Acaricidal and Repellent Efficacy of Cinnamomum verum Essential Oil Against Rhipicephalus microplus Ticks
Acaricidal and Repellent Efficacy of Cinnamomum verum Essential Oil Against Rhipicephalus microplus Ticks
Muhammad Salman1, Rao Zahid Abbas1*, Muhammad Kasib Khan1 and Muhammad Shahid Mahmood2
1Department of Parasitology, University of Agriculture, Faisalabad, Pakistan
2Institute of Microbiology, University of Agriculture, Faisalabad, Pakistan
ABSTRACT
The emergence of issues like non-target toxicity, drug residues and drug resistance has stressed the need to search for effective alternatives such as botanicals. Hence, the current research was aimed at the evaluation of acaricidal and repellent efficacies of the Cinnamomum verum bark essential oil against the Rhipicephalus microplus ticks of cattle. Moreover, the C. verum essential oil was also analysed using GC-FID analysis which revealed the cinnamaldehyde as its major component. The acaricidal and the repellent efficacies were evaluated at five concentrations (1, 2.5, 5, 10 and 20%) of the C. verum essential oil. Different parameters like adult tick mortality, fecundity index, egg hatchability, oviposition reduction, reproductive estimation, product effectiveness, larval mortality and tick repellency were determined. The results indicated a dose-dependent effect of C. verum essential oil showing 20% concentration to be the most effective in view of the acaricidal and the repellent activities. Hence, this essential oil can be considered as a potential candidate for the effective control of cattle tick (R. microplus).
Article Information
Received 19 May 2023
Revised 28 May 2023
Accepted 08 June 2023
Available online 26 June 2023
(early access)
Published 16 July 2024
Authors’ Contribution
MS methodology, research and writing first draft of the article. RZA methodology, supervision, review and editing. MKK methodology and supervision. MSM methodology and supervision.
Key words
Ticks, Rhipicephalus microplus, Essential oil, Cinnamomum verum, Acaricidal, Repellent
DOI: https://dx.doi.org/10.17582/journal.pjz/20230519150520
* Corresponding author: [email protected]
0030-9923/2024/0005-2107 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Parasitism with particular emphasis on tick infestation is the most important constraint towards profitable livestock rearing especially of large ruminants (Salman et al., 2020). Almost 80% of cattle population across the globe is at risk of contracting tick infestations and their associated diseases (Burrow et al., 2019). Ticks are the blood-sucking parasitic arthropods which are prevalent all over the world. However, the most important genus of hard ticks affecting large ruminants is the Rhipicephalus (Rooman et al., 2021). Rhipicephalus species inflict heavy financial losses on the large ruminant industry. They suck blood from their hosts causing a direct impact on the economics of the livestock production system in terms of reduced milk production, increased weight loss and physical damage to hides whereas indirect losses are associated with ticks-vectored protozoal, viral, and bacterial diseases (Saleem et al., 2019; Calvano et al., 2021; Ceylan et al., 2021; Hussain et al., 2021). Among these species, R. microplus is the most important tick in large ruminants. Its importance can be understood from the fact that an engorged female of R. microplus ticks can cause a 0.6g reduction in weight gain of beef calves (Zaman et al., 2012). Moreover, 22-30 billion dollars annual loss is estimated to be caused by alone R. microplus infestation globally (Lew-Tabor and Valle, 2016).
Control of ticks is mainly dependent upon synthetic acaricidal drugs. These drugs are also used prophylactically and serve as the epicentre for the control and eradication measures against ticks. These drugs provide an effective and relatively quick control over tick populations (Selles et al., 2021). However, the long-term and extensive use of these drugs has caused some serious problems including the development of drug resistance, the presence of these drugs’ residues in meat and milk of animals, and their toxicity to non-target organisms (Nath et al., 2018; Singh et al., 2019; Srisanyong et al., 2021; Goswami et al., 2022). Moreover, vaccination against tick infestation is another preventive strategy to overcome this problem but again there are some constraints limiting its effectiveness. Firstly, no single vaccine can prevent all the prevalent tick species and, secondly, there are antigenic strain variations which also undermine the vaccination’s significance. Furthermore, the higher cost of vaccine development is another limiting factor (De La Fuente and Estrada-Pena, 2019; Ndawula et al., 2019).
