Assessing the Validity of Fasciola hepatica ELISA Test for Immunodiagnosis of Small Ruminant Fasciolosis in Pothwar Region, Pakistan
Assessing the Validity of Fasciola hepatica ELISA Test for Immunodiagnosis of Small Ruminant Fasciolosis in Pothwar Region, Pakistan
Kiran Afshan1*, Sarwat Jahan1 and Mazhar Qayyum2
1Department of Animal Sciences, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, 45320, Pakistan
2Department of Zoology, Faculty of Sciences, PMAS-Agriculture University, Rawalpindi-46300, Pakistan
ABSTRACT
To improve the diagnostic efficacy of animal fascioliasis caused by Fasciola hepatica and Fasciola gigantica in Pakistan, we first time evaluated the diagnostic accuracy of commercially available bovine F. hepatica enzyme-linked immunosorbent assay (ELISA) for the detection of IgG against fascioliasis in sera of small ruminants. The result of current study indicated diagnostic accuracy of test 95.6%, while sensitivity and specificity of this assay for small ruminants was 97.83% (95% CI: 92.35% to 99.67%) and 93.75% (95% CI: 87.54% to 97.44%) respectively. In field this test indicated significant (p<0.05) values for sheep as compared to goat indicating high risk of infection in sheep. The current study showed that commercial bovine F. hepatica ELISA test is effective in Pakistan for small ruminants.
Article Information
Received 28 September 2015
Revised 18 February 2016
Accepted 06 March 2016
Available online 10 February 2017
Authors’ Contributions
KA conceived, designed and performed the experiments. KA, SJ and MQ analyzed the data and wrote the article.
Key words
ELISA, Immunodiagnosis, Fasciolosis, Ruminants, Pakistan.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.sc5
* Corresponding author: [email protected]
0030-9923/2017/0002-0737 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Fascioliasis cause deleterious loses in livestock due to reduction in weight, milk yield, fertility rate and condemned livers (Schweizer et al., 2005; Elitok et al., 2006; Charlier et al., 2007) and its control is limited due to absence of accurate and practicable tests for early diagnosis. Historically the microscopic examination of parasite eggs in faeces was common practice, this traditional diagnostic technique is still widely used and not effective until at least 10–12 week post infection (PI). The microscopic faecal examination has various drawbacks less sensitive, hard to perform, requires an appropriate amount of faeces, unable to diagnose infection at early stages, in chronic infection sporadic eggs release in faeces leads to misdiagnosis of infection (Anderson et al., 1999).
The so far estimated prevalence rate of fascioliasis based on coprological examination were, 25.46 per cent in Faisalabad (Khan et al., 2009), Punjab 14.71 per cent (Maqbool et al., 2002), Bahawalpur 17.68 per cent (Chaudhry and Niaz, 1984), Multan 23.97 per cent (Masud and Majid, 1984), Lahore 10.48 per cent (Sahar, 1996) and 55 per cent in Peshawar (Siddiqi and Shah, 1984).
Numerous immunological tests were applied for detection of anti-Fasciola antibodies in animal sera (Bossaert et al., 2000; Cornelissen et al., 2001; Phiri et al., 2006). From last two decades all over the world, investigations were made to search specific and sensitive methods for early diagnosis of fascioliasis in ruminants. Numerous ELISA tests were performed by using different antigens including whole F. hepatica excretory secretory antigens ESAs (Salimi-Bejestani et al., 2005), purified recombinant cathepsins (Sriveny et al., 2006) and other recombinant antigens (Arias et al., 2006).
Most of these immunological tests proved to be sensitive enough to detect fasciolids infection during the prepatent period in ruminants. The specificity and sensitivity of these tests is usually below 100 percent, and most of them are not commercially available (Mezo et al., 2007). Previously commercially available bovine F. hepatica IgG ELISA (DRG® Germany) test was successfully applied with high sensitivity and specificity in different part of world for bovines and ovine. This serological test which is a far more sensitive technique, can also detect antibodies to F. gigantica, the level of detection was much lower, since the two species might have marked dissimilarities in their antigenic epitopes (Aliyu et al., 2014). As different fasciolid strains exist in Pakistan (Mufti et al., 2011), it is very important to test the usefulness of commercially available diagnostic kits against these strains in different parts of the world. The current study was conducted to evaluate the usefulness of commercial DRG bovine F. hepatica IgG ELISA test against fasciolosis in small ruminants in Pakistan.
