Application of Amino Acids on Mango Tree (Mangifera indica L.) cv. ‘Rataul No. 12: Assessment of Fruit Fly Infestation and Postharvest Parameters
Research Article
Application of Amino Acids on Mango Tree (Mangifera indica L.) cv. ‘Rataul No. 12: Assessment of Fruit Fly Infestation and Postharvest Parameters
Muhammad Hasnain1*, Ghulam Mustafa2, Asif-ur-Rehman3, Ali Raza4, Abrar Ahmad4, Taj Muhammad4, Muhammad Rafiq Shahid4, Umair Faheem5, Muhammad Shahid4, Muhammad Akram4 and Muhammad Kashif Nadeem5
1Cotton Research Station, (AARI), Faisalabad, Pakistan; 2Mango Research Institute, Multan, Pakistan; 3Horticultural Research Station, Sahiwal, Pakistan; 4Cotton Research Institute, Multan, Pakistan; 5Entomological Research Sub Station, Multan, Pakistan; 6Adaptive Research Farms, Dera Ghazi Khan, Pakistan.
Abstract | Mango cv. ‘Rataul No. 12’ is one of Pakistan’s finest mango cultivars. It produces small to medium-sized fruits that are flavorful, fragrant, and well-suited for preserving. Assessment of the pre-harvest amino acids application on the postharvest life of mangoes cv. ‘Rataul No. 12’ was done. The effect of amino acids on mango cultivar ‘Rataul No. 12’ with the objective of studying and improving fruit retention, yield, or qualitative and quantitative changes in physio-chemical characteristics during ripening. The impact of spraying amino acids on fruit retention at various fruiting stages i.e., pea, marble, egg, at matures stages as well as yield at the end of harvesting as compared to the control. Increasing amino acid percentages (Promise 6.55%, Flagon 10.00%, and Izabion 62.55%) also increased the number of fruits at the fully mature stage i.e., 1.43%, 1.82%, and 2.20%, respectively. As compared to control, there were statistically significant differences. The increased the percentage of amino acids also increased the yield, Izabi on was 105.9kg/plant, whereas Promise and Flagon showed 96.65kg/plant and 95.86%, but both were non-significantly different. Fruit ripening caused changes in those factors, which were noted. Spraying amino acids had no appreciable impact on the physical features of fruit during storage, such as weight loss, fruit colour, hardness, length, and lenticel burn. Fruit fly punctures were non-significant among the different chemicals as compared to the control. The maximum number was in control, i.e., 3.06±0.48 and the minimum was in Promise®, followed by Flagon® and Izabion®, i.e., 0.45±0.21, 0.72±0.15 and 0.44±0.02, respectively. Whereas biochemical parameters, total soluble solids, pH, titratable acidity, vitamin C, rag weight, juice and stone weight were also non-significantly affected by amino acid spraying. The amino acids are essential for increasing the fruit retention and output of the fruit tree. Because amino acids are essential for the majority of biological processes, enhanced quantity and export quality should be utilized.
Received | June 26, 2023; Accepted | August 26, 2023; Published | September 26, 2023
*Correspondence | Muhammad Hasnain, Cotton Research Station, (AARI), Faisalabad, Pakistan; Email: hasnainaro@gmail.com
Citation | Hasnain, M., G. Mustafa, A. Rehman, A. Raza, A. Ahmad, T. Muhammad, M.R. Shahid, U. Faheem, M. Shahid, M. Akram and M.K. Nadeem. 2023. Application of amino acids on mango tree (Mangifera indica L.) cv. ‘Rataul No. 12: Assessment of fruit fly infestation and postharvest parameters. Sarhad Journal of Agriculture, 39(3): 745-756.
DOI | https://dx.doi.org/10.17582/journal.sja/2023/39.3.745.756
Keywords | Mango Rataul No. 12, Amino acids, Fruit set, Physical and biochemical parameters, Yield
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Mango (Mangifera indica L.) is referred to as “The King of Fruits” because of its remarkable nutritional content, superb flavour, alluring scent, and delectable taste (Nunes et al., 2007) has been well-known as a tropical crop for foreign exportation and local consumption (Sivakumar et al., 2005) in 87 countries around the world (Tharanathan et al., 2006) being the most valuable tropical fruit. Pakistan is the 5th largest mango producer with production of around one million tonnes per year, contributing a share of 7.6% to the world export market (Rehman et al., 2015). But the export volume is not significant (<10% of production) in many other mango export countries (FAOSTAT, 2011). Multan is the 6th largest city in Pakistan and is the hub of mango production (Tahir et al., 2012; Alam and Khan, 2001). Mango pests include stem borers, weevils, fruit flies, webworms, mealy bugs, and scale insects. Fruit flies significantly damage mangoes, affecting quality and marketability. Controlling them is challenging due to their rapid multiplication. Various methods, including pheromone traps and baits, are used to catch both male and female fruit flies. Food baits are frequently employed to catch both male and female fruit flies, whereas pheromone traps are utilized to draw only males (Khan et al., 2015). Although bagging the mango fruit and the entire tree can be a successful strategy for reducing fruit fly infestations, this technique is not widely used in Pakistan because of laborious, expensive, and adverse weather conditions, i.e., heavy rain and wind storms. Chemical spraying with various insecticides against insect pests, including the Tephritids, develops secondary pest resurgence and resistance (Hsu et al., 2006) and also leads to numerous types of chronic health problems in birds, humans and animals (Kamel and Hoppin, 2004). Anwar Rataul is Pakistan’s top mango cultivar, producing flavorful, fragrant, and preserved fruits. However, production faces challenges like insect pests, inadequate nutrients, and reduced yield due to heavy fruit drop and poor postharvest quality. Low fruit yield in orchards is an important factor due to heavy dropping of fruits, about 10% reaches maturity and only 0.1–0.25% reach the harvesting stage (Chadha, 1993).
Gardeners face severe problems like minor fruit set, reduced yield, and inferior quality due to fruit fly and diseases, requiring improved quality parameters (Jha et al., 2010). Additionally, flavour, volatiles, texture, and chemical contents are some of the essential factors that support the creation of high-quality fresh mangoes for the consumer’s acceptance (Mamiro et al., 2007; Gaaliche et al., 2012).