Hence, there is a need for other effective alternative methods which can help us overcome the tick problem. Among these alternatives, botanicals can prove to be a promising solution. More than 2000 plant species are estimated to have some sort of pest control potential (Mamun and Ahmed, 2011). Many plants or their products are known to have antiparasitic and anti-tick activities (Shnawa et al., 2017; Selles et al., 2021; Eltaly et al., 2022). Of these products, essential oils are very important. A number of investigations carried out on essential oils have proven their repellent and acaricidal efficacies against various tick genera such as Rhipicephalus, Dermacentor and Amblyomma (Benelli and Pavela, 2018; Salman et al., 2020; Selles et al., 2021). Similar experiments using essential oils from various species of the Cinnamomum genus have revealed these oils to possess potent acaricidal activities. For example, the essential oils of C. cassia and C. verum have known acaricidal activities against the Haemaphysalis longicornis ticks (Qiao et al., 2021; Nwanade et al., 2023). However, the repellent and the acaricidal efficacies of the essential oil extracted from the bark of C. verum against the cattle ticks have not been investigated so far. Hence, the current study was undertaken for the estimation of the repellent and the acaricidal efficacies of the C. verum essential oil against the R. microplus ticks of large ruminants.
Materials and Methods
Essential oil
The essential oil was extracted from the bark of C. verum using the hydro-distillation technique with the help of a clevenger apparatus. After extraction, the essential oil was analysed through the gas chromatography-flame ionization detection (GC-FID) procedure. The procedure was carried out at the University of Agriculture Faisalabad, Pakistan using the gas chromatograph apparatus of Shimadzu, GC-17A of the Central Hi-Tech Laboratory. This apparatus was attached to the flame ionization detector equipped with column DB WEX (30m×0.25) using nitrogen as its mobile phase having 20 ml/min flow rate. The oven temperature was maintained at 90°C for 2 min, 180°C for 2 min with the maximum temperature reaching 240°C for 3 min. The temperatures of the injector and detector were 250 and 270°C, respectively. The identification of the essential oils constituents was made on the basis of a comparison of retention times of both the samples’ constituents and the standards used (Belhachemi et al., 2022).
Ticks
Only naturally-infested buffaloes and cattle with strict compliance of no acaricidal treatment in the past 30 days were selected for ticks collection. The ticks were collected from the district of Faisalabad, Punjab, Pakistan during the summer season. The collected ticks were transported to the Laboratory of Parasitology in special aerated plastic bottles having cotton swabs soaked in water for provision of the moisture. These ticks were washed, dried, and identified with the help of a stereomicroscope (Olympus SZ61) at 10X magnification using the Walker (2003) identification guide.
Test compounds
Five different concentrations (1, 2.5, 5, 10 and 20% v/v) of the C. verum essential oil were prepared using acetone as the solvent and the results were compared to the negative and the positive control groups. The negative control was the acetone for both the acaricidal and the repellent experiments while 0.1% cypermethrin acted as the positive control for the acaricidal bioassays whereas the diethyltoluamide (DEET) was the positive control in the repellent bioassay.
Acaricidal experiment
Dipping test
The test was conducted following the procedure described by Koc et al. (2013). During the test, 10 adult ticks of mixed sexes were selected at random and subjected to each dilution for five min of dipping. After this, these ticks were then taken out, placed in the clean jars and put into the biological oxygen demand incubator at 27°C and 90% relative humidity for 24 h. After 24 h, the ticks were observed, and the mortality was recorded by counting the live and dead ticks. Each treatment was replicated thrice in this experiment. The following formula was used for the determination of percent mortality (Sousa et al., 2022):
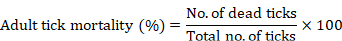
Adult immersion test
The adult immersion test was conducted on the engorged female ticks following the procedure of Drummond et al. (1973) for the estimation of efficacy of C. verum essential oil on the reproductive capability of ticks. The female ticks were selected on the basis of their size, mobility, and body integrity. The ticks were weighed and dipped in the respective test solutions for 30 sec. After dipping, these ticks were taken out and dried gently using tissue paper. Then, these ticks were put into the biological oxygen demand incubator having a relative humidity of 90% at 27°C for 20 days. After this period, the laid eggs were separated from dead female ticks to avoid microbial contamination. These eggs were weighed and again incubated at 27°C for 30 days at the same relative humidity level of more than 80% as mentioned above. Each of the test treatments comprised ten engorged female ticks and was replicated thrice. The parameters of fecundity index (FI), egg hatchability (EH), oviposition reduction (OR), reproductive estimation (RE), and product effectiveness (PE) were calculated as described previously (FAO, 2004; Castro et al., 2018; Jia et al., 2018):
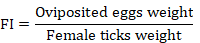
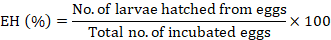

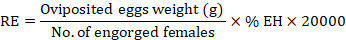
