Association of Serum Resistin with Indices of Obesity in Young Pakistani Subjects
Association of Serum Resistin with Indices of Obesity in Young Pakistani Subjects
Farah Ashfaq* and Tasnim Farasat
Department of Zoology, Lahore College for Women University, Lahore, Pakistan
ABSTRACT
The aim of this research work was to examine the relationship of serum resistin with metabolic markers in obese Pakistani subjects. Anthropometric parameters including age, body mass index (BMI), waist circumference, waist-hip ratio (WHR), diastolic and systolic blood pressure, lipid profile and fasting glucose, serum resistin and insulin of three hundred over weight, obese males and females, 17 to 30 years and 100 comparable control subjects were included. Serum resistin and insulin, fasting glucose, triglycerides and cholesterol were found significantly high in overweight and obese groups as compared to the normal weight group (p<0.01). In overweight group, resistin levels were significantly related with cholesterol, insulin levels and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) (r = 0.375, p = .000; r = 0.336, p < 0.01; r = 0.301, p<0.01). In obese group, resistin showed a significant positive correlation with BMI, fasting glucose, systolic blood pressure and diastolic blood pressure (r = 0.332, p < 0.01; r = 0.278, p<0.01; r = .279, p<0.01 and r = 0.329, p<0.01), respectively. To conclude this study suggests a positive association between serum resistin and metabolic markers of obesity in young Pakistani subjects.
Article Information
Received 28 September 2016
Revised 12 January 2017
Accepted 07 March 2017
Available online 11 August 2017
Authors’ Contribution
This paper is from PhD research work of FA. TF supervised the research work, helped in designing the research project, data analysis and final revision of the article.
Key words
Resistin, Obesity, Inflammation, Insulin resistance, BMI.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.5.1587.1593
* Corresponding author: farah_ashfaq@yahoo.com
0030-9923/2017/0005-1587 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Obesity is a major concern for public health throughout the world and it is one of the most common non communicable diseases. Obesity is the outcome of constant imbalance between intake and outflow of energy. It has significant impact in both developed and developing countries. Obesity is an epidemic, spreading globally and developing countries are gradually becoming more susceptible to it. In parallel with increase in adult obesity, obesity in adolescents and young adults is also increasing (Jafar et al., 2006). Overweight and obese people are prone to different disorders caused by a variety of metabolic diseases revealing an under lying pathophysiology.
Resistin is most likely in the form of a few splice variants and it is a 114 amino-acid peptide found in humans. The significance of resistin in inflammation has been reported and immune cells seem to be main source of this adipocytokine. Increased resistin levels have been documented in obese humans in numerous studies. Resistin contributes to increased secretion of several pro-inflammatory markers from monocytes. Resistin can participate in the regulation of inflammation and immunity and at least in case of humans, numerous features are common in resistin and pro-inflammatory cytokines (Tilg and Moschen, 2006). In view of previous studies, cytokines are considered to increase the resistin levels, which may contribute to numerous inflammatory disorders including insulin resistance in relation to obesity (Antuna-poenti et al., 2008).
Resistin expression, obesity and inflammation has complex association and there is a need for systematic studies in large and different populations to analyse its role in metabolic diseases (Gil et al., 2007). In rodents, adipose tissue is the main source of resistin and is considered as a link between obesity and diabetes by impairing insulin sensitivity and glucose tolerance (Steppan and Lazar, 2004). In humans, however, resistin is expressed primarily in macrophages and represents a potential novel link between inflammation and adipocytokine. Resistin is upregulated during monocyte macrophage differentiation, signifying a role of resistin in inflammation and obesity related disorders (Lehrke et al., 2004).
In spite of many recent studies that are concerned with pathophysiology of resistin, information regarding the role of resistin in the progression of inflammation is inadequate. Initially, resistin attained attention because of its potential link between obesity and glucose regulation. In rodents, resistin can cause insulin resistance, whereas its possible role in the control of insulin sensitivity is still a controversial issue in humans.
The purpose of this research was to investigate the correlation of serum resistin with indices of obesity including fasting glucose, insulin levels, lipid profile, systolic blood pressure, diastolic blood pressure and insulin resistance in young Pakistani subjects.
Materials and methods
We studied 300 young adults (including both males and females) aged 17 to 30 years from community in and around Lahore, Pakistan. One hundred comparable control subjects were included. The data was stratified on the basis of BMI into normal weight (Group 1), overweight (Group 2), Obese I (Group 3) and obese II (Group 4) following the WHO criteria for Asians (WHO, 2004). None of the subjects was taking any medication affecting the body weight or had evidence of metabolic disease other than obesity.