By overcoming this problem, some nutrients were found to be useful for improving the quantity of fruit setting, fruit quality and yield enhancement. Several researchers have made attempts to increase the postharvest quality and productivity of mangoes through foliar applications of micronutrients such as boron, calcium, amino acid, plant growth regulators, etc. Boron application increased the productivity of mango (Jutamanee et al., 2000). Calcium spraying increased the productivity of mangoes basically due to reducing abscission (Wahdan et al., 2011).
In recent years, the responses of amino acids and plant growth regulators have been extensively studied in fruit trees. Likewise, many researchers have demonstrated that many fruit trees can be easily improved by different amino acids and growth regulators (Drobek et al., 2019). Calcium, boron, and amino acids were reported to increase initial and final fruit set which subsequently affects yield and fruit quality (Khattab et al., 2016).
In order to boost the production of fruit trees throughout the world, new trends now employ a variety of cutting-edge techniques, including growth regulators, conventional and nano-fertilizers, and antioxidant chemicals (Orabi et al., 2018), as well as vitamins and organic acids, to increase the growth, production, and quality of these trees and their products (El-Motty and El-Faham, 2013).An alternate approach that is most efficient, cost-effective, and environmentally friendly is the study, and use of amino acid with micronutrient spray application techniques, i.e., improved growth, development, balance of the phenolic compounds, quality, and quantity (reduced dropping) of fruits due to increased primary and secondary metabolism. Amino acid content changes among cultivars during different stages of mango plant development (Augustin et al., 1978).The ripe state has been shown to include significant levels of amino acids such as alanine, arginine, glycine, serine, leucine, and isoleucine, while the remaining amino acids are all present in negligible amounts (Tharanathan et al., 2006). As intermediary molecules of endogenous plant hormones, amino acids contribute to protein synthesis and have a complexing influence on nutrients (Taiz et al., 2017). The combination of gibberellic acid and paclobutrazol (PBZ) increases the number of blooms, which boosts fruit set (Kurian and Iyer, 1993) in other mango cultivars. The glycine is an amino acid that prevents what seems to be photorespiration by C3 plants, such as the mango tree, and promotes photosynthetic efficiency with a greater sugar content and yield. Methionine controls blooming and fruit ripening because it is an ethylene precursor (Taiz and Zeiger, 2002).
Phenylalanine, glutamate, and asparagine amino acids are essential for fruit color and metabolism. They link carbon and nitrogen cycles, transport nutrients, and store nitrogen. Tryptophan is crucial for enzymes that catalyze auxin synthesis, suppressing early flower and fruit fall. The study aimed to assess the impact of amino acids on micronutrient spray application, fruit retention, and postharvest quality parameters in mango cv. ‘Rataul No. 12’.
Materials and Methods
This study was conducted during the year 2022 in a private orchard located at Jalal Pur Pirwala, Multan, Pakistan. Selected a one-acre block having the same variety of ‘Rataul No. 12’ mango cv., and 40 healthy, uniform-sized mango trees, The age of the trees may be more or less than 8–10 years old. All the cultural, agronomic, and horticultural practices were similar during the study period. In the field experiments, a randomized completely blocks with four treatments and ten replications was used. Trees under study were sprayed with Promise® (containing amino acids 6.55% w/v), Flagan® (containing 10% w/v amino acids), and Isabion® (containing amino acids 62.55% + micronutrients + bio-stimulant) commercial products manufactured by different companies, and control trees were sprayed with water only. Trees were sprayed four times; the first and second sprays were during the first and fourth weeks of June, respectively. The third spray was at full growth stage during the second week of July, and the fourth one was at maturity stage. The fruits were collected for the post-harvest experiment at maturity stage 2, and their properties were assessed while being stored at 23.8+5°C and 45 + 8% RH. The effectiveness of various, their impact on the postharvest physiology of mangoes, and their impact on the control of fruit flies were investigated.
The treatments were as follows:
T1= Promise (20ml/7Lof water/plant)
T2= Falgan (20ml/ 7L of water/plant)
T3=Izabian(20ml/ 7L of water/plant)
T4= Control (Water sprayed only)
The following Quantitative parameters (fruit retention (%), Tree yield (Kg), Physical quality parameters (Fruit fly puncture, physiological weight loss, fruit colour, firmness, length and width, lenticel burn) and Biochemical parameters pH, Vitamin C, Total Soluble Solids (TSS), Titratable Acidity (TA), %age of Rag weight, Juice contents, and stone weight of postharvest parameters were studied.
Quantitative parameters
Fruit retention (%): Ten terminal branches from each tree were randomly chosen and marked for morphological analysis during the early fruit set stage. Calculations were made for each panicle’s average initial fruit sets and fruit retention at four distinct sizes (pea, marble, egg, and mature with diameters of 1.0, 3.0, 5.0, and 7.0 cm, respectively). Percentage of fruit retention was determined by using this formula:
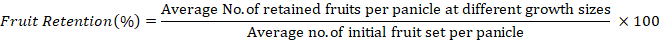
Yield (Kg): At mature stage, Yield was calculated by using the following formula:
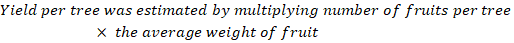
Physical parameters
Fruit fly punctures: Approximately 10kg of freshly picked mangoes fruits in each treatment packed with wooden box and placed under storage temperature at 23.8 + 2.8°C and 45 + 8% RH for 8-10 days for the purpose of emergence of maggots from infected fruits, maggots come out from the infected fruits for pupation then count the fruits punctures from each treatment separately.
The number of fruit fly puncture were counted on last day of examination
Physiological weight loss
Physiological weight loss (PWL) was calculated according to the formula:
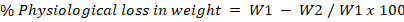
Where;
W1= Initial weight of unripe fruit (g); W2= Final weight of ripe fruit.
Fruit color
The gradual change in fruit color was measured using the score 1 =0% yellow, 2= 1-25% yellow, 3= 26-50% yellow, 4= 51-75% yellow and 5= 76-100% yellow (Malik et al., 2005).
Firmness
Fruits from each replication were selected for recording subjective (non-destructive) hand softness. Hand softness of fruit during ripening was scored daily according to the rating scale: 1, hard; 2, sprung; 3, slightly soft; 4, eating soft; and 5, over soft (Malik et al., 2005).
