary Sciences
Research Article
Evaluation of the Effectiveness of Various Vaccination Protocols for Pigs Against Aujeszky Disease
Dmytro Masiuk, Andrii Kokariev, Kateryna Holda, Tetiana Vasilenko*, Volodymyr Hlebeniuk
Research Center for Biosafety and Environmental Control of AIC Dnipro State Agrarian and Economic University, Dnipro, Ukraine.
Abstract | Vaccination of pigs against Aujeszky disease (AD) helps to prevent economic losses. But a number of factors, such as the method of immunization, the age of the pigs at the time of immunization, stress, etc., affect the effectiveness of the vaccination. Therefore, when forming the scheme of immunoprophylactic measures on the farm, it is necessary to take into account all these factors in order to obtain protective immunity against pseudorabies. The difference between infected animals and vaccinated animals (DIVA) based on the use of glycoprotein E (gE) negative vaccines and the detection of antibodies to marker and non-marker glycoproteins of the pseudorabies virus makes it possible to evaluate the effectiveness of immunization of pigs against AD. Consequently, the purpose of our work was to evaluate the effectiveness of various protocols for vaccination of pigs against Aujeszky’s disease in conditions of industrial production. For the experiment 3 similar groups of pigs were formed - control and two experimental, 220 pigs in each. To compare the effectiveness of different vaccination schemes in our work, we used two ELISA tests: general and discriminatory. The results obtained indicate that in herds of pigs parenterally vaccinated against AD, one of the main ways of infecting piglets with Aujeszky Disease virus (ADV) is through the nasal mucosa. Double intramuscular immunization of pigs after weaning with inactivated vaccine is insufficient to form an effective immune defense against ADV is. The combination of intranasal immunization of suckling pigs with live attenuated ADV is and intramuscular vaccination of pigs after weaning with inactivated ADV is the most effective scheme for the formation of protective immunity in piglets.
Keywords | Aujeszky disease, Pigs, Vaccination, Serological control, DIVA strategy
Received | October 27, 2020; Accepted | November 10, 2020; Published | February 15, 2021
*Correspondence | Tetiana Vasilenko, Research Center for Biosafety and Environmental Control of AIC Dnipro State Agrarian and Economic University, Dnipro, Ukraine; Email: vasilenkotanya81@gmail.com
Citation | Masiuk D, Kokariev A, Holda K, Vasilenko T, Hlebeniuk V (2021). Evaluation of the effectiveness of various vaccination protocols for pigs against aujeszky disease. Adv. Anim. Vet. Sci. 9(4): 483-489.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.483.489
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Tatiana et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Viral diseases are a widespread problem in the world among both humans and animals. The main mechanism in the control viral diseases is the immunization of animals and the formation of group immunity (Cavirani et al., 2019). This is the key to an effective scheme to control viral diseases (Masiuk et al., 2020). But vaccination does not always ensure the formation of protective immunity (Pol et al., 2011). This is due to many factors, the main of which are the biological properties of the virus itself (Mettenleiter et al., 2019).
Aujeszky disease (AD) is a highly contagious viral disease that causes significant economic damage to the pig industry (Liu et al., 2019; Colomer et al., 2020). Control of the spread of Aujeszky disease is carried out by vaccinating pigs with live attenuated or inactivated vaccines (Freuling et al., 2017).
AD virus is known to be highly contagious and can easily infect susceptible pigs. Due to its biological properties, after entering the body of a pig, the virus infects the nervous tissue in which it persists throughout life (Laval et al., 2020). This was one of the main factors that led to the development of gE-negative marker vaccines against Aujeszky disease (Mettenleiter, 2020). Removal of gE antigen from the virus is of important diagnostic value because it allows to differentiate infected pigs from immunized and virus-free. This has been achieved through the development of laboratory tests that detect antibodies to glycoproteins E and B of the p pseudorabies virus (Kinker et al., 1997; Silva-Junior et al., 2020). This has made it possible to significantly increase the effectiveness of disease control measures.Such measures can reduce the number of clinical manifestations of Aujeszky disease (Wang et al., 2016).