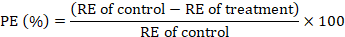
Syringe test
This test was conducted following the procedure of Sindhu et al. (2012). For this test, 3ml plastic syringes were cut and opened at their nozzle ends with the plunger partially pulled back. Around 200 (10 mg) eggs were placed in these syringes and their open ends were closed using organza fabric with tightly round rubber bands along the syringes barrels. These syringes were incubated at 27°C in the biological oxygen demand incubator having 90% relative humidity in the dark. After hatching, 14 days old larvae were used for estimation of the acaricidal potential of the essential oils. The syringes with larvae were filled with two millilitres of the test concentration and shaken for 30 sec. The test substance was then completely discarded and removed by pushing the plunger and with the help of tissue paper. Again, the plunger was drawn back to the marked 2 ml point. These syringes were then placed for one hour in the fume hood and then shifted to the biological oxygen demand incubator set at 27°C and 90% relative humidity under the dark conditions. After 24 h, the syringes were opened, and the mortality of larvae was recorded by counting the live and dead larvae. Only those larvae were considered live which had the ability of walking. The larval mortality (LM) was calculated using the following formula (FAO, 2004):
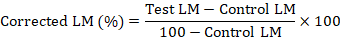
Repellency experiment: Tick climbing test
This experiment exploits the climbing behaviour of R. microplus ticks for the test of their repellence towards particular substances. The repellency test was conducted following the procedure of Ndungu et al. (1995). For this test, a specific apparatus was used consisting of aluminium base attached to aluminium rods. The aluminium rods were slide over with the glass tubes and the ends of these glass tubes were covered with moist cotton wool plugs. The purpose of these plugs was to block the ticks’ movement which climbed up to the top of these rods. These glass tubes provide the benefit of easy cleaning. These glass tubes were then wrapped around with filter paper strips of 1 cm width at 10 cm height from the base. This whole apparatus was then put into a tray having water in such a way that the upper surface of the base remained above water level. Ten ticks were then placed onto the base and the tubes were separated with a glass plate lifted 3 cm above base level to avoid mixing of odours. The test concentrations of the substances were then applied onto the filter paper strips. All this experiment was conducted at 27°C and almost 80% relative humidity and run for 1 hour. After one hour, the number of ticks above and below the filter paper strips were counted and the percentage repellence was calculated using the formula given below. This apparatus was cleaned thoroughly after running each experiment.
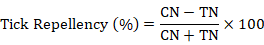
Where, CN is the number of ticks above the filter paper in the control while TN is the number of ticks above the filter paper in the respective treatment.
Statistical analysis
The obtained results for the experiments were analysed through the statistical procedures of the analysis of variance (ANOVA), Probit Analysis and Tukey’s means comparisons test. All these procedures were conducted by keeping a confidence level of 95% and the results were considered significant at P<0.05 (Barrios et al., 2022; Park et al., 2022). These statistical procedures were applied using the IBM SPSS statistical software.
Results and Discussion
The R. microplus ticks are the most important tick species throughout the globe, especially in the tropics, in terms of economic damage (Jabeen et al., 2022; Sultan et al., 2022). They are principally controlled by synthetic chemical acaricides. However, over the last few years, the research on botanicals including essential oils for investigation of their potential for control of ticks has been intensified. These botanicals are mixtures of a large number of active ingredients which possess diversified modes of action (Salman et al., 2020; Selles et al., 2021).
Essential oils, as the secondary metabolites, are the mixtures of different aliphatic and aromatic compounds produced by different families of the plant kingdom (Sharmeen et al., 2021). These essential oils may be extracted from the plants using different extraction techniques. However, in the current experiment, the essential oil was obtained from the C. verum bark through the process of hydro-distillation. When subjected to the phytochemical analysis, the C. verum essential oil was shown to be composed of a number of different components. These components were detected on the basis of their retention times using specific voltages. The major component revealed to have the highest concentration was cinnamaldehyde present at 33.6% concentration. These results were similar to the previous studies in the sense of cinnamaldehyde being the major component (Alizadeh et al., 2020; Pathak and Sharma, 2021). However, the difference observed in the detected concentration of cinnamaldehyde in the current experiment may be attributed to various factors like the age, soil nature, and the cultivar of the C. verum plant (Moghaddam and Mehdizadeh, 2017). Moreover, the chemical composition of the essential oils is also affected by the technique used for the essential oil’s extraction (Ayub et al., 2023). All the detected components along with their respective concentrations and retention times are listed in Table I.