Length and width
Fruit length and width were measured (mm) with a vernier caliper and recorded.
Lenticel burns
The lenticel burn was counted by using the scale 1= NILL, 2= <5%, 3= 10-25%, 4= 25-50%. 5= >50%.
Biochemical parameters
Total soluble solids: Total soluble solids (TSS) were determined using a digital hand-held refractometer. Juice was dropped into the refractometer’s prism, and TSS was calculated straight from the scale at room temperature (302°C) as °Brix.
pH: Using a pH meter and a large enough sample placed in a clean 50 mL beaker, pH was determined.
Titratable acidity
Fruit juice (10 mL) from each sample was collected in a beaker and diluted (1:4) with distilled water. After adding 2-3 drops of phenolphthalein as an indicator, the TA was calculated as citric acid by titration against 0.1 NaOH solution (Akhtar et al., 2010).
Titratable acidity (%) = ml of NaOH used x 0.0064 x 100 /Volume of sample used
Vitamin c
By using the technique, the ascorbic acid (vitamin C) content of the fruit sample was ascertained. Each sample’s juice that had been removed was run through Whatman® filter paper for this purpose. A 10 mL filtered aliquot was placed in a 100 mL round bottom flask, and the volume was increased with 0.4% oxalic acid to the required amount. A 5 mL sample from a 100 mL aliquot was placed in a beaker and titrated against newly made 2, 6-dichlorophenol indophenol until a bright pink end point was reached and remained for 10–15 seconds. To make the dye, 52 mg of 2, 6-dichlorophenol indophenol and 42 mg of baking soda (NaHCO3) were added to a 200 mL volumetric flask, and the volume was increased to the required amount by adding distilled water. Ascorbic acid was calculated by using the following formula:
Ascorbic acid (mg 100 mL-1)=R1xVx100/RxWxV1
Where; R1= mL dye used in titration of aliquot; R = mL of dye used in titration of 1mL standard ascorbic acid solution prepared by adding 1mL of 0.1% ascorbic acid + 1.5 mL of 0.4% oxalic acid; V1= mL of juice used; V = volume of aliquot made by addition of 0.4% oxalic acid; W = mL of aliquot used for titration.
% Rag weight
The rag weight was measured by weighing the pulp left after extraction of juice.
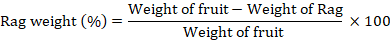
Juice contents
The juice contents were measured by weighing juice extracted from pulp. It was calculated by using the formula:
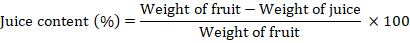
Stone weight
The stone weight was measured by weighing stone after removing pulp from its surroundings.
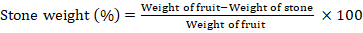
Data analysis
All the data was statistically analyzed under Randomized Complete Block Design (Steel et al., 1997). Analysis of variance (one-way analysis; ANOVA) and means comparisons by using LSD values at p>0.05 statistical software Statistix 8.1®.
Results and Discussion
Fruit retention (%)
Data revealed in Table 1, Izabion and Promise showed the highest number of initial fruit sets per panicle with 15.73% and 15.23%, respectively, as compared to the control tree. At pea size, the number of fruits retained per panicle showed a significant difference among the treatments; maximum retention was also observed in Izabion, followed by Flagon and Promise, as related to control values of 7.56%, 6.56%, 6.40%, and 5.59%, respectively. The highest values of retained fruit per panicle (4.66%, 3.45%, and 3.05%, respectively) were observed in Izabion, Flagon, and Promise at the marble size stage as compared to control values (2.57%). At egg and mature size, Izabion gave the highest retention value fruit per panicle (2.67% and 2.20%, respectively) compared to the control. Izabion showed the highest retention of fruit per panicle throughout the season during the years 2018.
According to the findings, spraying Izabion (62.55% amino acids + bio-stimulants) boosted fruit set, yield, and the maximum number of retained fruits per panicle, thus improving yield. The results obtained are consistent with those presented (El-Kosary et al., 2011; Khattab et al., 2012). The retained fruits per panicle increase at harvest due to effect of micronutrients and bio-stimulants on cell division, cell elongation and multiplication of cells occurred. The trees sprayed amino acids (8-16 gm/tree) results showed significantly increased vegetative (shoot length, number of leaves per shoot, leaf area) and reproductive growth (number of flowers per shoot, fruit set percentage, fruit retention percentage, fruiting yield (kg/tree) and number of fruits per tree (Singh and Maurya, 2004). The highest fruit retention was observed to Boramin Ca® (Amino acids+Calcium+Boron) treatment at 2000 ppm. Also, increased yield and number of fruits per tree was also detected (Khattab et al., 2016). Bio-stimulants containing nutrients, free amino acids, and Lithothamnium algae extract that benefit the nutritional values and increased the number of fruits per panicle and fruit production per mango tree ‘Kent’ if compared with non-treated plants (Lobo et al., 2019).
Yield (kg)
All the treatments on mango trees significantly increased the number of fruits per tree and subsequently increased the fruit yield (kg/tree) during the season as compared to the control. Izabion showed a higher number of fruits per tree (209.04%), and the average fruit weight (505.10 g) was significantly increased compared with all other treatments. As shown in Table 2, the tree yield increased significantly with all amino acid treated compounds as compared with the control. The highest significant yield, i.e., 105.59 kg/plant was recorded with Izabion treatments for ‘Rataul No. 12’ mango cultivars. However, Promise and Flagon treatments also increased yield, i.e., 96.65 kg/plant and 95.86 kg/plant, respectively, compared to the control.
Table 1: Effect of Promise, Flagon and Izabionon average number of initial fruit set and retained fruit per panicle at different growth sizes of ‘Rataul No. 12’ during season 2022.
|
Treatments |
Initial fruit set |
Pea |
Marble |
Egg |
Fruit retention |
|
Promise® (6.55% amino acids) |
15.23±0.39a |
6.40±0.19b |
3.05±0.02bc |
1.80±0.08b |
1.43±0.05c |
|
Flagan® (10.00% amino acids) |
15.06±0.12ab |
6.56±0.13b |
3.45±0.12b |
2.23±0.20ab |
1.82±0.04b |
|
Izabion® (62.55% amino acids+biostimulants) |
15.72±0.08a |
7.56±0.07a |
4.66±0.16a |
2.67±0.07a |
2.2±0.03a |
|
Control |
14.35±0.26b |
5.59±0.16c |
2.57±0.24c |
0.95±0.06c |
0.66±0.05d |
|
p Value |
0.0200 |
0.0007 |
0.0002 |
0.0004 |
0.000 |
Means in the same column followed by the same letter(s) are not significantly different at 5% level of probability.