Written informed consent was taken from all participants in the study. The study protocol was approved by the ethical committee and Advance study and Research Board of Lahore College for Women University, Lahore.
Anthropometry
The subjects were interviewed and the information regarding subject’s age, gender, smoking habits, family history of disease was collected through purposely designed questionnaire. Anthropometric parameters including age, BMI, waist and hip circumference, WHR, systolic and diastolic blood pressure were measured of all subjects participating in the present study by using standard procedures.
BMI was calculated according to the formula:
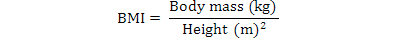
Waist-to-hip ratio (WHR) was calculated by dividing waist circumference to hip circumference. Systolic and diastolic blood pressure values were recorded after at least five minutes in seating position by means of sphygmomanometer apparatus (BD, Germany) under the supervision of a trained paramedical staff. The average of the three measurements was calculated.
Biochemical analysis
Blood sample (5.0 ml) was drawn from each subject, after an overnight fast. All samples were collected, processed, divided into aliquots and stored at −40 °C for analysis. Cholesterol was determined by CHOD-PAP method (Biolab). High density lipoprotein cholesterol (HDLc) was determined by using HDLc fluid precipitation with phosphotungstic acid and triglycerides (TG) were measured by GPO-PAP method (Biolab). Cholesterol, TG, HDLc, LDLc and glucose were determined by using commercial kits (Centronic, GmbH) Germany on Chemistry Analyzer (URIT-800 analyzer). The HDL/LDL ratio and cholesterol vs HDL (TChol / HDL ratio) were calculated.
The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated by the formula (Mathews et al., 1985):

Immunoassays
Serum Insulin levels (µIU/ml) and resistin level(µg/L) were measured by using ELISA kits run on automated CODA. Insulin levels were determined by using kit (CALBIOTECH, Inc., (CBI) USA.). The intra- and inter-assay coefficients of variation were 6.3 -8.1% and 7.4-8.5%, respectively.
Quantitative determination of human RETN concentration was done by ELISA kit of Glory Science Co., Ltd USA. Detection range of the kit was 1 µg/l-20µg/l.
Statistical analysis
Results are expressed as Means ± SEM. Categorical variables are compared by X2 test. One way analysis of variance ANOVA, with Tukey’s Post hoc test, was applied for group comparison Relationship between the parameters was analyzed by Pearson’s Correlation coefficients. Statistical significance was set at p< 0.05. Analysis was performed using the (SPSS 13.0 version (SPSS, Chicago, IL, USA.)
Results
Base line values of demographic and biochemical characteristics of the subjects are presented in Table I. ANOVA revealed significant differences in BMI ,WC, WHR, systolic BP, diastolic BP, cholesterol, triglycerides and resistin between groups and within groups (p<0.01) and HDL-c and TChol/HDL ratio (p<0.05). A non-significant association was observed in LDL-c (p>0.05). Post hoc analysis (Tukey test) showed significant difference between group 1 and 2, 2 and 3(p<0.05); group 1 and 3; group 1 and 4; group 2 and 4; group 3 and 4 (p<0.01).
Correlation analysis
In normal weight group, resistin showed a significant correlation with Hip circumference, WHR, cholesterol level and T Chol / HDL ratio (r = 0.335, p<0.01 and r = -0.205, p<0.05; r = 0.284, p<0.01; r = 0.327, p<0.01), respectively. Significant negative association was shown by resistin with HDLc in this group (r = -0.218, p<0.05).