Table I. GC-FID analysis of Cinnamomum verum essential oil.
|
Name of the component |
Concentration (%) |
Retention time (min.) |
|
Acetaldehyde |
1.9 |
2.317 |
|
Benzaldehyde |
7.8 |
22.567 |
|
Cinnamaldehyde |
33.6 |
28.750 |
|
Citral |
4.7 |
37.300 |
|
Ethyl acetate |
1.7 |
1.883 |
|
Eugenol |
4.6 |
25.700 |
|
Gamma terpinene |
0.8 |
19.367 |
|
Gamma undecalactone |
1.1 |
7.783 |
|
Geraniol |
9.5 |
5.150 |
|
Isopropyl acetate |
9.3 |
13.050 |
|
Limonin |
4.6 |
32.967 |
|
Linalool |
2.7 |
31.133 |
|
Nerol |
4.7 |
40.550 |
|
Octanal |
8.9 |
16.567 |
|
Unknown |
1.7 |
1.433 |
|
Valerolactone |
1.4 |
44.750 |
Table II. Lethal concentrations for Cinnamomum verum essential oil against adult ticks and larvae.
|
χ2 |
LC50 |
95% CI |
LC90 |
95% CI |
|
|
Adult tick |
1.950 |
6.258 |
4.912-8.090 |
22.284 |
15.495-40.036 |
|
Larvae |
3.706 |
5.009 |
4.658-5.385 |
16.646 |
14.829-19.009 |
As far as the acaricidal effectiveness of the C. verum essential oil is concerned, it exhibited a dose-dependent effect on the R. microplus ticks. The LC50 and LC90 values for the adults and larvae calculated using the probit analysis at 24 h are mentioned in Table II. The results revealed the larvae to be substantially more susceptible to the essential oil than the adult ticks as per the discovery of Ellse and Wall (2014). This acaricidal effect could have been exerted mainly by the cinnamaldehyde which was found to be the major component of this essential oil. Cinnamaldehyde is reported to be very effective against the R. microplus and other ticks exhibiting 100% mortality at 2.5-20 µL/mL concentrations (Nwanade et al., 2021). However, the varied response of the C. verum essential oil from that of the cinnamaldehyde is the reflection of overall antagonistic or synergistic interactions of both its major and minor components (Akhtar et al., 2012; Soares et al., 2016; Abbas et al., 2018).
Table III. Effect of different treatments on fecundity index and oviposition reduction of adult female ticks.
|
Treatment |
Fecundity index |
Oviposition reduction (%) |
Egg hatchability (%) |
Reproductive estimation (×20000) |
|
A |
51.78±8.31A |
2.76±15.60A |
80.1±4.1A |
41.70±8.81A |
|
B |
48.69±4.18AB |
8.56±7.86AB |
77.5±3.9AB |
37.78±4.48A |
|
C |
34.57±8.59B |
35.08±16.13B |
67.2±2.5B |
23.37±6.66B |
|
D |
15.12±2.03C |
71.61±3.81C |
40.2±3.1C |
6.12±1.29C |
|
E |
5.75±1.13C |
89.20±2.12C |
18.9±4.0D |
1.07±0.20C |
|
F |
53.25±7.84A |
0.00±14.73A |
84.1±6.1A |
44.50±3.75A |
|
G |
1.14±0.83C |
97.86±1.56C |
11.2±5.7D |
0.10±0.02C |
A: C. verum oil 1%; B: C. verum oil 2.5%; C: C. verum oil 5%; D: C. verum oil 10%; E: C. verum oil 20%; F: Negative control; G: Positive control. Mean values (±SD) with same superscript letters within the column differ non-significantly from each other (P>0.05).
In the current experiment, the C. verum essential oil also had a negative impact on the reproductive performance of the R. microplus ticks. This reproductive effect had a direct relation with the essential oil’s concentration and was reflected in the reduced fecundity index, oviposition reduction, egg hatchability, and reproductive estimation (Table III). For most of these parameters, the obtained results for the 10% concentration varied non-significantly ( P> 0.05) from those of the positive control group.
Moreover, the overall reproductive effect of the C. verum essential oil estimated through product effectiveness indicated the minimum 10% concentration of the essential oil to have similar results (P>0.05) to that of the positive control treatment (Fig. 1). The mechanism behind the acaricidal and reproductive effects of essential oils is very complicated. As the essential oils come into contact with the cuticle of ticks, these penetrate the haemolymph, thus, affecting the internal organs. Normally, ticks concentrate the ingested blood with the help of their salivary glands. Thus, any damage to these salivary glands leads to the poor absorption of nutrients. There exists a direct correlation between the ticks’ reproductive and digestive systems. Hence, the impaired digestive system results in the reduced reproductive performance by the ticks (Remedio et al., 2016). As the R. microplus ticks possess a high biotic capability, hence, the products which can affect the reproductive performance of these ticks are of great importance for tick control (De Oliveira et al., 2016). These substances affect the ovaries, thus, reducing the female tick’s capability to lay viable eggs (Remedio et al., 2015; Wang et al., 2020). Moreover, these oils may block the respiratory spiracles of ticks and disintegrate their cuticular waxes, thus, leading to suffocation and water stress (Agwunobi et al., 2020). Additionally, the central nervous system of the ticks may also get affected, thus, leading to the neurotoxic effect (Selles et al., 2021).