Table 2: Effect of Promise, Flagon and Izabion on yield of ‘Rataul No. 12’ during season 2022.
|
Treatments |
Number of fruits/ trees |
Fruit weight (g) |
Yield (kg) |
|
Promise® (6.55% amino acids) |
193.71±2.67b |
499.00±2.68b |
96.65±0.93b |
|
Flagon® (10.00% amino acids) |
191.23±1.03b |
501.27±0.93ab |
95.86±0.69b |
|
Izabion® (62.55% amino acids) |
209.04±1.57a |
505.10±1.71a |
105.59±0.85a |
|
Control |
174.55±3.25c |
485.49±2.25c |
84.75±1.96c |
|
p Value |
0.0001 |
0.0003 |
0.000 |
Means in the same column followed by the same letter(s) are not significantly different at 5% level of probability.
Yield significantly was increased by increasing the amino acids foliar application. The results are in accordance with (Fathi et al., 2002) on peach, (Eissa et al., 2003) on apricot, (Abbas et al., 2006) on grapevines and (Ismail et al., 2007) on Le-Conte pear.
Fruit fly punctures (%)
According to the statistical analysis, there were no appreciable differences between the chemical treatments and the control group (Table 3). The maximum number of average fruit fly punctures was found more in control, i.e., 3.06±0.4867 and minimum was in Promise®, followed by Flagon® and Izabion®, i.e., 0.453±0.2107, 0.7267±0.1588 and 0.4467±0.0267, respectively. Many researchers reported the beneficial effects of amino acids on different fruit tree metabolisms, low infection and infestation, and the growth and development of fruit trees (Walch-Liu and Forde, 2007). The Glutamic acid (amino acid) was evaluated in plum and mango orchards to control the Mexican fruit fly (Anastrophe ludens) in Mexico (Aluja et al., 2009). Timely application of aminoethoxy vinylglycine reduced the pre-harvest dropping and improved the fruit quality and quantity fruit trees (Schupp and Greene, 2004). Increasing dose of amino acids enhanced the number of fruits per plant and also reduced the infestation level of fruit fly (Do C. Mouco et al., 2006). Our result also correlated with above researches.
Physical weight loss (%)
The higher the water content, the higher the weight loss and the faster the fruit loses its firmness and develops a speedy fruit colour change. Weight loss is directly related to the transpiration rate and concentration of O2(respiration) in mangoes (Domis et al., 2002). The control of gas diffusion reduced respiratory rates and decreased mango weight loss (Dang et al., 2008). Physical weight loss in all treatments of mangoes increased with time. The weight loss was found to be statistically non-significant, as illustrated in Table 3. More weight loss was observed in fruits of treatment 4 i.e., 2.26%while fruits in treatment 3 exhibited less weight loss i.e., 1.66%.
Fruit colour
Fruit colour is an important quality parameter for mango and a ripeness and maturity indicator (Saranwong et al., 2004), it influences customer acceptability (Maskan, 2001). During ripening, colour changes from green to yellow because of an increase in the synthesis of carotenoids (Rathore et al., 2007) in the fruit and the decomposition of chlorophyll by ethylene (Blankenship and Dole, 2003) and the enzyme chlorophyllase activity (Ketsa et al., 1999). Ripening (change of colour) also results in flesh softening, loss of acidity, conversion of starch to sugars and development of ripe flavour and aromas (Hofman et al., 1997). Mango peel colour development compared to the control was found to be non-significantly different among the treatments as shown in Table 3. Our result confirms non-significant differences in the mango peel of ‘Tommy Atkins’ fruit (Jacobi et al., 2001). Colour development is the continuing process shown in Figure 1, from green to peeling yellow, as indicated in the colour score chart.
Table 3: Relative abundance of the fruit flies punctures, weight loss and fruit color on different treatments on cv. ‘Rataul No. 12’.
|
Treatments |
Fruit fly punctures |
Weight loss (%) |
Fruit color |
|
Promise® (Amino acids) |
0.45±0.21b |
1.95±0.12a |
2.47±1.27a |
|
Flagon® (Amino acids) |
0.72±0.15b |
2.09±0.24a |
2.23±1.11a |
|
Izabion® (Amino acids +micronutrients + bio stimulants) |
0.44±0.02b |
1.66±0.08a |
2.16±0.80a |
|
Control |
3.06±0.48a |
2.26±0.48a |
1.99±1.03a |
|
P value |
0.0008 |
0.5068 |
0.0831 |
Different letters on columns are indicative of statistical difference (LSD, P< 0.05).
During the first 5 days mangoes ripening process gradually increased 25%, after 6th and 7th days ethylene production increased caused 50% raping takes place. During the study period 8th day 70 to 80 % ripening was occurred and fruit ready for consumption for consumers.
Firmness
The firmness was found to be non-significantly different among the treatments, as shown in Table 4. The firmness was found to be increasing as well as when the fruits were ripening, i.e., 2.28, 2.22, 2.01, and 2.24 of treatments 1, 2, 3, and 4, respectively. With the passage of time, the softness of the mango fruits was observed day by day, as described in Figure 2. The fruits in treatment 1 were found to be softer as compared to others on the last day of assessment, and the least firmness was found in Izabion. Treated mangos, so it increased the shelf life of the mango cultivars.
During the first to 5 days mangoes firmness was gradually increased hard to sprung, during the study period 6 days to 8 days mango firmness becomes slightly soft to eating soft also associated with ripening of fruits and ready for consumption for consumers. The interaction between amino acids application and cultivars on fruit firmness had significantly increased fruit firmness. The result was confirm that the pulp firmness of mango cv. Tommy Atkins was non-significantly affected by amino acid applications at different concentrations (do C. Mouco et al., 2006).