|
Variables
|
Normalweight (G1) |
Over weight (G2) |
Obese 1 (G3) |
Obese II(G4) |
ANOVA |
|
Mean ± SEM |
Mean ±SEM |
Mean ±SEM |
Mean ± SEM |
P value
|
|
|
n = 100 |
n = 100 |
n = 101 |
n = 98 |
||
| Age (yrs) |
23.17 ± 0.46 |
23.56 ± 0.43 |
23.96 ± 0.44 |
24.78 ± 0.43 |
P>0.05NS |
| Height (cm) |
158.24±0.53 |
159.34±0.67 |
159.38±0.71 |
160.52±0.54 |
P>0.05NS |
| Weight (kg) |
52.54±0.48 |
61.48±0.54 |
71.05±0.67 |
91.61±1.14 |
P<0.01** |
|
BMI (kg/m2) |
20.96±0.12 |
24.17±0.04 |
27.94±0.13 |
35.53±0.39 |
P<0.01** |
| Waist circumference (cm) |
72.58±0.76 |
74.79±0.88 |
87.81±0.86 |
103.19±1.13 |
P<0.01** |
| Hip circumference (cm) |
87.04±0.95 |
90.91±1.18 |
102.66±0.94 |
112.23±0.96 |
P<0.01** |
| WHR |
0.84±0.01 |
0.83±0.01 |
0.86±0.01 |
0.92±0.01 |
P<0.01** |
| Fasting Glucose (mg/dL) |
81.89±0.48 |
84.63±0.50 |
86.10±0.68 |
88.42±0.64 |
P<0.01** |
| Systolic B.P (mmHg) |
116.14±0.74 |
119.65±0.94 |
125.67±1.14 |
129.13±1.20 |
P<0.01** |
| Diastolic B.P. (mm Hg) |
80.65±0.47 |
81.56±0.47 |
84.60±0.61 |
87.48±0.56 |
P<0.01** |
| Triglycerides (mg/dL) |
122.07±2.80 |
133.97±4.0 |
140.93±4.88 |
150.77±4.44 |
P<0.01** |
| Cholesterol (mg/dL) |
165.80±2.65 |
175.55±2.84 |
190.17±4.13 |
205.03±3.75 |
P<0.01** |
|
HDL_c (mg/dL) |
43.95±0.67 |
42.57±0.62 |
41.96±1.02 |
40.29±0.71 |
P<0.05* |
|
LDL_c (mg/dL) |
117.67±1.69 |
118.23±1.92 |
117.59±2.43 |
124.53±2.62 |
P>0.05NS |
| HDL vs LDL |
0.38±0.01 |
0.37±0.01 |
0.38±0.02 |
0.34±0.01 |
P<0.05* |
| T Chol /HDL ratio |
3.86±0.09 |
4.20±0.09 |
4.75±0.14 |
5.22±0.12 |
P<0.01** |
| Resistin (µg/L) |
5.25±0.16 |
7.24±0.32 |
8.06±0.30 |
10.29±0.75 |
P<0.01** |
| Insulin (µIU/ml) |
6.93±0.38 |
7.92±0.42 |
10.12±0.56 |
15.20±0.71 |
P<0.01** |
| HOMA IR |
1.43±0.08 |
1.68±0.09 |
2.16±0.12 |
3.47±0.16 |
P<0.01** |
BMI, body mass index; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; WHR, waist hip ratio; IL6, interleukin 6; hsCRP, high sensitivity creative protein; Homa IR, homeostatic model of assessment of insulin resistance; *, significant (P<0.05);**, significant (P<0.01); NS, non-significant.
In overweight group, resistin was significantly correlated with Hip circumference, triglycerides, cholesterol, TChol/ HDL ratio and insulin (r = 0.237, p<0.05; r = 0.265, p<0.01), (r = 0.375, p<0.01 and r = 0.347, p<0.01) and (r = 0.336, p<0.01). Resistin significantly correlated with insulin resistance (r =0.301, p<0.01).
In obese I group, resistin showed significant correlations with WHR and fasting glucose (r = -0.214, p< 0.05; r = -0.196, p< 0.05) and a non-significant association (p>0.05) was observed between resistin and other parameters of the study.
In Obese II group, resistin was significantly correlated with BMI, fasting glucose, systolic BP, diastolic BP, serum insulin and insulin resistance (r = 0.332, p<0.01; r = 0.278, p<0.01; r=0.279, p<0.05; r = 0.329, p<0.01; r = .360, p<0.01) and (r = 0.354, p<0.01), respectively (Figs. 1, 2, 3, 4, 5, 6, 7, 8).
Discussion
Immune cells are considered as very important source of resistin, which highlights the significance of this adipocytokine regarding inflammation. In the adipose tissue, infilteration of the immune cells in fat create an environment that enables the progression of inflammation and lead to the stimulation of adipocytes to secrete inflammatory mediators, like adipokines. A cycle of inflammation related obesity is completed in this manner (Fantuzzi, 2005). Nagaev et al. (2006) demonstrated in a human study that experimental endotoxemia resulted in hyper resistinemic condition.
Resistin showed no correlation with BMI in normal weight, overweight and obese I groups (p>0.05). These results are consistent with some previous studies in human subjects, in which resistin was not correlated with body mass index (Heilbronn et al., 2004). However, resistin showed significant correlation with BMI in obese II subjects (r = 0.332, P<0.01). Previous studies also supported these findings (Azuma et al., 2003). Some other studies showed a positive correlation between distribution of body fat and resistin levels (Coutinho et al., 2015).
