Advances in Animal and Veterinary Sciences
Research Article
Lumpy Skin Disease in Calves: The Association Between Clinical Signs and Biochemical Alterations
Sherin R. Rouby1*, Olfat Shehata2, Ahmed S. Abdel-Moneim3, Khaled H. Hussein1, Mourad Mahmoud Mahmoud4
1Department of Veterinary Medicine (Infectious Diseases), Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Department of Clinical Pathology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 3Department of Microbiology, College of Medicine, Taif University, Al-Taif, Saudi Arabia; 4Department of Animal Medicine, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt.
Abstract | Lumpy skin disease (LSD) is an acute, sub-acute and chronic devastating disease of cattle. The current study was performed to determine various alterations in some haemato-biochemical parameters in calves naturally infected with lumpy skin disease virus (LSDV). Two groups of calves were enrolled in the study. The first group included nine Laboratory confirmed LSDV infected calves and the second group included five healthy calves. Laboratory confirmation was conducted by polymerase chain reaction (PCR) flanking the partial RPO30 gene. Blood samples were collected from all calves and subjected to hematological and biochemical analyses. The total mean leukocytic counts, lymphocytes, and monocytes in the LSDV infected calves were significantly higher than those in the control group. In addition, the total protein, total creatine kinase (CK), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine and potassium levels showed significant high values in LSDV infected calves while cholesterol levels were significantly lowered when compared to healthy ones. Serum gamma glutamyl transferase (GGT) activity, serum albumin, globulins and sodium levels did not show significant difference in calves in different groups. Results of both hematology and biochemistry profiles obtained from calves reflect the severe inflammatory process associated with natural LSD infection.
Keywords | Hemato-biochemical, Calves, Lumpy skin disease, PCR, RPO30 gene
Received | July 06, 2021; Accepted | July 27, 2021; Published | September 25, 2021
*Correspondence | Olfat Shehata, Department of Clinical Pathology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; Email: [email protected]
Citation | Rouby SR, Shehata O, Abdel-Moneim AS, Hussein KH, Mahmoud MM (2021). Lumpy skin disease in calves: The association between clinical signs and biochemical alterations. Adv. Anim. Vet. Sci. 9(11): 1863-1868.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.1863.1868
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Rouby et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Lumpy skin disease (LSD) is one of the most economically important cattle diseases not only in Africa, Middle East but also in Europe and Asia. Although the mortality rate is usually low (10%), economic losses result from damaged hides, decreased milk production, abortion, loss of traction power, poor growth, weight loss, and infertility (Tuppurainen and Oura, 2012). The disease is caused by a lumpy skin disease virus (LSDV). LSDV is a member of the genus Capripoxvirus (CaPV) within the family Poxviridae (Buller et al., 2005). Sheep pox virus (SPPV) and goat pox virus (GTPV) share LSDV in the genus and are closely related to it (Maclachlan and Dubovi, 2011).
LSD may occur as an acute, sub-acute or chronic form and cause mild to severe symptoms. Clinical signs of LSD include fever, the appearance of raised, circular, firm nodules on the skin, skin edema, and lymphadenitis (Tuppurainen et al., 2005). Lesions also develop on the mucous membranes of mouth, nostrils, and respiratory tract, with subsequent pneumonia (Tuppurainen and Oura, 2012). The severity of clinical signs of LSD and its timeline of infection depend on the host immune status, age, sex and breed type (OIE, 2010). Diseases and factors which overwhelmed the immune status of the animal such as blood parasite in Egypt might add to the severity of LSD infection. In general, young animals often have more severe disease and develop lesions within 24 to 48 h (Al-Salihi, 2014). Fine-skinned breeds are considered the more vulnerable breeds to LSD infection such as Holstein-Friesian (H-F) and Jersey breeds (Davies, 1991; Babiuk et al., 2008).
Clinical signs are highly suggestive and pathognomonic to LSD. The clinical diagnosis is confirmed by using either conventional polymerase chain reaction (PCR) (Ireland and Binepal, 1998; Heine et al., 1999) or real time PCR (Lamien et al., 2011b). Several reports shed light on the possibility of utilizing the alterations in some hematological and biochemical parameters to help better understanding the pathogenesis and prognosis of the disease.