Length and width of fruit
Illustrated results in Table 4 showed that fruit width of ‘Rataul No. 12 ’mango cultivars was significantly affected by different treatments during the seasons of study. The maximum width was also found in Izabion (75.69 mm), while the minimum width was found in control (63.67mm). The mango cultivar treated with Izabion foliar application of amino acids recorded a higher fruit width than that of the other treatments. Similar results were obtained by (Saleh and Eman, 2003; Dutta, 2004).
Table 4 cleared showed that results of fruit length were non-significantly affected by different treatments but the interaction between the treatment and cultivar clearly showed that amino acid with combination of micronutrients and bio-stimulants application improved the length of the mango fruits. The maximum length was found in Izabion (114.71 mm) while minimum found in control (91.2mm). Our results are in line with findings of (Banik et al., 1997) on mango trees cv. Fazli and on mango cv. Fagri Kalan (Dutta and Dhua, 2002).
Lenticel burn
Data regarding lenticel burn was shown to be non-significantly affected by amino acid application at different treatments. More lenticel burn was counted on Promise, followed by Flagon and Izabion, which had values of 3.46%, 3.213%, and 3.083, respectively, as compared to control, i.e., 3.030%. Discoloration is the primary condition of lenticels (Bezuidenhout et al., 2005). It is most likely an indication of the fruit tissue around the lenticel stoma’s physiological stress response (Grassmann et al., 2002). The accumulation of phenolics in the cell walls and vacuoles of the tissue surrounding the lenticel cavity was linked to the coloring of lenticels (Du Plooy et al., 2006).
Biochemical parameters
Total soluble solids: Biochemical parameters study of fruits also showed significant differences among different mango cultivars. TSS contents were not
Table 4: Relative abundance of the fruit flies punctures, weight loss and fruit color on different treatments on cv. ‘Rataul No. 12’.
|
Treatments |
Firmness (%) |
Length (mm) |
Width (mm) |
Lenticel burn (%) |
|
Promise®(Amino acids) |
2.28±0.26a |
91.83±0.60a |
65.17±0.90b |
3.46±0.08a |
|
Flagon®(Amino acids) |
2.22±0.24a |
90.37±0.70a |
63.67±0.48b |
3.21±0.28a |
|
Izabion® (Amino acids +micronutrients + bio stimulants) |
2.01±0.23a |
114.71±22.74a |
75.693±0.45a |
3.08±0.42a |
|
Control |
2.24±0.25a |
91.2±0.89a |
63.67±0.48b |
3.03±0.21a |
|
P value |
0.8597 |
0.4377 |
0.0001 |
0.6358 |
Different letters on columns are indicative of statistical difference (LSD, P< 0.05).
significantly increased of mango cultivars Rataul No. 12’ during the initial postharvest examinations as shown in Table 5. Increase in TSS usually occurs during fruit ripening process due to the accumulation of certain free sugars due to the hydrolysis of starch contents by the action of amylase enzymes that are triggered based upon ethylene production (White, 2002). TSS, primly used to estimate the sugar content in a particular fruits and it provide the degree of sweetness. There is an inverse relationship between the TSS and TA; as the value of sugar content (TSS) increases, that for the acidity (TA) decreases. Total soluble solid contents were found non- significantly different from each other as shown in the Table 5. The highest TSS (22.433°Brix) was found in control whereas lowest among the amino acids treatments was TSS (21.3°Brix) in Flagon. The same result was found that control treated fruit have higher TSS % than chemically treated fruits because; the adversely chemical effect reduces TSS percentage. Langra and Amropali mango fruits contained the highest total sugar content that sprayed by single or combined foliar applications of different trace element (Zn, Fe, Mn, B and Cu) conversion of starch to sugar an important ripening process in mango and other climacteric fruits and further hydrolysis decreased the TSS (Kittur et al., 2001).
Juice pH
The pH was found significantly different among the treatments as shown in the Table 5. The maximum pH was found in Promise followed by Flagon and Izabion having values i.e., 5.37, 5.29 and 5.17 as compared by control having values i.e., 5.03, respectively.
Titratable acidity
The titratable acidity was found statistically non- significantly different in all the treatment as described in Table 5. There was a consistent increase in total soluble solids content during ripening, but a significant decrease in titratable acidity was only observed at 20 oC (Medlicott and Thompson, 1985). Total titratable acidity was not significantly affected by amino acids application spraying our result confirmed by (do C. Mouco et al., 2009).
Vitamin c
The vitamin C was found statistically non-significantly different among all the treatments. The maximum vitamin C (128.5mg/100g) was found in Promise and Izabion while minimum (126.98 mg/100g) was found in Flagon as compared to the control. Vitamin C (ascorbic acid) content increase on mango fruit and showed that the acidity increased during maturity (Venkatachalam et al., 2018), which is closely associated with the production of higher amounts of anti-oxidants. Our study showed an increase in ascorbic acid content in treated fruit agreed with.
Rag weight (%)
The rag weight was found to be statistically non-significantly different in all the treatments, as shown in Table 6. The maximum rag weight percentage was found in Flagon (10.99%), followed by Izabion (8.79%) and Promise (7.66%) as compared to control (7.63%).
Table 5: Relative abundance of the fruit flies punctures, weight loss and fruit color on different treatments on cv. ‘Rataul No. 12’.
|
Treatments |
TSS (°Brix) |
pH |
TA |
Vitamin C (mg/100g) |
|
Promise®(Amino acids) |
21.46±0.14a |
5.37±0.10a |
0.39±0.05a |
128.57±5.49a |
|
Flagon®(Amino acids) |
21.30±0.25a |
5.29±0.02a |
0.40±0.02a |
126.98±4.19a |
|
Izabion® (Amino acids +micronutrients + bio stimulants) |
21.63±0.18a |
5.17±0.33ab |
0.42±0.05a |
128.57±11.98a |
|
Control |
22.43±0.21a |
5.03±0.06b |
0.40±0.02a |
125.40±3.17a |
|
P value |
0.6408 |
0.0444 |
0.9714 |
0.9718 |
Different letters on columns are indicative of statistical difference (LSD, P< 0.05).