There are different schemes for vaccinating pigs against Aujeszky disease, which differ in several variables, such as the method of immunization, the age of the pigs at the time of immunization, and others (Casal et al., 2004; Yefimov et al., 2016). These variables make it possible to optimize the vaccination schedule, which helps to completely free the herd from ADV. It should be noted that the choice of vaccine is important in the formation of the vaccination scheme. Typically, eradication programs use gE-negative marker vaccines (Mettenleiter, 2020).
They make it possible to separate infected pigs from vaccinated animals with protective specific immunity against ADV (Kinker et al., 1997). Due to the biological characteristics of the pseudorabies virus, it is almost impossible to completely stop the spread of the pathogen and the development of a latent form of the disease induced by the field strain (Yoon et al., 2006). The development of effective vaccination schemes for pigs that would provide protective immunity against pseudorabies virus is a promising avenue for herd recovery today.
Therefore, the main goal of this paper is to evaluate the effectiveness of various protocols for vaccination of pigs against Aujeszky’s disease in industrial settings.
MATERIALS AND METHODS
Laboratory studies were conducted on the basis of the Scientific Research Centre of Biosafety and Environmental Control Agro-Industrial Complex in the Laboratory of Immunochemistry and Molecular Genetic Analysis of the Dnipro State Agrarian and Economic University.
Animals. Experimental studies were performed on the basis of a pig complex of the farrowing-fattening cycle, which numbered about 11,000 pigs.
Six hundred and sixty piglets of the same age originating from a commercial herd of pigs were used for the experiment.
Animals were mixed and randomly divided into three groups - control and two experimental, 220 piglets each. The piglets were not divided by sex.
Vaccination programs. The piglets of the control group were vaccinated intramuscularly once at the age of 75 days according to the scheme of treatment and prevention measures at the enterprise. Piglets in the experimental group I were vaccinated intramuscularly twice. First time at the age of 65 days of life and revaccinated for 75 days of life. Pigs of the experimental group II were immunized twice. The first time intranasally at 14 days of age, and the second time intramuscularly at the age of 65 days.
It should be noted that the sows from which the piglets were derived were vaccinated against Aujeszky disease intramuscularly 3 times a year by mass vaccination.
An inactivated gE-negative vaccine against Aujeszky disease from strain “77/ЗВ» (clone of strain Bartha K61) ≥ 8 lg TCІD50 per ml at a dose of 2 ml was used for intramuscular vaccination.
For intranasal vaccination, an attenuated gE-negative vaccine against Aujeszky disease from strain «KB» (strain Bart K61) ≥ 5 lg TCІD50 at a dose of 1 ml in each nostril was used.
Ethical approval. Animal studies have been conducted within the framework of the “General Ethical Principles of Animal Experiments, which have been approved by the National Congress of Bioethics (Kyiv, 2001) and in line with the provisions of the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes (Strasbourg, 1986).
Samples collection. Blood samples were drawn weekly from the piglets in the control and experimental groups from 63 to 154 days of age in vacuum blood collection systems with a coagulation activator. From each age group were taken 7 blood samples. Blood samples from piglets were taken from cranial vena cava. The puncture site was treated with 70% ethyl alcohol. All blood samples from piglets were taken randomly.
ELISA test. Serum IgG specific for glycoprotein B (gB) and glycoprotein E (gE) antigens of ADV were determined by commercial ELISA kits - «ID Screen Aujeszky gB Antibody Competition» (IDVet, France) and «ID Screen Aujeszky gE Antibody Competition» (IDVet, France) using a BioTek ELx800 photometer. According to the recommendations for diagnostic kits, blood serum before testing was diluted in a ratio of 1/4 for IgG specific to gB antigen of ADV and 1/5 for IgG specific to gE antigen of ADV.
According to the instructions for the gB kit, the sample was considered positive when the calculation the competition percentage (S/N %) value is less or equal to 30% and in case of gE measurement the sample is considered positive if the result is less than or equal to 60%.
Statistical analysis. Variation and statistical processing of the obtained results were performed by using the Statistica 6.0 specialized software (StatSoft Inc., USA). The signifiance of diffrences was assessed after verifying the experimental data which was obtained by using Student t-test or its non-parametric counterpart – the Wilcoxon test. Selective parameters presented in the work have the following designations: x is the sample average; SE is the standard error of the average value; CV is the coeffient of parameter variation in the group.
RESULTS
Research IgG specific to gB antigens of Aujeszky disease virus.