These alterations can be observed when cellular or organ damage occurs. In case of viral diseases cellular damage takes place when the virus replicates in essential cells sufficiently and destroys them directly or damages organ function indirectly through cytotoxic immune response towards viral infected cells (Baron et al., 1996).
There is a lack of knowledge about the hematological and serum biochemical findings of calves naturally infected with LSDV. The purpose of the current study was to investigate the changes in hematological and biochemical parameters in calves naturally infected with LSDV.
MATERIALS AND METHODS
Epidemiological data
The present study was conducted in a small village in EL-Wasta, Beni-Suef, Egypt where a sporadic outbreak of LSD occurred during September: November 2019 after arrival of an apparently health bull purchased from El-Beheira, Egypt on 22 of August 2019. By September 1, 2019 the bull suffered from clinical signs typical to LSD. After one week (7/9/2019) the bull died. By September 15, 2019 a calf in-contact with the bull suffered from the same signs. From 17/9/2019 through 26/11/2019 a total of eight cases were observed in the neighboring premises. The village had a history of vaccination against LSD with sheep pox vaccine (Yugoslavian RM65 strain) nine months before the onset of the outbreak.
Animals
A total of 14 male and female calves ageing 8-11 months were included in the present work. Nine calves showed the characteristic clinical signs of LSD were selected as LSDV infected group. Five healthy calves of the same age and breed free from blood parasites were used as control group. Calves were thoroughly examined, and the clinical findings were recorded (Table 1).
| No. | Animal data | Clinical signs | |
| Species/breed | Age | ||
| 1 | Calf/Friesian | 9 Month | Fever, skin nodules, edema in limb, Pneumonia followed by death after a course of one week |
| 2 | Calf/Friesian | 10 Month | Fever, skin nodules and edema in limb |
| 3 | Heifer/Baladi | 8 Month | Fever, skin nodules |
| 4 | Heifer/Baladi | 11 Month | Fever, skin nodules |
| 5 | Heifer/Crossbreed | 11 Month | Fever, skin nodules and edema in limb |
| 6 | Calf/Baladi | 8 Month | Fever, skin nodules |
| 7 | Heifer/Crossbreed | 9 Month |
Fever, skin nodules and edema in limb and dewlap |
| 8 | Calf/Baladi | 11 Month | Fever, skin nodules |
| 9 | Calf/Friesian | 10 Month | Fever, skin nodules |
Ethical approval
All clinical samples were collected as per standard sample collection procedure without giving any harm or stress to the animals. The present work was approved by the Ethical Commetee for Medical Research at the College of Veterinary Medicine, Beni-Suef University and Animal Care Guidelines of the General Organization for Veterinary Services, Egypt.
Samples
Tissue samples
Skin nodules were surgically extirpated after the skin was locally anesthetized with 2% lidocaine then placed in glycerol saline and stored at −20°C for PCR analysis.
Blood samples
Approximately, 6ml of blood per calf was drawn aseptically from Jugular vein, of which 2 ml of blood was transferred to a sterile vial containing disodium ethylenediaminetetraacetic (EDTA) for leukocytic examination and PCR. The remaining 4 ml of blood was transferred to vacutainer tube for serum separation. Serum samples were kept at –20 oC till used for biochemical examination.
PCR
DNA was extracted from collected skin lesions and blood using a DNA Mini Kit (Thermo, Germany) according to the manufacturer’s instructions.
PCR run was performed using primer set targeting RP030 gene with following sequences; forward primer: F 5’-TCTATGTCTTGATATGTGGTGGTAG-3 and reverse primer: R 5’-AGTGATTAGGTGGTGTATTATTTTCC-3 and expected amplicon size 172bp (Lamien et al., 2011a). PCR amplification was conducted using PCR master mix (Thermo, USA) in a total volume of 25μl/reaction. The thermal profile started by an initial denaturation at 94°C for 4 minutes, followed by 35 cycles of denaturation at 94°C for 45 second, annealing at 55°C for 45 second and extension at 72°C for 1 minute and a final extension at 72°C for 7 minutes. PCR products were electrophoresed in 2% agarose gel containing ethidium bromide at 100V for 30 minutes and visualized in trans-illuminator.