Table 6: Relative abundance of the fruit flies punctures, weight loss and fruit color on different treatments on cv. ‘Rataul No. 12’.
|
Treatments |
Rag weight (%) |
Juice weight (%) |
Stone weight (g) |
|
Promise®(Amino acids) |
7.66±3.84a |
45.13±5.91a |
23.49±3.67a |
|
Flagon®(Amino acids) |
10.99±2.95a |
53.95±8.81a |
31.12±9.85a |
|
Izabion® (Amino acids +micronutrients + bio stimulants) |
8.79±2.61a |
43.29±2.07a |
23.92±1.67a |
|
Control |
7.63±1.41a |
33.13±8.84a |
24.77±0.36a |
|
P value |
0.8723 |
0.3197 |
0.7242 |
Different letters on columns are indicative of statistical difference (LSD, P< 0.05).
Juice weight (%)
There was a non-significant difference in juice contents among different amino acid treatments. The maximum juice weight was found in Flagon (53.95%), followed by Izabion (43.29%), and Promise (45.13%), as compared to the control treatment (33.13%). So, on the basis of the results, amino acids increased the juice weight whenever the fruit size at maturity increased. Our result is consistent: an increase in fruit size with maturity could be the result of an increase in juice weight at fully ripe mulberry fruits (Gunes and Cekic, 2004).
Stone weight (g)
Stone weight remained consistent across treatments, with Flagon having the highest (31.12g) and Promise having the lowest (23.49%). Exogenous amino acid application did not affect stone weight size.
Conclusions and Recommendations
The production of proteins is decreased by the spontaneous synthesis of high quantities of free amino acids, which also has an adverse effect on the plant’s development. Due to the fact that proteins are involved in all of the processes (drought, disease, extreme temperature changes, etc.), the use of free amino acids in plants is advantageous. The majority of biological functions depend heavily on amino acids, which are chemical compounds found in proteins. There are several other non-protein amino acids having metabolic and physiological purposes. Plants use a significant amount of energy to synthesize the amino acids from the nitrogen taken up by their roots. In Pakistan, farmers have not sprayed any amino acids on the mango orchards due to this the production of mangoes and plant vigor decreased day by day and increased the bacterial and fungal diseases. Foliar application of amino acids three to four time at flowering, blooming and at fruit setting stages were the most effective treatment for increasing fruit set, yield and improving export quantity as well as quality of mangoes fruit and Anwar Rataul No. 12 trees due to the induction of enzymatic antioxidants in fruit.
Acknowledgements
Authors are thankful to the Administration of Mango Research Institute Multan, Pakistan for providing research work opportunities.
Novelty Statement
Amino acids’ primary purpose is to act as the building blocks of proteins. Amino acid foliar sprays applied at the onset of flowering, during full bloom, and after setting were the most successful treatments for enhancing Anwar Rataul’s production (Kg/tree). The effects of amino acids on plant cell division and multiplication, as well as cell elongation, may account for the increase in retained fruits per panicle quantity and quality at harvest, resulting in improved fruit fly-free mango exports to the rest of the globe.
Author’s Contribution
Muhammad Hasnain: Principal author did research and wrote the 1st draft of the manuscript.
Ghulam Mustafa and Asif-ur-Rehman: Helped in data collection.
Muhammad Kashif Nadeem: Reviewed literature and chemicals arrangement
Ali Raza and Abrar Ahmad: Helped in data analysis and review draft.
Taj Muhammad and Muhammad Rafiq Shahid: Helped in performing quantitative data analysis.
Umair Faheem, Muhammad Shahid and Muhammad Akram: Provided practical assistance and helped in the writing.
Each author assisted equally in analysis, revising, feedback, and endorsing the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Abbas, E., S. Bondok and V. Girgis. 2006. Effect of foliar with some nutrients and humic acid on fruit set, yield and quality of roomy ahmar grapevines. J. Agric. Sci. Manso. Univ., 31: 7847-7857. https://doi.org/10.21608/jpp.2006.236424
Akhtar, M.J., M. Ahamed, S. Kumar, H. Siddiqui, G. Patil, M. Ashquin and I. Ahmad. 2010. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology, 276: 95-102. https://doi.org/10.1016/j.tox.2010.07.010
Alam, S. and M. Khan. 2001. Mango-An important fruit of Pakistan. Indus. Econol.,
Aluja, M., F. Díaz-Fleischer, E. Boller, J. Hurter, A. Edmunds, L. Hagmann, B. Patrian and J. Reyes. 2009. Application of feces extracts and synthetic analogues of the host marking pheromone of Anastrepha ludens significantly reduces fruit infestation by A. obliqua in tropical plum and mango backyard orchards. J. Eco. Entomol., 102: 2268-2278. https://doi.org/10.1603/029.102.0632
Augustin, J., S. Johnson, C. Teitzel, R. True, J. Hogan, R. Toma, R. Shaw and R. Deutsch. 1978. Changes in the nutrient composition of potatoes during home preparation: II. Vitamins. Am. Potato J., 55: 653-662. https://doi.org/10.1007/BF02852138
Banik, B., S. Mitra, S. Sen and T. Bose. 1997. Interaction effects of zinc, iron and boron sprays on flowering and fruiting of mango cv. Fazli. Indian Agric., 41: 187-192.
Bezuidenhout, J., P. Robbertse and C. Kaiser. 2005. Anatomical investigation of lenticel development and subsequent discolouration of ‘Tommy Atkins’ and ‘Keitt’mango (Mangifera indica L.) fruit. J. Hortic. Sci. Biotech., 80: 18-22. https://doi.org/10.1080/14620316.2005.11511884
Blankenship, S.M. and J.M. Dole. 2003. 1-Methylcyclopropene: A review. Posthar. Bio. Technol., 28: 1-25. https://doi.org/10.1016/S0925-5214(02)00246-6
Chadha, K., 1993. Fruit drop in mango. Adv. Hortic., 3: 1131-1166.