Materials and methods
This study was conducted at small ruminants farms located in the semi-arid climate of Pothwar region (latitude 30 and 34° N and longitude 70 and 74° E) Pakistan. The animals were kept under extensive and semi-extensive management systems. In summer animals are usually taken out for grazing while in winter stall feed because of the lack of forage. The animal data was obtained from the owners of farm. The sample size for an expected prevalence of at least 70% at the 5% absolute precision level for a 95% confidence interval (CI) was calculated at 323 animals (Thrusfield, 2007). The total animals (n=350) of which sheep belonging to Salt range (n = 88) and Afghani (n = 74) breeds and goats belonging to Local Hairy (n = 86), Beetal (n = 62), a crossbreed of Beetal and Hairy (n = 40) breeds. A stratified random sampling method was used to select animals according to species and their breeds.
Blood samples were obtained from the jugular vein of animals in non-EDTA coated vacutainers, centrifuged at 3000 rpm for 15 min and sera was separated and kept at −20°C until used for antibodies detection.
Fascioliasis positivity of the serum samples used in the test as positive control (n= 92) was confirmed through adult fluke collection from liver of slaughtered animals and through repeated parasitological techniques. The sera positive for parasitic infection (n=54) other than Fasciola mainly: Haemonchus contortus; Strongyloides spp.; Ostertagia spp.; Paramphistomum spp.; coccidian; hydatidosis and schistosomiasis were taken from clinically, serologically and parasitologically confirmed cases from veterinary centres to check the cross reactivity of the test. The sera for negative control (n=58) were taken from young calves having no exposure to infection and proved parasitologically negative animals for infection.
Serum IgG-antibodies were assayed for specific F. hepatica antigens by using a commercially available indirect ELISA Kit (DRG® instruments GmbH’ Germany). The ELISA was performed according to manufacture instructions on 96 well micro titration plates, whose odd columns were coated with specific F. hepatica antigens whereas even columns were used to control the specificity of the test.
The sensitivity and specificity was determined with data obtained from positive and negative sheep and goats sera from fluke and fluke free animals. The diagnostic sensitivity and specificity values were calculated with its 95% confidence interval (MedCalc Software). The positive predictive value (PPV) was calculated using the following formula:
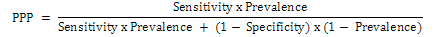
The negative predictive value (NPV) was calculated using the following formula:
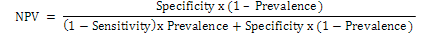
The Software Graph Pad Prism V. 5 was used for graphical representation of DU values of each sample. The Maximum likelihood estimation of a binormal Receiver-operating characteristic (ROC) curve from categorical rating data at 95% confidence interval was calculated (JROCFIT Version 1.0.2).
Results and discussion
The true positive and false negative test results in fascioliasis cases of animals are provided in Figure 1. The sensitivity of the F. hepatica IgG bovine ELISA test was found to be 97.83% (95% CI: 92.35% to 99.67%) detecting 2 false negative cases animal.
Specificity of the test was calculated by using the data from numbers of false negative and true positive test results in infected sera other than fascioliasis and negative cases. These results can be grouped as follows: infected positive cases other than fascioliasis (infected+) / seropositive by F. hepatica IgG ELISA test (test+) and infected positive cases other than fascioliasis (infected+)/ seronegative by F. hepatica IgG ELISA test (test-) (Fig. 2A). These values were used to calculate specificity, presenting a value of 93.75%
(95% CI: 87.54% to 97.44%). The sera free from infection were used as control negative also analysed through F. hepatica IgG ELISA test for finding the sensitivity of the test (Fig. 2B).
The diagnostic accuracy of the test is 95.6% along with PPVs and NPVs of test is: 92.78% (95% CI: 85.69 % to 97.04%), 98.13% (95% CI: 93.40% to 99.72%), respectively. For the measurement of accuracy of the test area under the ROC curve is 0.998, which represents a perfect test. The false positive and true positive friction is shown in Figure 3.
The test was applied in field to determine the prevalence of infection in sheep and goats. The mean of DU values for all sheep and goats sera is shown in Figure 4A, B. DU values were significantly higher in animal groups infected with fascioliasis other than non-infected animals (P<0.001). In sheep DU values were found higher in Afghani breed as compared to Salt range breed, while in goat low DU values obtained indicating low level of infection among goat breeds.