Leukogram analysis
Leucogram profile was done within one hour after sample collection. Both total and differential leucocytic counts were estimated according to Feldman et al. (2000).
Biochemical analysis
The harvested serum was used to measure total protein, albumin, aspartate aminotransferase (AST), GGT, CK, blood urea nitrogen (BUN), creatinine, cholesterol and serum sodium and potassium concentrations. Biochemical analyses were conducted by spectrophotometrically using commercially available diagnostic kits (Diamond diagnostics, Holliston, United states) according to manufacturer’s instructions.
RESULTS
Clinical findings
The LSDV infected calves (LSD group) exhibited the following clinical signs; inappetence, dullness, high fever (40.70±0.25°C vs. 38.68±0.24°C in affected and control calves, respectively), increased respiratory and heart rates (39.46±1.16 vs. 25.62±1.18 and 91.60±1.24 vs. 57.20±1.64 in affected and control calves, respectively). Firm circumscribed skin nodules about 0.5-5 cm in diameter in skin accompanied with enlargement of superficial lymph nodes especially prescapular and precrural lymph nodes were detected. Skin nodules were distributed in various body parts involving the neck, chest, abdomen, limbs, perineal area, and muzzle (Figure 1). Excessive salivation, lacrimation and nasal discharge were also noted in all naturally infected calves. Edematous swelling of dewlap one or more legs with lameness were observed in four animals (two Friesian calves and two crossbreed heifer) and one of them were suffered from pneumonia followed by death after a course of one week.
Detection of viral DNA by PCR
Viral DNA was identified in all skin lesions collected from diseased calves (n= 9) and was negative in all control animals (n= 5). The RPO30 gene-based PCR generated PCR products with a length of 172 bp (Figure 2).
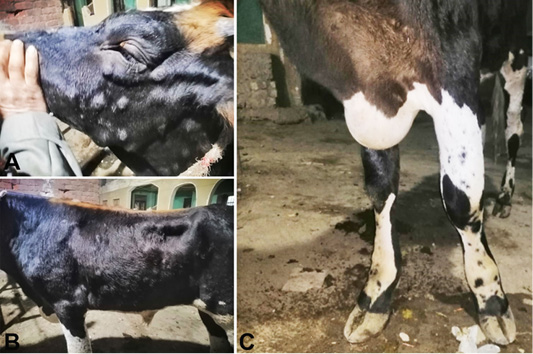
Figure 1: Clinical sings of LSD, A. Cutaneous nodules on the head and neck regions, B. lumps on different body regions with enlargement of superficial lymph nodes. C. Edema in the dewlap and fore limb
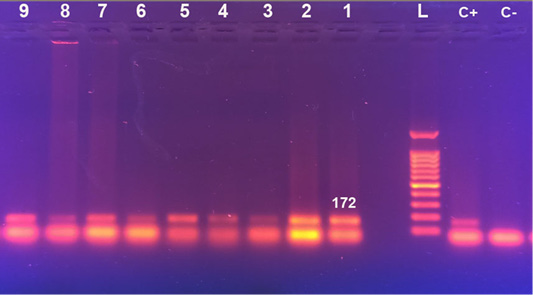
Figure 2: Gel electrophoresis of RPO30 gene-based PCR C-: control negative, C+: control positive, L: 100bp DNA ladder, Lane 1:9: naturally LSDV infected calves samples
Leukogram and biochemical analysis
Results of leucogram showed significant leukocytosis accompanied with lymphocytosis and monocytosis in LSDV infected calves when compared to control group (Table 2). The total protein, total CK, AST, BUN, creatinine and potassium levels showed significant high values while cholesterol levels significantly lowered in diseased calves when compared to healthy one. Serum albumin, globulins, A/G ratio, Serum GGT activity and sodium levels were not significantly altered (Table 2).