Dang, K.T., Z. Singh and E.E. Swinny. 2008. Edible coatings influence fruit ripening, quality, and aroma biosynthesis in mango fruit. J. Agric. Food Chem., 56: 1361-1370. https://doi.org/10.1021/jf072208a
Do C. Mouco, M., M. De Lima, A. Da Silva, S. Dos Santos and F. Rodrigues. 2006. Amino acids on mango yield and fruit quality at Submedio Sao Francisco Region, Brazil. VIII Int. Mango Symp., 820: 437-442. https://doi.org/10.17660/ActaHortic.2009.820.54
Do C. Mouco, M., E. Ono and J. Rodrigues. 2009. Mango flower induction in the Brazilian Northeast Semi-arid with gibberellin synthesis inhibitors. XI Int. Symp. Plant Bioregulat. Fruit Prod., 884: 591-596. https://doi.org/10.17660/ActaHortic.2010.884.77
Domis, M., A. Papadopoulos and A. Gosselin. 2002. Greenhouse tomato fruit quality. Hortic. Rev., 26: 239-349.
Drobek, M., M. Frąc and J. Cybulska. 2019. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress. A review. Agronomy, 9: 335. https://doi.org/10.3390/agronomy9060335
Du Plooy, G., C. Van Der Merwe and L. Korsten. 2006. Lenticel discolouration in mango (Mangifera indica L.) fruit. A cytological study of mesophyll cells from affected tissue. J. Hortic. Sci. Biotech., 81: 869-873. https://doi.org/10.1080/14620316.2006.11512152
Dutta, P., 2004. Effect of foliar boron application on panicle growth, fruit retention and physico-chemical characters of mango cv. Himsagar. Indian J. Hortic., 61: 265-266.
Dutta, P. and R. Dhua. 2002. Improvement on fruit quality of Himsagar mango through application of zinc, iron and manganese. Hortic. J., 15: 1-9.
Eissa, F., M. Fathi and M. Yehia. 2003. Response of Canino apricot to foliar application of some biostimulants. Minia J. Agric. Res. Dev., 23: 69-82.
El-Kosary, S., I. El-Shenawy and S. Radwan. 2011. Effect of microelements, amino and humic acids on growth, flowering and fruiting of some mango cultivars. J. Hortic. Sci. Ornament. Plants, 3: 152-161.
El-Motty, E. and S. El-Faham. 2013. Effect of oil coating and different wrapping materials on prolonging storage periods of Florida prince peach fruits. J. Appl. Sci. Res., 9: 2927-2937.
Faostat, F., 2011. Agriculture organization of the United Nations–faostat. B.
Fathi, M., F.M. Eissa and M. Yehia. 2002. Improving growth, yield and fruit quality of “Desert Red” peach and “Anna” apple by using some biostimulants. Minia J. Agric. Res. Dev., 22: 519-534.
Gaaliche, B., M. Trad, L. Hfaiedh, W. Lakhal and M. Mars. 2012. Pomological and biochemical characteristics of fig (Ficus carica L.) cv. Zidi in different agro-ecological zones of Tunisia. Pak. J. Agric. Sci., 49: 425-428. https://doi.org/10.5402/2012/326461
Grassmann, J., S. Hippeli and E.F. Elstner. 2002. Plant’s defence and its benefits for animals and medicine: role of phenolics and terpenoids in avoiding oxygen stress. Plant Phys. Biochem., 40: 471-478. https://doi.org/10.1016/S0981-9428(02)01395-5
Gunes, M. and C. Cekic. 2004. Some chemical and physical properties of fruits of different mulberry species commonly grown in Anatolia, Turkey. Asian J. Chem., 16: 1849-1855.
Hofman, P., L. Smith and G. Meilberg. 1997. What causes green, ripe mangoes. Mango Care, 20: 13-15.
Hsu, J.C., D.S. Haymer, W.J. Wu and H.T. Feng. 2006. Mutations in the acetylcholinesterase gene of Bactrocera dorsalis associated with resistance to organophosphorus insecticides. Insect Biochem. Mol. Biol., 36: 396-402. https://doi.org/10.1016/j.ibmb.2006.02.002
Ismail, A., S. Hussien, S. El-Shall and M. Fathi. 2007. Effect of irrigation and humic acid on Le-Conte pear. J. Agric. Sci. Mansoura Univ., 32: 7589-7603. https://doi.org/10.21608/jpp.2007.220648
Jacobi, K.K., E.A. Macrae and S.E. Hetherington. 2001. Postharvest heat disinfestation treatments of mango fruit. Sci. Hortic., 89: 171-193. https://doi.org/10.1016/S0304-4238(00)00240-5
Jha, S., S. Sethi, M. Srivastav, A. Dubey, R. Sharma, D. Samuel and A. Singh. 2010. Firmness characteristics of mango hybrids under ambient storage. J. Food Eng., 97: 208-212. https://doi.org/10.1016/j.jfoodeng.2009.10.011
Jutamanee, K., S. Eoomkham, A. Pichakum, K. Krisanapook and L. Phavaphutanon. 2000. Effects of calcium, boron and sorbitol on pollination and fruit set in mango cv. Namdokmai. Int. Symp. Trop. Subtrop. Fruits, 575: 829-834. https://doi.org/10.17660/ActaHortic.2002.575.98
Kamel, F. and J.A. Hoppin. 2004. Association of pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect., 112: 950-958. https://doi.org/10.1289/ehp.7135
Ketsa, S., W. Phakawatmongkol and S. Subhadrabhandhu. 1999. Peel enzymatic activity and colour changes in ripening mango fruit. J. Plant Physiol., 154: 363-366. https://doi.org/10.1016/S0176-1617(99)80181-3
Khan, S., S. Hussain, F. Maula, M.A. Khan and I. Shinwari. 2015. Efficacy of different lures in male annihilation technique of peach fruit fly, Bactrocera zonata (Diptera: Tephritidae). J. Entomol. Zool. Stud., 3: 164-168.
Khattab, M., A. Shaban and A.E. Hassan. 2016. Impact of foliar application of calcium, boron and amino acids on fruit set and yield of Ewais and Fagry Kelan mango cultivars. J. Hortic. Sci. Ornamental. Plants, 8: 119-124.
Khattab, M.M., A.E. Shaban, A.H. El-Shrief and A.E.-D. Mohamed. 2012. Effect of humic acid and amino acid on pomegranate trees under deficit irrigation. I: Growth, flowering, and fruiting. J. Hortic. Sci. Ornament. Plants, 4: 253-259.