Several investigations have been made to search specific and sensitive methods for the serodiagnosis of fascioliasis in ruminants from last two decades. Several antigenic fractions of Fasciola has been effectively applied in ELISA tests (Mezo et al., 2003; Sanchez-Andrade et al., 2008; Demerdash et al., 2011), whole excretory secretory antigens ESAs of F. hepatica (Espino and Dume´Nigo, 1987; Rivera-Marrero et al., 1988; Itagaki et al., 1995; Ortiz et al., 2000; Salimi-Bejestani et al., 2005), purified antigens (O’Neill et al., 1999; Rokni et al., 2002) and more recently, purified and recombinant cathepsins antigens (O’Neill et al., 1999; Cornelissen et al., 2001; Neyra et al., 2002; Espinoza et al., 2005; Sriveny et al., 2006). The most frequently used target antigens for detecting anti-Fasciola antibodies are Cathepsins L (Rokni et al., 2002; Mezo et al., 2004, 2007, 2010; Intapan et al., 2005; Wongkham et al., 2005; Valero et al., 2009b; Muin˜o et al., 2011), as circulating antibodies remain at high levels to these molecules for long periods (Valero et al., 2009b).
The other recombinant antigens are also used for detection of anti-Fasciola antibodies (Silva et al., 2004; Paz-Silva et al., 2005; Arias et al., 2006).
The present study emphasized on the detection of Fasciola-antibodies in serum samples of naturally infected sheep and goats by using commercially available DRG bovine Fasciola hepatica IgG ELISA kit. The commercial bovine Fasciola hepatica IgG ELISA kit was proved as consistent and easy to use diagnostic tool for screening large number of bovines. The commercial DRG test was evaluated in cattle, obtaining a sensitivity and specificity of 98% (96–100%) and 96% (93–98%) respectively at a cut-off value of 15% positivity (Salimi-Bejestani et al., 2005). The sensitivity and specificity values of the DRG test here in small ruminants is 97.83% (95% CI: 92.35% to 99.67%) and 93.75% (95% CI: 87.54% to 97.44%), respectively, at a cut-off value of 13% positivity. The results of current study indicated that the DRG bovine F. hepatica IgG kit is equally useful for ovine as proved in rest of the world.
The result showed the test to be sensitive and specific for ovine. However, the result of other authors for F. hepatica IgG ELISA in-house assays: 92.4% and 83.6% (Espinoza et al., 2007), 97.2% and 100% (Rahimi et al., 2011), 97.0% and 96.6% (Cornejo et al., 2010) and 100 and 95.6% (Figueroa-Santiago et al., 2011) are in agreement with present study sensitivity and specificity values of DRG kit results for ovine.
The diagnostic accuracy of test proved high (95.6%) as the large positive predictive value (PPV=92.78%) indicates that many of the positive results from this testing procedure are true positives. Thus it proved as a more reliable test to obtain a more accurate assessment as to whether fascioliasis is present. Similarly its negative predictive value (NPV= 98.13%) gives us a high confidence that its negative result is true.
The use of commercial kits are advantages as they save time and provide quality control reagents for better reproducibility as compared to in-house assays. Furthermore, only very few commercial kits, such as the DRG F. hepatica IgG ELISA test evaluated here, are presently available for the diagnosis of animal fascioliasis.
Conclusion
In conclusion, this study is first implication of DRG bovine F. hepatica IgG ELISA test against serodiagnosis of fascioliasis in small ruminants grazing in Pothwar region of Pakistan. The currently used commercial test is recommended for mass screening on large-scale epidemiological studies of ovine fascioliasis in other agro-ecological zones of Pakistan.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Afshan, K., Qayyum, M., Rizvi, S.S.R., Mukhtar, M., Mushtaq, M. and Miller, J.E., 2013a. Small Rumin. Res., 113: 267-272. https://doi.org/10.1016/j.smallrumres.2013.01.020
Aliyu, A.A., Ajogi, I.A., Ajanusi, O.J. and Reuben, R.C., 2014. Scholars J. Agric. Vet. Sci., 1: 13-19.