Similarly, the C. verum essential oil also exhibited a dose-dependent repellent effect against the R. microplus ticks. The probit analysis revealed the EC50 and the EC90 concentrations for C. verum essential oil to be 7.575 and 27.098%, respectively. The probit repellency graph for different concentrations of the C. verum essential oil is shown as Figure 2.
As mentioned earlier in the discussion, the current repellent activity of the C. verum essential oil may also be attributed to the cinnamaldehyde which is already known to have repellent activity against insects (Deletre et al., 2019; Wahab et al., 2020). The volatile chemical substances of the essential oils are responsible for the exertion of repellent effects owing to their vapour barrier. They produce a driving impact on their target arthropod species which are then forced to move away from these odorous substances, thus, imparting a repellent potential to these substances. However, this volatile nature of the essential oils leads to the faded activity with time (da Silva Lima et al., 2016; Chen et al., 2019; Salman et al., 2020; Gupta et al., 2022).
Conclusion
From the results of the current experiment, it is very clear that the C. verum essential oil is capable of both killing and repelling the R. microplus ticks. However, trials on animals are suggested before recommending the use of this essential oil under the field conditions. Moreover, research should also be conducted on improving the techniques for essential oils extraction and prolonging the residual life of these volatile oils.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abbas, A., Abbas, R.Z., Masood, S., Iqbal, Z., Khan, M.K., Saleemi, M.K. and Khan, J.A., 2018. Acaricidal and insecticidal effects of essential oils against ectoparasites of veterinary importance. Bol. Latinoam. Caribe. Plantas. Med. Aromat., 17: 441–452.
Agwunobi, D.O., Hu, Y., Yu, Z. and Liu, J., 2020. Cymbopogon citratus essential oil induced ultrastructural and morphological changes in the midgut, cuticle and Haller’s organ of the tick Haemaphysalis longicornis (Acari: Ixodidae). Syst. appl. Acarol. 25: 2047–2062. https://doi.org/10.11158/saa.25.11.10
Akhtar, Y., Pages, E., Stevens, A., Bradbury, R.O.D., da Camara, C.A. and Isman, M.B., 2012. Effect of chemical complexity of essential oils on feeding deterrence in larvae of the cabbage looper. Physiol. Ent., 37: 81–91. https://doi.org/10.1111/j.1365-3032.2011.00824.x
Alizadeh, B.B., Falah, F., Lavi, A.F., Vasiee, M. and Tabatabaee, Y.F., 2020. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid-based. Complement. Altern. Med., 5190603. https://doi.org/10.1155/2020/5190603
Ayub, M.A., Goksen, G., Fatima, A., Zubair, M., Abid, M.A. and Starowicz, M., 2023. Comparison of conventional extraction techniques with superheated steam distillation on chemical characterization and biological activities of Syzygium aromaticum L. essential oil. Separations, 10: 27. https://doi.org/10.3390/separations10010027
Barrios, H., Flores, B., Düttmann, C., Mora–Sánchez, B., Sheleby–Elías, J., Jirón, W. and Balcázar, J.L., 2022. In vitro acaricidal activity of Piper tuberculatum against Rhipicephalus (Boophilus) microplus. Int. J. Acarol., 48: 187–191. https://doi.org/10.1080/01647954.2022.2050808
Belhachemi, A., Maatoug, M.H. and Canela–Garayoa, R., 2022. GC–MS and GC–FID analyses of the essential oil of Eucalyptus camaldulensis grown under greenhouses differentiated by the LDPE cover–films. Ind. Crops Prod., 178: 114606. https://doi.org/10.1016/j.indcrop.2022.114606
Benelli, G. and Pavela, R., 2018. Repellence of essential oils and selected compounds against ticks. A systematic review. Acta Trop., 179: 47–54. https://doi.org/10.1016/j.actatropica.2017.12.025
Burrow, H.M., Mans, B.J., Cardoso, F.F., Birkett, M.A., Kotze, A.C., Hayes, B.J. and Djikeng, A., 2019. Towards a new phenotype for tick resistance in beef and dairy cattle: A review. Anim. Prod. Sci., 59: 1401–1427. https://doi.org/10.1071/AN18487
Calvano, M.P.C.A., Brumatti, R.C., Barros, J.C., Garcia, M.V., Martins, K.R. and Andreotti, R., 2021. Bioeconomic simulation of Rhipicephalus microplus infestation in different beef cattle production systems in the Brazilian Cerrado. Agric. Syst., 194: 103247. https://doi.org/10.1016/j.agsy.2021.103247
Castro, K.N.D.C., Canuto, K.M., Brito, E.D.S., Costa–Júnior, L.M., Andrade, I.M.D., Magalhães, J.A. and Barros, D.M.A. 2018. In vitro efficacy of essential oils with different concentrations of 1, 8–cineole against Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet., 27: 203–210. https://doi.org/10.1590/s1984-296120180015
Ceylan, O., Uslu, A., Ceylan, C. and Sevinc, F., 2021. Predominancy of Rhipicephalus turanicus in tick-infested sheep from Turkey: A large-scale survey. Pak. Vet. J., 41: 429–433.