Kittur, F., N. Saroja and R. Tharanathan. 2001. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol., 213: 306-311. https://doi.org/10.1007/s002170100363
Kurian, R.M. and C. Iyer. 1993. Chemical regulation of tree size in mango (Mangifera indica L.) cv. Alphonso. II. Effects of growth retardants on flowering and fruit set. J. Hortic. Sci., 68: 355-360. https://doi.org/10.1080/00221589.1993.11516362
Lobo, J.T., K.D.S.M. De Sousa, V.B.D.P. Neto, R.N. Pereira, L.D.S. Silva and Í.H.L. Cavalcante. 2019. Biostimulants on fruit yield and quality of mango cv. kent grown in semiarid. J. Am. Pomol. Soc., 73: 152-160.
Malik, A., Z. Singh and S. Tan. 2005. Exogenous application of polyamines improves shelf life and fruit quality of mango. I Int. Symp. Improv. Perf. Supply Chains Trans. Econ., 699: 291-296. https://doi.org/10.17660/ActaHortic.2006.699.34
Mamiro, P., L. Fweja, B. Chove, J. Kinabo, V. George and K. Mtebe. 2007. Physical and chemical characteristics of off vine ripened mango (Mangifera indica L.) fruit (Dodo). African J. Biotechnol., 6. https://doi.org/10.5897/AJB2007.000-2392
Maskan, M. 2001. Kinetics of colour change of kiwifruits during hot air and microwave drying. J. Food Eng., 48: 169-175. https://doi.org/10.1016/S0260-8774(00)00154-0
Medlicott, A.P. and A.K. Thompson. 1985. Analysis of sugars and organic acids in ripening mango fruit (Mangifera indica L. var. Keitt) by high-performance liquid chromatography. J. Sci. Food Agric., 36: 561–566.
Nunes, M.C.N., J. Emond, J.K. Brecht, S. Dea and E. Proulx. 2007. Quality curves for mango fruit (cv. Tommy Atkins and Palmer) stored at chilling and nonchilling temperatures. J. Food Qual., 30: 104-120. https://doi.org/10.1111/j.1745-4557.2007.00109.x
Orabi, S., M. Hussein, E.Z. Abd El-Motty and S.Y. El-Faham. 2018. Effect of Alpha-tochophyrol and glutamic acid on total phenols, antioxidant activity, yield and fruit properties of mango trees. Middle East G. Appl. Sci., 8: 1229-1239.
Rathore, H.A., T. Masud, S. Sammi and A.H. Soomro. 2007. Effect of storage on physico-chemical composition and sensory properties of mango (Mangifera indica L.) variety Dosehari. Pak. J. Nutr., 6: 143-148. https://doi.org/10.3923/pjn.2007.143.148
Rehman, A., M. Alam, A. Malik, H. Ali and B. Sarfraz. 2015. Preharvest factors influencing the postharvest disease development and fruit quality of mango. J. Environ. Agric. Sci., 3: 42-47.
Saleh, M. and A.M. Eman. 2003. Improving productivity of Fagri Kalan mango trees grown under sandy soil conditions using potassium, boron and sucrose as foliar spray. Ann. Agric. Sci. Cairo., 48: 747-756.
Saranwong, S., J. Sornsrivichai and S. Kawano. 2004. Prediction of ripe-stage eating quality of mango fruit from its harvest quality measured nondestructively by near infrared spectroscopy. Posth. Bio. Tech., 31: 137-145. https://doi.org/10.1016/j.postharvbio.2003.08.007
Schupp, J.R. and D.W. Greene. 2004. Effect of Aminoethoxyvinylglycine (AVG) on preharvest drop, fruit quality, and maturation of Mcintosh apples. I. Concent. Timing Dilute Appl. AVG. Hortic. Sci., 39: 1030-1035. https://doi.org/10.21273/HORTSCI.39.5.1030
Singh, J. and A. Maurya. 2004. Effect of micronutrients on bearing of mango (Mangifera Indica) CV Mallika. Prog. Agric., 4: 47-50.
Sivakumar, D., T. Regnier, B. Demoz and L. Korsten. 2005. Effect of different post-harvest treatments on overall quality retention in litchi fruit during low temperature storage. J. Hortic. Sci. Biotechnol., 80: 32-38. https://doi.org/10.1080/14620316.2005.11511887
Steel, R.G.D., J.H. Torrie, and Mickey, D.A. 1997. Analysis of variance I: The one-way classification. Principles and procedures of statistics: a biometrical approach, 139–203.
Tahir, W.A., M. Ahmad and M. Iftikhar. 2012. An analysis of the effectiveness of extension work conducted by public sector with special reference to mango in the southern Punjab, Pakistan. Pak. J. Agric. Sci., 49: 229-232.
Taiz, L. and E. Zeiger. 2002. Photosynthesis: Physiological and ecological considerations. Plant Physiol., 9: 172-174.
Taiz, L., E. Zeiger, I. Moller and A. Murphy. 2017. Physiology and plant development. Porto Alegre: Artmed. 858.
Tharanathan, R., H. Yashoda and T. Prabha. 2006. Mango (Mangifera indica L.). The king of fruits. An overview. Food Rev. Int., 22: 95-123. https://doi.org/10.1080/87559120600574493
Venkatachalam, K., I. Muthuvel, S. Sundaresan, K.S. Subramanian, J.G. Janaki, J.A. Sullivan, G. Paliyath and J. Subramanian. 2018. Post-harvest dip of enhanced freshness formulation to extend the shelf life of banana (Musa acuminata cv. Grand Naine) in India. J. Fac. Food Agric., 95: 1-13.
Wahdan, M., S. Habib, M. Bassal and E. Qaoud. 2011. Effect of some chemicals on growth, fruiting, yield and fruit quality of” Succary Abiad” mango cv. J. Am. Sci., 7: 651-658.
Walch-Liu, P. and B.G. Forde. 2007. L-Glutamate as a novel modifier of root growth and branching: What’s the sensor? Plant Signal. Behav., 2(4): 284-286. https://doi.org/10.4161/psb.2.4.4016
White, P.J. 2002. Recent advances in fruit development and ripening: an overview. J. Exp. Bot., 53 (377): 1995– 2000. https://doi.org/10.1093/jxb/erf105.
To share on other social networks, click on any share button. What are these?








