Advances in Animal and Veterinary Sciences
Research Article
Detection of Brucella Antibodies in Sheep with Special Aspect of Clinicalstatus and Breed
Kirit B. Patel1*, Harshad C. Chauhan2, Sandip S. Patel3, Bharat K. Patel4, Mehul D. Shrimali5, Arun C. Patel6, Bharatsingh S. Chandel7
1*,2,4,5,7Department of Animal Biotechnology, College of Veterinary Science & A.H, SDAU, Sardarkrushinagar-385506, Banaskantha, Gujarat, India; 3,6Department of Veterinary Microbiology, College of Veterinary Science & A.H, SDAU, Sardarkrushinagar-385506,Banaskantha,Gujarat, India.
Abstract | Brucellosis is an important zoonosis and a significant cause of reproductive losses in animals. To ascertain the serological status of the disease, a total of 1373 samples were screened for the presence of Brucella antibodies by RBPT and i-ELISA. Overall seroprevalence recorded in sheep was 6.84% (94 samples out of 1373) and 4.80% (66 out of 1373) by RBPT and i-ELISA respectively. Clinical status wise seroprevalence was recorded in heifer 4.65% and 6.97%, clinically healthy animals 2.62% and 1.79%,animals with the history of abortion 16.58% and 13.98%, hygroma 27.27% and 18.18%,pregnant 4.59% and 2.29%, non-pregnant 6.80% and 4.18%, status unknown 10.78% and 6.86%, still birth 17.64% and 5.88%, retention of placenta 20.00% and 20.00%,orchitis16.12% and 9.67% respectively by RBPT and i-ELISA, respectively. Breedwise highest seroprevalence was recorded in Patanwadi 7.82% and 5.99% followed by Marwadi 6.35% and 4.16%,Magra 3.84% and 1.28%, Avikalin 4.76% and 0.00%and Chokhla 1.78% and 0.00%by RBPT and i-ELISA, respectively. Present study revealed that the Patanwadi breed of the sheep is relatively more susceptible and high rate of seroprevalence was observed from the animals having history of abortion, retention of placenta and hygroma.
Keywords | Seroprevelence, Sheep, Brucellosis, RBPT, I-ELISA
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 05, 2017; Accepted | August 18, 2017; Published | November 10, 2017
*Correspondence | Kirit B Patel, Department of Animal Biotechnology, College of Veterinary Science & A.H, SDAU, Sardarkrushinagar-385506, Banaskantha, Gujarat, India; Email: [email protected]
Citation | Patel KB, Chauhan HC, Patel SS, Patel BK, Shrimali MD, Patel AC, Chandel BS (2017). Detection of brucella antibodies in sheep with special aspect of clinicalstatus and breed. Adv. Anim. Vet. Sci. 5(12): 486-490.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.12.486.490
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Kirit Patel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Brucellosis is caused by various bacterial species of the genus Brucella, which is the second most widespread zoonosis worldwide (Dawood, 2008).It is one of the infectious diseases, which poses major constraints for animal production. The disease is an important public health problem in many parts of the world including India (Pal, 2007; Hadush and Pal, 2013). The disease is manifested by late term abortions, weak calves, still births, infertility and characterized mainly by placentitis, epididymitis and orchitis, with excretion of the organisms in uterine discharges and milk (England et al., 2004). In addition to its direct effects on animals, brucellosis causes economic losses through abortions, stillbirths or the death of young stock. The disease can also have a blow on exports and have negative impact on the efforts to improve breeding. Brucellosis has a considerable impact on animal and human health, as well as wide socio-economic impacts, especially in countries in which rural income relies largely on livestock breeding and dairy products (Maadi et al., 2011). As signs and symptoms of brucellosis are unspecific, culture and serology are necessary for diagnosis (Colmenero et al., 1996). Although India is largest milk producer in the world (102 million tons), animal resource in the country is threatened by reproductive disorders viz., infertility, retained placenta, abortion, endometritis etc., causing considerable economic losses. Brucellosis has been one of the most important reproductive diseases among different livestock species as well as animal handlers (Verma et al., 2000; Chahota et al., 2003). It is an emerging disease since the discovery of Brucellamelitensis as the cause of Malta fever by Bruce in 1887 and the isolation of B. abortus from aborted cattle by Bang in 1897 (McMahan, 1944). Brucellosis is essentially a disease of sexually matured animals this indicates its impact on human and animal health, as well as socioeconomic status, especially, where rural income relies largely on animal husbandry and animal byproduct industry. Brucellosis is found worldwide. Though it has been eradicated from many countries, it is one of the most serious diseases in developing countries. The rate of infection varies greatly from one country to another and between regions within the country, with highest prevalence in dairy cattle. In India, brucellosis was first reported in 1942 and is now endemic throughout the country (Renukaradhya et al., 2002). In general, risk factors such as unrestricted trade and movements of animals, free grazing and movement with frequent mixing of flocks of sheep and goats also attribute to the high prevalence and wide distribution of brucellosis. In addition, increasing demand for dairy products and animal protein, changing agricultural methods and movement of animals have caused high prevalence of disease. Accurate diagnosis of brucellosis is essential for institution of control strategies, either disease as a whole or as species-specific. The most widely used serological tests for diagnosis of brucellosis in animals are rose bengal plate test (RBPT), standard tube agglutination test (STAT) and enzyme linked immunosorbent assay (ELISA ). Since, neither a single serological test nor a combination of tests detects all infected animals, nor also due to high homology among Brucella species, the detection of brucellosis remains a major problem. Hence the present study was planned with an objective to study the seroprevalence of Brucellosis among various sheep breeds and their correlation with clinical signs. Present study will help to identify the resistant breed/animal, strong positive reactor from the heathy heard, which may help in segregation of such animals which facilitate the easy control of diseases and on long run become economic to farmer. For these reason we focus on the detection of Brucella antibodies with special aspect of clinical status and breed.