As a result of the study, the age dynamics of specific antibodies to gB antigens of the Aujeszky disease pathogen was determined against the background of the use different vaccination schemes. It was found that 100% of animals in the control group from 63 to 70 days of life have specific antibodies whose level gradually decreases at the end of 11 weeks of birth (Table 1). In animals of the control group at the age of 84 days of life there is a decrease in S/N by more than 5.5 times compared with those obtained at 77 days of age. In the future, the level of antibodies to the gB antigen of ADV in the blood of 100% of piglets is maintained at a high level until 147 days of age. This is indicated by the low value of S/N, which fluctuated in the piglets of the control group in the range of 5-15%. From 154 days of life in piglets found high values of S/N.
Considering the results of the experimental groups, it was found that at 77 days of age the level of S/N in the piglets of the I and II experimental groups significantly (p≤0.05) differs from the control group. Forwards, the animals of the experimental groups showed a gradual decrease in S/N to 98 days of age. It should be noted that the S/N rate is higher at the age of 84 and 91 days of life in animals of the experimental group I by 48% and 46%, and in pigs of the experimental group II by 56% and 49% compared to the values of animals in the control group.
In piglets of experimental groups I and II from 98 to 147 days of life, a significant difference between the values of S/N with animals of the control group was not detected. A probable decrease in S/N in animals of the experimental groups I and II at the age of 154 days of life by 60% and 59% (p≤0.05) compared with the values of the control group of pigs are detected.
The degree of dispersion of the S/N index, in the study of IgG to gB antigens in pigs of the control and experimental groups, indicates the absence of a significant difference between the groups (Figure 1).
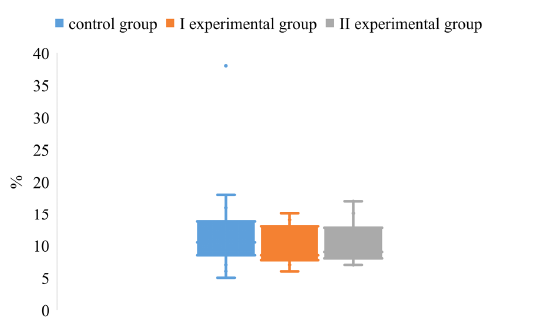
Figure 1: The dispersion of the S/N index for the determination of IgG to the antigen gB in the blood of pigs on the background of different vaccination schemes against the pseudorabies virus
It was found that the upper quartile of the control group is within 14%, and the lower - 8%. The maximum value of the control group reaches 19%, while the minimum value is 5%. The above data indicate a low level of S/N values swing in animals of the control group. The values obtained from pigs in the experimental groups did not differ significantly from the values of animals in the control group.
Analyzing the value of the CV index, it was found that in animals of experimental groups I and II the degree of dispersion is much lower compared to the control group (Figure 2).
It was found that the upper quartile of the CV of control group is about 62%, the lower quartile - 38%. The maximum value of the control group is about 92%. The minimum CV level reaches 22%. The above data indicate a high degree of variance of the values of the CV of animals in the control group.
Comparing the degree of dispersion of the CV index for different schemes of immunoprophylaxis of Aujeszky disease, it was found that the upper quartile of the experimental groups I and II are lower by 36% and 50%, respectively,
Table 1: The level of IgG to gB antigens of ADV in the pig’s blood of different age groups under different vaccination schemes (M±m, %, n=7)