DIscussion
Transfer of subclinically infected animal already incubating the disease is considered one of the main risk factors for LSD spread (Tuppurainen and Oura, 2012). In the current study the illegal movement of an apparently health bull is assumed to be responsible for the development of such outbreak. The appearance of severe clinical signs in calves is attributed to the lack of either passive or active immunity against LSD. The last vaccination against LSD in the village was conducted in January 2019, at that time the calves were aging less than 3 months of age therefore they received no vaccine shots with no history of exposure to field infection until the beginning of the outbreak. On clinical examination, calves were suffered from high fever, firm circumscribed skin nodules about 0.5-5 cm in diameter in skin accompanied with enlargement of superficial lymph nodes especially prescapular and precrural lymph nodes. Excessive salivation, lacrimation and nasal discharge were also noted in all naturally infected calves. Edematous swelling of one or more legs with lameness were observed in four animals (two Friesian calves and two crossbreed heifer) and one of them was suffered from pneumonia followed by death after a course of two weeks. All these lesions were reported previously by Prozesky and Barnard (1982); Coetzer (2004); Awadin et al. (2011) and prove that LSD is more severe in Friesian and crossbreed than native breed.
Table 2: Alteration in some haematological and biochemical parameters in calves naturally infected with LSDV (mean ± SE).
| Control | Naturally infected calves | |
|
TLC (x 103//ul) |
6.11 ± 0.20 | 8.45 ± 0.15 ** |
|
Lymphocytes (x 103//ul) |
3.47 ± 0.11 | 4.54 ± 0.09 * |
|
Neutrophils (x 103//ul) |
2.17 ± 0.28 | 3.13 ± 0.23 |
|
Monocytes (x 103//ul) |
0.27 ± 0.02 | 0.42 ± 0.05 * |
|
Eosinophils (x 103//ul) |
0.20 ± 0.04 | 0.36 ± 0.09 |
| AST (U/L) | 55.33 ± 2.26 | 142.12 ± 3.92*** |
| CK (U/L) | 98.67 ± 1.86 | 183.31 ± 8.82** |
| GGT (U/L) | 24.66 ± 2.40 | 20.00 ± 1.52 |
| Creatinine (mg/dl) | 1.14 ± 0.01 | 1.31 ± 0.05* |
| BUN (mg/dl) | 18.33 ± 0.88 | 26.67 ± 2.23* |
| Cholesterol (mg/dl) | 212.00 ± 1.53 | 61.33 ± 0.89*** |
| Na (mmol/L) | 131.50 ± 1.76 | 148.67 ± 7.86 |
| K (mmol/L) | 5.03 ± 0.023 | 6.03 ± 0.37 |
| Total protein (g/dl) | 6.37 ± 0.19 | 7.54 ± 0.29* |
| Albumin (g/dl) | 3.46 ± 0.13 | 4.10 ± 0.28 |
| Globulin (g/dl) | 2.91 ± 0.23 | 3.43 ± 0.36 |
| A/G ratio | 1.19 ± 0.02 | 1.20 ± 0.10 |
Significant *P ≤ 0.05 **P ≤ 0. 01 ***P ≤ 0.001.
PCR is the best technique for quickly detecting and identifying the causative agent of the examined viral outbreak. PCR targeting RPO30 gene were confirm the presence of LSDV in skin lesions collected from affected calves. RPO30 gene-based PCR assay according to Lamien et al. (2011a) affords a simply approach for CaPV classification and aids in the swift differentiation between GTPV/ LSDV and SPPV without the requisite of DNA sequencing (Rouby, 2018).