Chen, Z., van Mol, W., Vanhecke, M., Duchateau, L. and Claerebout, E., 2019. Acaricidal activity of plant–derived essential oil components against Psoroptes ovis in vitro and in vivo. Parasit. Vectors, 12: 1–11. https://doi.org/10.1186/s13071-019-3654-x
da Silva Lima, A., De Carvalho, J.F., Peixoto, M.G., Blank, A.F., Borges, L.M.F. and Costa Junior, L.M., 2016. Assessment of the repellent effect of Lippia alba essential oil and major monoterpenes on the cattle tick Rhipicephalus microplus. Med. Vet. Ent., 30: 73–77. https://doi.org/10.1111/mve.12140
De La Fuente, J. and Estrada–Peña, A., 2019. Why new vaccines for the control of ectoparasite vectors have not been registered and commercialized? Vaccines, 7: 75. https://doi.org/10.3390/vaccines7030075
De Oliveira, P.R., de Carvalho Castro, K.N., Anholeto, L.A. and Camargo Mathias, M.I., 2016. Cytotoxic effects of extract of Acmella oleraceae (Jambú) in Rhipicephalus microplus females ticks. Microsc. Res. Tech., 79: 744–753. https://doi.org/10.1002/jemt.22693
Deletre, E., Martin, T., Duménil, C. and Chandre, F., 2019. Insecticide resistance modifies mosquito response to DEET and natural repellents. Parasit. Vectors, 12: 1–10. https://doi.org/10.1186/s13071-019-3343-9
Drummond, R.E.A., Ernst, S.E., Trevino, J.L., Gladney, W.J. and Graham, O.H., 1973. Boophilus annulatus and B. microplus: Laboratory tests of insecticides. J. econ. Ent., 66: 130–133. https://doi.org/10.1093/jee/66.1.130
Ellse, L. and Wall, R., 2014. The use of essential oils in veterinary ectoparasite control: A review. Med. Vet. Ent., 28: 233–243. https://doi.org/10.1111/mve.12033
Eltaly, R., Mohamed, M.B., Ibrahim, T.R., Mohamed, Y., Hosam, S.A., Aabdelfattah, S. and Khater, H.F., 2022. Novel acaricidal activity of Vitex castus and Zingiber officinale extracts against the camel tick, Hyalomma dromedarii. Int. J. Vet. Sci., 11: 479–483.
FAO, 2004. Guidelines resistance management and integrated parasite control in ruminants. Food and Agriculture Organization of the United Nations Rome, pp. 25–77.
Goswami, R., Arora, N., Mrigesh, M., Verma, P. and Rajora, V.S., 2022. Effect of eucalyptus oil against tick infestation in cattle. Pharm. Innov. J., 11: 804–807.
Gupta, D.K., Gupta, R. and Tiwari, A., 2022. Novel formulation and evaluation of poly herbal mosquito repellent. Res. Square, pp. 1–20. https://doi.org/10.21203/rs.3.rs-1900478/v1
Hussain, S., Saqib, M., Ashfaq, K. and Sindhu, Z.U.D., 2021. First molecular evidence of Coxiella burnetii in ticks collected from dromedary camels in Punjab, Pakistan. Pak. Vet. J., 42: 276–280.
Jabeen, F., Mushtaq, M., Qayyum, M., Ul Hasan, M., Zafar, M.A., Riaz, A. and Nasir, F., 2022. Tick taxonomy and nucleotide sequence analysis by internal transcribed spacer 2 (ITS 2) in large ruminants of Pothohar, Pakistan. Pak. Vet. J., 42: 554–560.