| Age, days | Group of animals | ||||||||
| Control |
experimental І |
experimental ІІ |
|||||||
| pos. | S/N | CV | pos. | S/N | CV | pos. | S/N | CV | |
| 63 | 100 | 15,57±1,36 | 23 | 100 | 13,86±1,72 | 33 | 100 | 16,86±0,94 | 15 |
| 70 | 100 | 17,57±1,89 | 28 | 100 | 15,29±1,51 | 26 | 100 | 16,71±2,49 | 39 |
| 77 | 71 | 37,57±8,00 | 56 | 100 | 13,29±1,52* | 30 | 100 | 14,57±2,72* | 49 |
| 84 | 100 | 6,57±0,84 | 34 | 100 | 12,71±1,58** | 33 | 100 | 12,14±1,22** | 27 |
|
91 |
100 | 5,43±1,21 | 59 | 100 | 12,43±1,09** | 23 | 100 | 10,71±1,30* | 32 |
| 98 | 100 | 11,29±2,69 | 63 | 100 | 10,14±1,06 | 27 | 100 | 9,14±1,22 | 35 |
| 105 | 100 | 8,71±1,29 | 39 | 100 | 8,00±2,06 | 68 | 100 | 8,57±1,07 | 33 |
| 112 | 100 | 9,86±2,03 | 54 | 100 | 7,29±0,68 | 25 | 100 | 7,57±1,31 | 46 |
| 119 | 100 | 13,14±2,66 | 54 | 100 | 6,43±0,43 | 18 | 100 | 7,29±0,68 | 25 |
| 126 | 100 | 10,86±2,44 | 60 | 100 | 8,57±1,19 | 37 | 100 | 9,57±1,13 | 31 |
| 133 | 100 | 10,43±2,11 | 54 | 100 | 8,29±1,08 | 35 | 100 | 7,71±1,46 | 50 |
| 140 | 100 | 5,86±1,45 | 66 | 100 | 8,43±1,21 | 38 | 100 | 8,14±1,58 | 51 |
| 147 | 100 | 9,00±1,72 | 51 | 100 | 7,57±0,65 | 23 | 100 | 8,00±1,69 | 56 |
| 154 | 87 | 12,29±2,25 | 91 | 100 | 7,43±2,75* | 27 | 100 | 7,29±0,84* |
30 |
Note: changes are considered to be statistically signifiсant at * – Р<0,05, ** – Р<0,01 relative to the parameters of the animals of the control group; pos. - percentage of seropositive animals; S/N - the calculation the competition percentage; CV is the coefficient of parameter variation in the group
Table 2: The level of IgG to gE antigens of ADV in the pig’s blood of different age groups under different vaccination schemes
| Age, days | Group of animals | ||||||||
| Control |
experimental І |
experimental ІІ |
|||||||
| pos. | S/N | CV | pos. | S/N | CV | pos. | S/N | CV | |
| 63 | 71 | 33,71±10,14 | 80 | 86 | 32,29±10,41 | 85 | 86 | 31,14±7,28 | 62 |
| 70 | 71 | 35,57±12,15 | 90 | 71 | 43,86±8,77 | 53 | 71 | 58,43±5,22 | 24 |
| 77 | 71 | 39,14±14,14 | 96 | 71 | 32,43±10,25 | 84 | 57 | 53,57±6,52 | 32 |
| 84 | 86 | 37,29±7,50 | 53 | 57 | 45,57±12,21 | 71 | 29 | 65,71±12,1 | 49 |
|
91 |
100 | 28,57±5,52 | 51 | 71 | 42,57±9,78 | 61 | 0 | 82,71±4,3*** | 14 |
| 98 | 86 | 23,71±8,09 | 90 | 71 | 33,29±11,31 | 90 | 14 | 68,86±9,04** | 35 |
| 105 | 71 | 31,43±13,16 | 111 | 57 | 42,71±12,24 | 76 | 0 | 85,14±3,18** | 10 |
| 112 | 100 | 20,43±6,24 | 81 | 71 | 40,71±10,46 | 68 | 0 | 83,86±3,58*** | 11 |
| 119 | 86 | 22,14±10,97 | 131 | 71 | 45,14±11,55 | 68 | 0 | 80,14±4,11*** | 14 |
| 126 | 86 | 22,71±10,98 | 128 | 86 | 23,29±9,05 | 103 | 14 | 71,86±6,84** | 25 |
| 133 | 86 | 20,71±11,12 | 142 | 100 | 18,57±7,23 | 103 | 0 | 83,86±1,96*** | 6 |
| 140 | 100 | 13,86±3,43 | 66 | 100 | 27,71±6,87 | 66 | 0 | 75,14±1,98*** | 7 |
| 147 | 86 | 18,29±10,97 | 159 | 71 | 36,14±9,81 | 72 | 0 | 72,71±2,78*** | 10 |
| 154 | 71 | 33,71±13,49 | 106 | 57 | 44,86±12,51 | 74 | 0 | 79,29±2,49** |
8 |
Note: changes are considered to be statistically signifiсant at * – Р<0,05, ** – Р<0,01 relative to the parameters of the animals of the control group; pos. - percentage of seropositive animals; S/N - the calculation the competition percentage; CV is the coefficient of parameter variation in the group
compared with the control group. The lower quartiles of the arrays of CV values do not have significant differences.