Anderson, A., Luong, T.T., Vo, N.G., Bui, K.L., Smooker, P.M. and Spithillt, W., 1999. Vet. Parasitol., 83: 15-24. https://doi.org/10.1016/S0304-4017(99)00026-6
Arias, M., Hillyer, G.V., Sanchez-Andrade, R., Suarez, J.L., Pedreira, J., Lomba, C., Diaz, P., Morrondo, P., Diez-Banos, P. and Paz-Silva, A., 2006. Vet. Parasitol., 137: 67-73. https://doi.org/10.1016/j.vetpar.2005.12.015
Bossaert, K., Farnir, F., Leclipteux, T., Protz, M.L., Onneux, J.F. and Losson, B., 2000. Vet. Parasitol., 87: 103-123. https://doi.org/10.1016/S0304-4017(99)00177-6
Charlier, J., Duchateau, L., Claerebout, E., Williams, D. and Vercruysse, J., 2007. Prev. Vet. Med., 78: 57-66. https://doi.org/10.1016/j.prevetmed.2006.09.010
Chaudhry, A.H. and Niaz, M., 1984. Pak. Vet. J., 4: 42–43.
Cornejo, H., Oblitas, F., Cruzado, S. and Quispe, W., 2010. Rev. Peru. Med. Exp. Salud. Publica., 27: 569-574. https://doi.org/10.1590/S1726-46342010000400012
Cornelissen, J.B.V.J., Gaasenbeek, C.P.H., Borgsteede, F.H.M., Holland, W.G., Harmsen, M.M. and Boersema, W.J.A., 2001. Int. J. Parasitol., 31: 728-737. https://doi.org/10.1016/S0020-7519(01)00175-8
Demerdash, Z.A., Diab, T.M. and Aly, I.R., 2011. Parasit. Vect., 4: 176. https://doi.org/10.1186/1756-3305-4-176
Elitok, B., Elitok, O.M. and Kabu, M., 2006. Vet. Parasitol., 135: 279-285. https://doi.org/10.1016/j.vetpar.2005.10.008
Espino, A.M. and Dume´Nigo, B.E., 1987. Am. J. trop. Med. Hyg., 37: 605-608.
Espinoza, J.R., Maco, V. and Marcos, L., 2007. Am. J. trop. Med. Hyg., 76: 977–982.
Espinoza, J.R., Timoteo, O. and Herrera-Velit, P., 2005. J. Helminthol., 79: 235-240. https://doi.org/10.1079/JOH2005303
Figueroa-Santiago, O., Delgado, B. and Espino, A.M., 2011. Diagn. Microbiol. Infect. Dis., 70: 355–361. https://doi.org/10.1016/j.diagmicrobio.2011.03.016
Intapan, P.M., Tantrawatpan, C., Maleewong, W., Wongkham, S., Wongkham, C. and Nakashima, K., 2005. Diagn. Microbiol. Infect. Dis., 53: 125-129. https://doi.org/10.1016/j.diagmicrobio.2005.05.010
Itagaki, T., Sakamoto, T. and Itagaki, H., 1995. J. Vet. med. Sci., 57: 511–513. https://doi.org/10.1292/jvms.57.511
Khan, M.K., Sajid, M.S., Khan, M.N., Iqbal, Z. and Iqbal M.U., 2009. Res. Vet. Sci., 87: 70-75. https://doi.org/10.1016/j.rvsc.2008.12.013
Maqbool, A., Hashmi, H.A., Shafique, M., Tanveer, A., Ahmad, M. and Mahmood, M., 2002. Ind. J. Anim. Res., 1: 33-36.
Masud, F.S. and Majid, A., 1984. Incidence of fascioliasis in buffaloes and cattle of Multan division. Pak. Vet. J., 4: 33-34.
Mezo, M., Gonza´ Lez-Warleta, M. and Ubeira, F.M., 2003. J. Parasitol., 89: 843-849. https://doi.org/10.1645/GE-74RI.1
Mezo, M., Gonza´ Lez-Warleta, M. and Ubeira, F.M., 2007. J. Parasitol., 93: 65-72. https://doi.org/10.1645/GE-925R.1
Mezo, M., Gonza´ Lez-Warleta, M., Carro, M.C. and Ubeira, F.M., 2004. J. Parasitol., 90: 845-852. https://doi.org/10.1645/GE-192R
Mezo, M., Gonzalez–Warleta, M., Castro-Hermida, J.A., Carro, M.C. and Ubeira, F.M., 2010. Vet. Parasitol., 168: 36-44. https://doi.org/10.1016/j.vetpar.2009.10.007
Mufti, S., Ahmad M.M., Zafar, Y. and Qayyum, M., 2011. Pakistan J. Zool., 43: 1069-1077.