Jia, M., He, Q., Wang, W., Dai, J. and Zhu, L., 2018. Chemical composition and acaricidal activity of Arisaema anurans essential oil and its major constituents against Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol., 261: 59–66. https://doi.org/10.1016/j.vetpar.2018.08.006
Koc, S., Oz, E., Cinbilgel, I., Aydin, L. and Cetin, H., 2013. Acaricidal activity of Origanum bilgeri PH Davis (Lamiaceae) essential oil and its major component, carvacrol against adults Rhipicephalus turanicus (Acari: Ixodidae). Vet. Parasitol., 193: 316–319. https://doi.org/10.1016/j.vetpar.2012.11.010
Lew–Tabor, A.E. and Valle, M.R., 2016. A review of reverse vaccinology approaches for the development of vaccines against ticks and tick borne diseases. Ticks Ticks Borne Dis., 7: 573–585. https://doi.org/10.1016/j.ttbdis.2015.12.012
Mamun, M.S.A. and Ahmed, M., 2011. Prospect of indigenous plant extracts in tea pest management. Int. J. Agric. Res. Innov. Technol., 1: 16–23. https://doi.org/10.3329/ijarit.v1i1-2.13924
Moghaddam, M. and Mehdizadeh, L., 2017. Chemistry of essential oils and factors influencing their constituents. In: Soft chemistry and food fermentation. Academic Press, pp. 379–419. https://doi.org/10.1016/B978-0-12-811412-4.00013-8
Nath, S., Mandal, S., Pal, S., Jadhao, S., Ottalwar, N. and Sanyal, P., 2018. Impact and management of acaricide resistance–pertaining to sustainable control of ticks. Int. J. Livest. Res., 8: 46. https://doi.org/10.5455/ijlr.20180402121612
Ndawula, J.C., Sabadin, G.A., Parizi, L.F. and da Silva Vaz, J.I., 2019. Constituting a glutathione S–transferase cocktail vaccine against tick infestation. Vaccine, 37: 1918–1927. https://doi.org/10.1016/j.vaccine.2019.02.039
Ndungu, M., Lwande, W., Hassanali, A., Moreka, L. and Chhabra, S.C., 1995. Cleome monophylla essential oil and its constituents as tick (Rhipicephalus appendiculatus) and maize weevil (Sitophilus zeamais) repellents. Ent. Exp. Appl., 76: 217–222. https://doi.org/10.1111/j.1570-7458.1995.tb01965.x
Nwanade, C.F., Wang, M., Wang, T., Zhang, X., Zhai, Y., Zhang, S. and Liu, J., 2021. The acaricidal activity of cinnamon essential oil: current knowledge and future perspectives. Int. J. Acarol., 47: 446–450. https://doi.org/10.1080/01647954.2021.1936632
Nwanade, C.F., Wang, M., Yu, Z. and Liu, J., 2023. Biochemical and molecular mechanisms involved in the response of Haemaphysalis longicornis (Acari: Ixodidae) to Cinnamomum cassia essential oil and its major constituent. J. Pest Sci., pp. 1–13. https://doi.org/10.1007/s10340-023-01602-y
Park, J.H., Kim, H.J., Wimalasena, S.H.M.P. and Shin, G.W., 2022. In vitro repellent efficacy of Pogostemon cablin (Blanco) Benth. (Lamiaceae) essential oil and its nanoemulsion against Haemaphysalis longicornis (Acari: Ixodidae). Int. J. Acarol., 48: 466–471. https://doi.org/10.1080/01647954.2022.2134925
Pathak, R. and Sharma, H., 2021. A review on medicinal uses of Cinnamomum verum (Cinnamon). J. Drug. Deliv. Ther., 11: 161–166. https://doi.org/10.22270/jddt.v11i6-S.5145
Qiao, Y., Yu, Z., Bai, L., Li, H., Zhang, S., Liu, J. and Yang, X., 2021. Chemical composition of essential oils from Thymus mongolicus, Cinnamomum verum, and Origanum vulgare and their acaricidal effects on Haemaphysalis longicornis (Acari: Ixodidae). Ecotoxicol. Environ. Saf., 224: 112672. https://doi.org/10.1016/j.ecoenv.2021.112672
Remedio, R.N., Nunes, P.H., Anholeto, L.A., Oliveira, P.R. and Camargo–Mathias, M.I., 2015. Morphological effects of neem (Azadirachta indica A. Juss) seed oil with known azadirachtin concentrations on the oocytes of semi–engorged Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol. Res., 114: 431–444. https://doi.org/10.1007/s00436-014-4200-6
Remedio, R.N., Nunes, P.H., Anholeto, L.A., Oliveira, P.R., Sá, I.C.G. and Camargo–Mathias, M.I., 2016. Morphological alterations in salivary glands of Rhipicephalus sanguineus ticks (Acari: Ixodidae) exposed to neem seed oil with known azadirachtin concentration. Micron, 83: 19–31. https://doi.org/10.1016/j.micron.2016.01.004
Rooman, M., Assad, Y., Tabassum, S., Sultan, S., Ayaz, S., Khan, M.F. and Ali, R., 2021. A cross–sectional survey of hard ticks and molecular characterization of Rhipicephalus microplus parasitizing domestic animals of Khyber Pakhtunkhwa, Pakistan. PLoS One, 16: e0255138. https://doi.org/10.1371/journal.pone.0255138
Saleem, M.Z., Akhtar, R., Aslam, A. and Rashid, M.I., 2019. Histopathological investigation of skin and hides damage of small and large ruminants due to naturally infested ticks. Trop. Biomed., 36: 1081–1086.