The present study revealed a nonsignificant correlation between resistin and waist circumference, but significant correlation with hip circumference, in overweight group (p<0.05) in comparison to obese group which demonstrated anon significant correlation. A significant negative relationship was observed between resistin and WHR in normal weight and obese I group. According to findings of a previous study, resistin was significantly correlated with measures of central obesity like waist circumference and waist to height ratio in a study in Spanish adolescents (Codoner-Franch et al., 2014). However a contradiction was observed in previous study which reported a weak association between waist or hip circumference and resistin in obese subjects due to varied amount of abdominal fat (Bajaj et al., 2004).
Resistin was significantly correlated with systolic and diastolic BP in obese II subjects (r = 0.279, p < 0.01) and (r = 0.329, p < 0.01) while no significant association was observed in other BMI groups. Furuhashi et al. (2003) reported a positive relationship between elevated serum resistin levels and mean blood pressure. According to another study, the secretion of factors from adipocytes is synchronized in hypertension associated with obesity and they also contribute in regulation of chronic blood pressure (Yiannkouris et al., 2010).
In the current study, resistin was significantly correlated with triglycerides, cholesterol and Tchol/HDL ratio in over weight subjects (p < 0.01) in contrast to the subjects of group. These results are consistent with another findings by (Filkowa et al., 2009) who reported the contribution of resistin in the arterial inflammation and endothelial dysfunction along with accumulation of cholesterol and triglycerides in macrophages. Inflammatory responses could be amplified by resistin in liver through central nervous, signifying a unique mechanism causing decrease in insulin sensitivity and development of dyslipidemia (Muse et al., 2004).
The results of present studies revealed significant correlation with fasting glucose, insulin and homa IR in obese II subjects (p<0.01). Previous studies reported an association between serum resistin and insulin resistance in humans (Wasim et al., 2006). According to other study in Pima Indians, resistin was not correlated to insulin-resistance in healthy normal-weight individuals (Vozarova et al., 2004).
Resistin stimulates output of hepatic glucose and exerts its glucoregulatory effect in this way (Yang et al., 2009). Moreover, human resistin stimulates insulin resistance when expressed in mouse macrophages suggesting that human and mouse resistin may have comparable function regardless of their different sources of production (Qatanani et al., 2009).
Conclusion
These studies revealed a positive relationship between resistin and metabolic markers of obesity in young Pakistani subjects. Research on resistin has shown some contradictory results, and the specific role of resistin in advancement of different diseases remains to be clarified.
Acknowledgements
This paper is a part of HEC Funded Research Project (Ref # 20_1650/R & D/09 (2809) (Molecular mechanisms involved in the interaction of Obesity, Type 2 Diabetes Mellitus and Hypertension). We acknowledge the financial support of HEC to carry out our research work.
Statement of conflict of interest
No conflict of interest
References
Antuna-Puente, B., Feve, B., Fellahi, S. and Bastard, J.P., 2008. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab., 34: 2–11. https://doi.org/10.1016/j.diabet.2007.09.004
Azuma, K., Katsukawa, F., Oguchi, S., Murata, M., Yamazaki, H., Shimada, A. and Saruta, T., 2003. Correlation between serum resistin level and adiposity in obese individuals. Obes. Res., 11: 997–1001. https://doi.org/10.1038/oby.2003.137
Bajaj, M., Suraamornkul, S., Hardies, L.J., Pratipanawatr, T. and DeFronzo, R.A., 2004. Plasma resistin concentration, hepatic fat content and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients. Int. J. Obes. Relat. Metab. Disord., 28: 783–789. https://doi.org/10.1038/sj.ijo.0802625
Codoñer-Franch, P., Tavárez-Alonso, Porcar-Almela, M., Navarro-Solera, M., Arilla-Codoner, A. and Alonso-Iglesias, E., 2014. Plasma resistin levels are associated with homocysteine, endothelial activation, and nitrosative stress in obese youths. Clin. Biochem., 44: 44–48. https://doi.org/10.1016/j.atherosclerosis.2010.12.035