Differential leucocytic count of the blood samples obtained from naturally infected calves showed a significant leukocytosis accompanied with neutrophilia, lymphocytosis and monocytosis as compared to control group. This result in agreement with Abutarbush (2015). On the contrary, El-Shoukary et al. (2019) reported leucopenia in a LSD infected bull. This is probably related to the stage and severity of infection (Ahmed, 2015). In cattle, leukopenia is usually seen in the developmental stage of the acute infection, after which the production of neutrophils is intensified, leading to leukocytosis (Morris, 2002). Leukocytosis could be due to secondary acute bacterial infections, especially pyogenic bacterial infections as reported by Ahmed (2015). Viral diseases and chronic inflammatory condition might be causes for lymphocytosis. Monocytosis can occur any time that neutrophilia occurs, because both cell lines are derived from a common bipotential stem cell. Monocytosis may be observed in both acute and chronic stages of disease (Latimer, 2011).
Regarding serum biochemical findings, most calves naturally infected with LSDV had hyperproteinemia. Hyperproteinemia in the present study could be due to dehydration. On the other hand, hyperproteinemia associated with the inflammatory leukogram confirm the presence of severe inflammatory condition as reported by Baron et al. (1996). It is known that LSDV replicates in pericytes, endothelial cells and probably some cells in blood vessel and lymph vessel walls. This fact results in severe vasculitis and lymphangitis in affected areas with proliferation of lymphocyte (Prozesky and Barnard, 1982). Vasculitis was also reported in a small number of vessels (<10) in organs other than the skin and associated musculature, including the kidney, small intestine, and cardiac muscle which called Extracutaneous LSDV lesions (Bernardo et al., 2020). Extracutaneous lesions have been reported previously in muscle, liver, rumen and lung of premature calf with LSD (Rouby and Aboulsoud, 2016). This explains the elevation of AST, total CK, creatinine and the potassium levels in calves suffered from LSD. AST present in the hepatocytes, skeletal muscle and muscular cardiac cells and its elevation may be attributed to the muscular injuries. LSD lesions was previously reported in the muscle fascia, in the skeletal muscles (Barnard et al., 1994) and cardiac muscles (Vasatova et al. 2013; Sevik et al., 2016).
The hyperkalemia and the significant increase in the total CK detected in the current study might be attributed to the muscular damage (Hoffmann and Solter, 2008) or metabolic acidosis associated with LSD infection (Radostitis et al., 2000; Carlson, 2002). However, Marmor et al. (1988) indicated that CK levels might be elevated due to cardiac injury. Renal affections might be a cause of hypekalemia.
A significant increase in the level of serum creatinine and blood urea nitrogen in diseased calves were observed in comparison with apparently healthy animals. It has been stated that an increase in creatinine level reflects a decrease in the glomerular filtration rate (Gowda et al., 2010; Samra and Abcar, 2012). High serum urea and creatinine levels in LSDV naturally infected animals agree with other previously published results (Helmy et al., 2017). According to Morris and Johnston (2002), A significant increase in blood urea nitrogen in diseased animals might be attributed to anorexia, the direct effect of LSDV on the kidneys, loss of muscle mass, increased protein catabolism and reduced renal blood flow during the viraemic stage of LSD. A decrease in serum total cholesterol level in the diseased calves is in agreement with that found in other viral infectious diseases and stressful conditions in the ruminant animals (Fernandez et al., 2011). Hypocholesterolemia may be due to impaired cholesterol absorption as a sequela of vasculitis in small intestine of LSDV infected animals as Bernardo et al. (2020). Under stressful diseased condition, hypocholesterolemia may occur as a result of enhanced rate of macrophage-specific reverse cholesterol transport, increased transit of cholesterol through the large intestine, and increased fecal bile acid excretion (Silvennoinen et al., 2015).
CONCLUSIONS AND RECOMMENDATIONS
Application of some laboratory investigations (including some hematological and biochemical parameters) in correlation with clinical signs may serve in diagnosis of LSD.
Novelty Statement
The study describes The Association Between Clinical Signs of LSD and Biochemical Alterations in naturally infected calves.
Author’s Contribution
Sherin Rouby, Olfat Shehata and Morad Mahmoud designed the study and performed laboratory works, Ahmed S. Abdel-Moneim reviewed the manuscript, Khaled Hussein collected the Field samples.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