Salman, M., Abbas, R.Z., Israr, M., Abbas, A., Mehmood, K., Khan, M.K. and Shah, S., 2020. Repellent and acaricidal activity of essential oils and their components against Rhipicephalus ticks in cattle. Vet. Parasitol., 283: 109178. https://doi.org/10.1016/j.vetpar.2020.109178
Selles, S.M.A., Kouidri, M., González, M.G., González, J., Sánchez, M., González–Coloma, A. and Valcárcel, F., 2021. Acaricidal and repellent effects of essential oils against ticks: A review. Pathogens, 10: 1379. https://doi.org/10.3390/pathogens10111379
Sharmeen, J.B., Mahomoodally, F.M., Zengin, G. and Maggi, F., 2021. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules, 26: 666. https://doi.org/10.3390/molecules26030666
Shnawa, B.H., Gorony, S. and Khalid, K.M., 2017. Efficacy of Cyperus rotundus rhizomes-tubers extracts against protoscoleces of Echinococcus granulosus. World J. Pharm. Res., 6: 157–179. https://doi.org/10.20959/wjpr20178-9053
Sindhu, Z.U.D., Jonsson, N.N. and Iqbal, Z., 2012. Syringe test (modified larval immersion test): A new bioassay for testing acaricidal activity of plant extracts against Rhipicephalus microplus. Vet. Parasitol., 188: 362–367. https://doi.org/10.1016/j.vetpar.2012.03.021
Singh, N.K., Abhijit, N. and Harkirat, S., 2019. Detection of multi–acaricide resistance in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Explor. Anim. med. Res., 9: 24–28.
Soares, A.M.D.S., Penha, T.A., Araújo, S.A.D., Cruz, E.M.O., Blank, A.F. and Costa–Junior, L.M., 2016. Assessment of different Lippia sidoides genotypes regarding their acaricidal activity against Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet., 25: 401–406. https://doi.org/10.1590/s1984-29612016087
Sousa, A.B.B.D., Bianchi, D., Santos, E.M., Dias, S.R., Peleja, P.L., Santos, R.R. and Minervino, A.H.H., 2022. First description of acaricide resistance in populations of Rhipicephalus microplus tick from the lower Amazon, Brazil. Animals, 12: 2931. https://doi.org/10.3390/ani12212931
Srisanyong, W., Bunyaluk, D., Srinontong, P. and Chitsanoor, S., 2021. Acaricidal activity of phenolic crude extract from Artocapus lakoocha leaves against cattle tick Rhipicephalus (Boophilus) microplus. Int. J. Vet. Sci., 10: 307–311. https://doi.org/10.47278/journal.ijvs/2021.063
Sultan, S., Zeb, J., Ayaz, S., Rehman, S.U., Khan, S., Hussain, M. and Sparagano, O.A., 2022. Epidemiologic profile of hard ticks and molecular characterization of Rhipicephalus microplus infesting cattle in central part of Khyber Pakhtunkhwa, Pakistan. Parasitol. Res., 121: 2481–2493. https://doi.org/10.1007/s00436-022-07596-3
Wahab, I.A., Jaliuddin, A.F. and Anuar, N.A., 2020. Mosquito repellency effects of the essential oils from Cinnamomum iners leaves and barks. IOP Conf. Ser. Earth Environ. Sci., 596: 012079. https://doi.org/10.1088/1755-1315/596/1/012079
Walker, A.R., 2003. Ticks of domestic animals in Africa: A guide to identification of species. Bioscience Reports, Edinburgh. pp. 3–210.
Wang, M., Hu, Y., Li, M., Xu, Q., Zhang, X., Wang, X. and Wang, H., 2020. A proteomics analysis of the ovarian development in females of Haemaphysalis longicornis. Exp. appl. Acarol., 80: 289–309. https://doi.org/10.1007/s10493-020-00469-3
Zaman, M.A., Iqbal, Z., Abbas, R.Z., Khan, M.N., Muhammad, G., Younus, M. and Ahmed, S., 2012. In vitro and in vivo acaricidal activity of a herbal extract. Vet. Parasitol., 186: 431–436. https://doi.org/10.1016/j.vetpar.2011.11.018
To share on other social networks, click on any share button. What are these?