Research IgG specific to gE antigens of Aujeszky disease virus.
It was found that 84% of piglets in the control group, 74% of animals in the experimental group I and 19% of the experimental group II have specific immunoglobulins to gE antigens of the pathogen Aujeszky disease (Table 2).
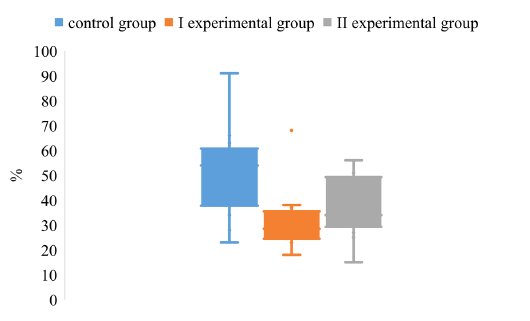
Figure 2: The dispersion of the CV index for the determination of IgG to the antigen gB in the blood of pigs on the background of different vaccination schemes against the pseudorabies virus
Comparing the degree of dispersion of the data of the S/N index of animals by antibodies to the gE antigen, a significant difference was found between the values of experimental groups II and the control group. (Figure 3).
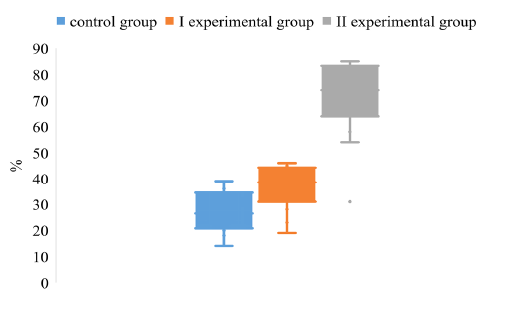
Figure 3: The dispersion of the S/N index for the determination of IgG to the antigen gE in the blood of pigs on the background of different vaccination schemes against the pseudorabies virus
The array of S/N values obtained from the piglets of the experimental group II is in negative limits in contrast to the control and experimental groups I, which is due to its high value in animals seronegative for gE antigen.
Analyzing the degree of dispersion of CV in animals of control and experimental groups on the background of different vaccination schemes, it was found that the degree of dispersion of CV in animals of the I and II experimental groups is much smaller compared to animals in the control group (Figure 4).
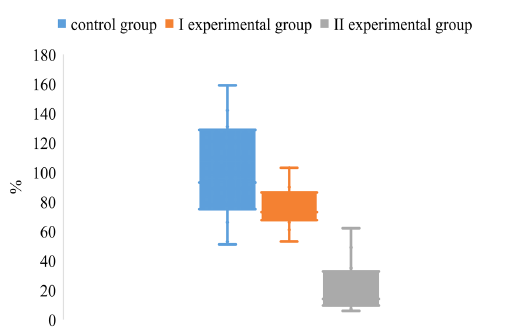
Figure 4: The dispersion of the CV index for the determination of IgG to the antigen gE in the blood of pigs on the background of different vaccination schemes against the pseudorabies virus
It was found that the upper quartile of the CV of the experimental group II is about 35%, which is 3.7 and 2.8 times lower, respectively, compared to the values of pigs in the control and experimental groups I. The lower quartile values of the control and the experimental groups I do not differ significantly, while the values of the experimental group II are much smaller than those of the animals of the control and the experimental groups I.
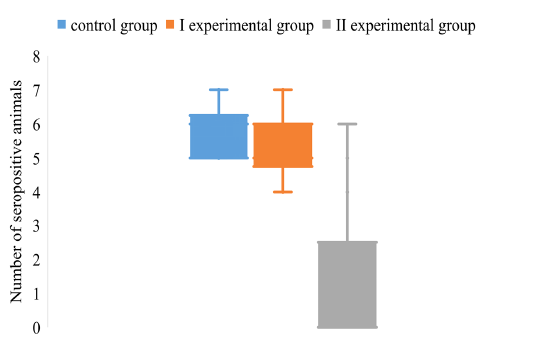
Figure 5: The dispersion of the positive pigs on the background of different vaccination schemes against the pseudorabies virus
Figure 5 shows the distribution of seronegative animals between groups by the gE marker antigen of the pseudorabies virus.