MATERIALS AND METHODS
Collection of Samples
A total of 1373 sera were collected from sheep screened by RBPT and i-ELISA for detection of Brucella antibodies. Approximately 10 ml of blood was collected from individual animal aseptically from jugular vein using plain vacutainer (BD vacutainer). The vacutainer tubes were kept in slanting position at room temperature for two hours and centrifuged at 3000 rpm for 10 minutes. The separated serum was collected in screw capped plastic vial and held at –200C temperature till further use.
Rose Bengal Plate Test (RBPT)
The RBPT antigen was procured from the Institute of Animal Health and Veterinary Biological (IAH and VB), Hebbal, Bangalore. One drop (0.03 ml) of serum was taken on a glass slide by micropipette. The antigen bottle was shaken well to ensure homogenous suspension and then one drop (0.03 ml) of the antigen was added. The antigen and serum were mixed thoroughly with sterile tooth picks and then the slide was rotated for four minutes and result was read immediately. Definite clumping/agglutination was considered as positive reaction, whereas no clumping/agglutination was considered as negative.
Indirect-enzyme linked Immunosorbent Assay (i-ELISA)
Indirect ELISA kit was procured from National institute of Veterinary Epidemiology and Disease informatics (NIVEDI) Bangalore, and used as per manufacturer’s protocols. The kit detects the antibodies against Brucella lipopolysaccharide (LPS) in serum samples.
Procedure
Interpretation of Results
Percent positivity (PP) values which were used for acceptance of test sera data and for diagnostic interpretation are calculated as follows:
PP = (Average OD value of test wells / Median OD of C++ well (Positive control) X 100
The mean of any sample that gave a PP value between 55 to 65 per cent was moderate positive and below 55 per cent was taken as negative. If sample showed a PP value of that below 55 per cent was negative. On the other hand, when a sample showed a PP value of 54 per cent, then, it was retested.
RESULTS AND DISCUSSION
OverallSseroprevalence
In the present study RBPT and i-ELISA used for detection of brucella antibodies in sheep sera. Out of 1373 sera tested, 94 (6.84 %) and 66 (4.80 %) were found to be positive for Brucella antibodies by RBPT and i-ELISA, respectively (Figure 1 and Figure 2). Similarly, Lone et al., (2013) reported 6.50% and Hassanain and Ahmed (2012) reported 5.71% seroprevalence in sheep by RBPT. Panchasara et al. (2015) recorded more or less similar overall seroprevalence was 10.66%, 10.29% and 9.38% by RBPT, STAT and i ELISA, respectively in North Gujarat. Tayshete (2001) detected 4.0% seroprevalence in north Gujarat by i-ELISA.

Figure 1: Rose Bengal test
In contrast to the present findings Awandkae et al. (2012), Azmi (2012) and Raju (2005) reported 28.10%, 21.10% and 12.00% seroprevalence in sheep by RBPT, respectively. Sulima et al. (2010) and Al-Mariri et al. (2011) was detect 20.35% and 60.00% seroprevalence in sheep by i-ELISA, respectively.