Muiño, L.1., Perteguer, M.J., Gárate, T., Martínez-Sernández, V., Beltrán, A., Romarís, F., Mezo, M., González-Warleta, M. and Ubeira, F.M., 2011. Mol. Biochem. Parasitol., 179: 80-90. https://doi.org/10.1016/j.molbiopara.2011.06.003
Neyra, V., Chavarry, E. and Espinoza, J.R., 2002. Vet. Parasitol., 105: 21-32. https://doi.org/10.1016/S0304-4017(02)00002-X
O’Neill, S.M., Parkinson, M., Dowd, A.J., Strauss, W., Angles, R. and Dalton, J.P., 1999. Am. J. trop. Med. Hyg., 60: 749-751.
Ortiz, P.L., Claxton, J.R., Clarkson, M.J., McGarry, J. and Williams, D.J.L., 2000. Vet. Parasitol., 93: 121-134. https://doi.org/10.1016/S0304-4017(00)00360-5
Paz-Silva, A., Hillyer, G.V., Sanchez-Andrade, R., Rodriguezmedina, J.R., Arias, M., Morrondo, P. and Diez-Banos, P., 2005. Parasitol. Res., 95: 129-135. https://doi.org/10.1007/s00436-004-1202-9
Phiri, A.M., Phiri, I.K. and Monrad, J., 2006. J. Helminthol., 80: 65-68. https://doi.org/10.1079/JOH2005313
Rahimi, M.T., Ashrafi, K., Koosha, S., Abdi, J. and Rokni, M.B., 2011. Arch. Iranian Med., 14: 18–21.
Rivera-Marrero, C.A., Santiago, N. and Hillyer, G.V., 1988. J. Parasitol., 74: 646-652. https://doi.org/10.2307/3282184
Rokni, M.B., Massoud, J., O’neill, S.M., Parkinson, M. and Dalton, J.P., 2002. Diagn. Microbiol. Infect. Dis., 44: 175–179. https://doi.org/10.1016/S0732-8893(02)00431-5
Sahar, R., 1996. A study on the epidemiological aspects of fascioliasis in buffaloes in Lahore district. M.Sc. thesis, College of Veterinary Sciences, Lahore.
Salimi-Bejestani, M.R., McGarry, J.W., Felstead, S.M., Ortiz, P., Akca, A. and Williams, D.J.L., 2005. Res. Vet. Sci., 78: 177-181. https://doi.org/10.1016/j.rvsc.2004.08.005
Sanchez-Andrade, A., Suarez, J.L., Arias, M., Francisco, I.. Diez. C., Cortinas, J, Romasanta, A., Morrondo, P., Diez-Banos P., Paz-Silva, A. and Sanchez-Andrade, R., 2008. Annu. trop. Med. Parasitol., 102: 489-498.
Schweizer, G., Braun, U., Deplazes, P. and Torgerson, P.R., 2005. Vet. Rec., 157: 188-193. https://doi.org/10.1136/vr.157.7.188
Siddiqi, M.N. and Shah, S.A.U., 1984. Pak. Vet. J., 4: 100-107.
Silva, E., Castro, A., Lopes, A., Rodrigues, A., Dias, C., Conceic A.O.A., Alonso, J., Correia, D.A., Costa, J.M., Bastos, M., Parra, F., Moradas-Ferreira, P. and Silva, M., 2004. J. Parasitol., 90: 746-751. https://doi.org/10.1645/GE-136R
Sriveny, D., Raina, O.K., Yadav, S.C., Chandra, D., Jayraw, A.K., Singh, M., Velusamy, R. and Singh, B. P., 2006. Vet. Parasitol., 135: 25-31. https://doi.org/10.1016/j.vetpar.2005.10.016
Thrusfield, M., 2007. Veterinary epidemiology, 3rd erd. University of Edinburg, Blackwell Science.
Urquhart, G.M., Armour, J., Duncan, J.L., Dunn, A.M. and Jennings, F.W., 1996. Veterinary parasitology 2nd ed. Longman Scientific and Technical Press, Oxford, UK, pp. 100-109.
Valero, M.A., Ubeira, F.M., Khoubbane, M., Artigas, P., Muiño, L., Mezo, M., Pérez-Crespo, I., Periago, M.V. and Mas-Coma, S., 2009. Vet. Parasitol., 159: 77-81. https://doi.org/10.1016/j.vetpar.2008.10.014
Wongkham, C., Tantrawatpan, C., Intapan, P.M., Maleewong, W., Wongkham, S. and Nakashima, K., 2005. Clin. Diagn. Lab. Immunol., 12: 1152-1156.
To share on other social networks, click on any share button. What are these?