It was found that among the piglets of the control group 84% contain antibodies to gE antigen. The number of gE positive pigs in experimental group I is 10% less than the control group. The most pronounced are the results of experimental groups II. The number of pigs containing IgG to gE antigen of ADV is 19%, which is 4.4 times less than the control group of pigs.
DISCUSSION
To compare the effectiveness of different vaccination schemes in our work, we used two ELISA tests. The first is general, which detects antibodies to the structural antigen gB of the ADV, and the second is discriminatory, which detects antibodies to the antigen gE (Mettenleiter, 2020). The results of the IgG study for gB virus antigen in control piglets indicate a decrease in antibody levels and the appearance of seronegative animals at 77 days of age, which leads to the formation of a «serological window» in the group immunity of the herd. This is evidenced by the high values of S/N. Such changes may be due to the natural catabolism of colostral antibodies, which according to Wright et al. (1984) and Casal et al. (2004) may persist for 8-14 weeks from birth. With age, the pigs of the control group showed a decrease in S/N, due to the seroconversion of specific antibodies in response to the action of ADV antigens. The absence of a «serological window» among pigs of experimental groups I and II can be associated with earlier vaccination of these piglets, which promotes timely post-vaccination seroconversion.
It should be noted that the presence of a «serological window» in the herd on the background of vaccination of pigs contributes to an increase in infected with the field strain ADV individuals. This is indicated by results obtained in the study of antibodies to the discriminating gE antigen of the pseudorabies virus. It was found that in experimental group I the number of infected pigs with field ADV is 10% less compared to the control group. This is consistent with the results of other researchers (Pensaert et al., 1982; De Leeuw and Van Oirschot, 1985), who indicate infection of pigs during fattening despite intramuscular vaccination.
The most effective scheme for the formation of protective immunity was the double vaccination of pigs through intranasal immunization with attenuated pseudorabies virus at day 14 of life and intramuscular at day 65 of life. The number of seronegative pigs in the experimental group II is about 81%, which is 65% more than the control group and 55% more than the value of experimental group I. It should be noted that the difference in the vaccination schemes of the experimental groups I and II is only in the intranasal vaccination of piglets on the 14th day of life with an attenuated vaccine. In the first days of life, piglets are protected from the pathogenic action of ADV by maternal antibodies (Kritas et al., 1997), but their level decreases over time, which weakens the resistance of neonatal piglets and makes them more vulnerable to pseudorabies virus. In view of this, it can be concluded that the main gateway to Aujeszky disease infection is the mucous membrane of the nasal cavity of piglets older than 14 days of age. In their studies, Labarque et al. (1999) concluded that the clinical and virological protection of pigs, created by two-fold parenteral vaccination and intranasal intramuscular vaccination, did not differ significantly. But they suggested that with a vaccination schedule that included intranasal immunization, ADV transmission would be interrupted earlier and more efficiently than with the classic vaccination schedule.
Thus, the results obtained in this study results confirm the theory that immunization of pigs with intranasal antigen administration at 14 days of age is most effective in the herd infected with ADV, as indicated by the minimum number of seropositive gE antigen animals against a sufficiently high serum IgG level for gB virus antigen.
CONCLUSIONS
The obtained results suggest that in herds of pigs that are parenterally vaccinated against Aujeszky disease, one of the main ways of infecting piglets with the pseudorabies pathogen is the penetration of the virus through the mucous membrane of the nasal or/and oral cavities.
Double intramuscular immunization of pigs after weaning with an inactivated vaccine is not sufficient to form an effective immune defense against Aujeszky disease virus.
The combination of intranasal immunization of suckling pigs with live attenuated ADV and intramuscular vaccination of pigs after weaning with inactivated AD virus is the most effective scheme for the formation of protective immunity in piglets and group immune protection in the herd, which allows the successful implementation of Aujeszky disease eradication program.
ACKNOWLEDGEMENTS
This work was supported by the Scientific Research Centre of Biosafety and Environmental Control Agro-Industrial Complex in the Laboratory of Immunochemistry and Molecular Genetic Analysis of the Dnipro State Agrarian and Economic University, Ukraine.
AUTHORS CONTRIBUTION
All authors contributed equally to this work.
CONFLICT OF INTEREST
There is no conflict of interest.
REFERENCES