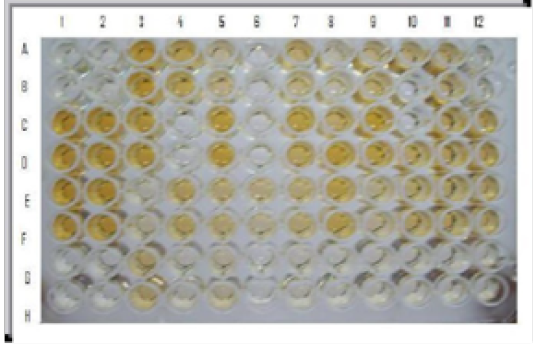
Figure 2: i-ELISA: Wells A1, B1, A2, B2: Negative control; Wells C1, D1, C2, D2: Moderately positive control; Wells E1, F1, E2, F2 : Strong positive control; Wells A3, B3, C3, D3, C5, D5 etc. field sera indicate positive reaction
Clinical Status Wise Seroprevalence
In the present study, serum sample collected from sheep showing different clinical condition. The rate of seroprevalence was highest in hygroma (27.27% and 18.18%), followed by retention of placenta (20.00% and 20.00%), still birth (17.64% and 5.88%), abortion (16.58% and 13.98%), orchitis (16.12% and 9.67%), status unknown (10.78% and 6.86%), non-pregnant (6.80% and 4.18%), pregnant(4.59% and 2.29%) and Clinically healthy status (2.62% and 1.79%) of sheep in Gujarat by RBPT and i-ELISA, respectively (Table 1). Patel (2015), who found highest seroprevalence was obtained in abortion of 57.14% and 46.43% followed by hygroma (50.00% and 37.50%) and orchitis (40.00% and 20.00%) by RBPT and i-ELISA, respectively.
Breed Wise Seroprevalence
In the present study, five breeds of sheep were included. The rate of seroprevalence was highest in Patanwadi (7.82% and 5.99%), followed by Marwadi (6.35% and 4.16%), Magra (3.84% and 1.28%), Avikalin (4.76% and 0.00%) and Chokhla (1.78% and 0.00%) by RBPT and i-ELISA, respectively (Table 2). In support to present findings of Patel (2015) highest seroprevalence was obtained in patanwadi sheep of 14.80% and 13.27% followed by 12.50% and 11.41% in Marwari; 8.82% and 5.88% in Magra; 6.45% and 3.23% in Avikalin and no seroprevalance in Chokhla breed by RBPT and I-ELISA, respectively. However, Kotadiya (2012) reported 15.68% and 23.52% seroprevalence of brucellosis in Marwari breed followed by Patanwadi breed (9.60 and 15.25%) and Magra breed (8.82% and 11.76%) of sheep in Gujarat by RBPT and i-ELISA, respectively.
Table 1: Clinical status wise seroprevalence
| Status | No. of tested | RBPT Positive | i-Elisa Positive |
| Clinically healthy | 725 | 19(2.62) | 13(1.79) |
| Abortion | 193 | 32(16.58) | 27(13.98) |
| Hygroma | 22 | 06(27.27) | 04(18.18) |
| Pragnant | 87 | 04(4.59) | 02(2.29) |
| Non-pragnant | 191 | 13(6.80) | 08(4.18) |
| Status unknown | 102 | 11(10.78) | 07(6.86) |
| Still birth | 17 | 03(17.64) | 01(5.88) |
| Retention of Placenta | 05 | 01(20.00) | 01(20.00) |
| Orchitis | 31 | 05(16.12) | 03(9.67) |
| Total | 1373 | 94(6.84%) | 66(4.80%) |
Table 2: Breedwise seroprevelence
| Breed | No.of tested | RBPT Positive | i-ElisaPositive |
| Patanwadi | 767 | 60(7.82%) | 46(5.99%) |
| Marwadi | 456 | 29(6.35%) | 19(4.16%) |
| Magra | 78 | 03(3.84%) | 01(1.28%) |
| Chokhla | 56 | 01(1.78%) | 00(0.00%) |
| Avikalin | 21 | 01(4.76%) | 00(0.00%) |
| Total | 1373 | 94(6.84%) | 66(4.80%) |
CONCLUSIONS
The present study indicates prevalence of brucellosis in sheep in Gujarat. Overall seroprevalence detected in sheep was 6.84% and 4.80% respectively by RBPT and i-ELISA. The high rate of prevalence recorded might be the reason of samples collected from the herds/flocks having history of reproductive disorder. Moreover, the variation in breed wise seroprevalence rate of sheep might be due to disease resistance.
ACKNOWLEDGMENTS
The authors are thankful to the Animal Biotechnology, College of Veterinary Science & A.H, Sardarkrushinagar Dantiwada Agricultural University, Sardar krushinagar for providing necessary facility. We are highly thankful to DBT, Govt. of India for financial assistance for the project.
CONFLICT OF INTEREST
There is no conflict of interest with any of the party either directly or indirectly to the content of this article.
Authors Contribution
Kirit B. Patel carried out the experiment, analyzed epidemiological data and prepared the draft manuscript, Harshad C. Chauhan and Bharatsingh S. Chandel provided guidance and technical support, Bharat K. Patel carried out the experiment and analyzed epidemiological data, Sandip S. Patel and Arun C. Patel edited the manuscript. Mehul D. Shrimali carried out the sample collection.
REFERENCES