Coutinho, P.R., Leite, N., Lopes, W.A., da Silva, L.R., Consentino, C.M., Araújo, C.T., Moraes Jr., F.B., de Jesus, I.C., Cavaglieri, C.R. and Radominski, R.B., 2015. Association between adiposity indicators, metabolic parameters and inflammatory markers in a sample of female adolescents. Arch. Endocrinol. Metab., 59: 325-334. https://doi.org/10.1590/2359-3997000000070
Fantuzzi, G., 2005. Adipose tissue, adipokines, and inflammation. J. Allergy clin. Immunol., 115: 911–919. https://doi.org/10.1016/j.jaci.2005.02.023
Filkova, M., Haluzik, M., Gay, S. and Senolt, L., 2009. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin. Immunol., 133: 157-170. https://doi.org/10.1016/j.clim.2009.07.013
Furuhashi, M., Ura, N., Higashiura, K., Murakami, H. and Shimamoto, K., 2003. Circulating resistin levels in essential hypertension. Clin. Endocrinol., 59: 507–510. https://doi.org/10.1046/j.1365-2265.2003.01879.x
Gil, A., Aguilera, C.M., Gil-Campos, M. and Canete, R., 2007. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Br. J. Nutr., 98: 121-126. https://doi.org/10.1017/S0007114507838050
Heilbronn, L.K., Rood, J., Janderova, L., Albu, J.B., Kelley, D.E., Ravussin, E. and Smith, S.R., 2004. Relationship between serum resistin concentrations and insulin resistance in non obese, obese, and obese diabetic subjects. J. clin. Endocrinol. Metab., 89: 1844–1848. https://doi.org/10.1210/jc.2003-031410
Jafar, T.H., Chaturvedi, N. and Pappas, G., 2006. Prevalence of overweight and obesity and their association with hypertension and diabetes mellitus in an Indo-Asian population. Can. med. Assoc. J., 175: 1071-1077. https://doi.org/10.1503/cmaj.060464
Lehrke, M., Reilly, M.P., Millington, S.C., Iqbal, N., Rader, D.J. and Lazar, M.A., 2004. An inflammatory cascade leading to hyper-resistinemia in humans. PLoS Med., 1: e45. https://doi.org/10.1371/journal.pmed.0010045
Matthews, D.R., Hosker, J.P., Rudenski, A.S., Naylor, B.A., Treacher, D.F. and Turner, R.C., 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 28: 412-419. https://doi.org/10.1007/BF00280883
Muse, E.D., Obici, S., Bhanot, S., Monia, B.P., McKay, R.A., Rajala, M.W., Scherer, P.E. and Rossetti, L., 2004. Role of resistin in diet-induced hepatic insulin resistance. J. clin. Invest., 114: 232-239. https://doi.org/10.1172/JCI200421270
Nagaev, M., Bokarewa, A., Tarkowski, U. and Smith, 2006. Human resistin is a systemic immune-derived pro inflammatory cytokine targeting both leukocytes and adipocytes. PLoS One, 1: e31. https://doi.org/10.1371/journal.pone.0000031
Qatanani, M., Szwergold, N.R., Greaves, D.R., Ahima, R.S. and Lazar, M.A., 2009. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J. clin. Inevest., 119: 531–539. https://doi.org/10.1172/JCI37273
The current biology of resistinJ. Int. Med., 255https://doi.org/10.1111/j.1365-2796.2004.01306.x
Tilg, H. and Moschen, A.R., 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol., 6: 772-783. https://doi.org/10.1038/nri1937
Vozarova de Courten, B., Degawa-Yamauchi, M., Considine, R.V. and Tataranni, P.A., 2004. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes, 53: 1279–1284. https://doi.org/10.2337/diabetes.53.5.1279
Wasim, H., Al-Daghri, N.M., Chetty, R., McTernan, P.G., Barnett, A.H. and Kumar, S., 2006. Relationship of serum adiponectin and resistin to glucose intolerance and fat topography in South-Asians. Cardiovasc. Diabetol., 5: 10-49. https://doi.org/10.1186/1475-2840-5-10
WHO, 2004. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet, 363: 157-163. https://doi.org/10.1016/S0140-6736(03)15268-3
Yang, M., Xiao, Y., Mao, H., Li, S., Zhao, Y., Gu, R., Wang, J., Yu, X., Zhang, D.M., Irwin, G., Niu, H. and Tan, 2009. Resistin and insulin resistance in hepatocytes: resistin disturbs glycogen metabolism at the protein level. Biomed. Pharmacother., 63: 366–374. https://doi.org/10.1016/j.biopha.2008.06.033
Yiannikouris, F., Gupte, M., Putnam, K. and Cassis, L., 2010. Adipokines and blood pressure control. Curr. Opin. Nephrol. Hypertens., 19: 195–200. https://doi.org/10.1097/MNH.0b013e3283366cd0
To share on other social networks, click on any share button. What are these?










