Biochar Improves Viability of Arbuscular Mycorrhizal Fungi (Amf) in Soil and Roots of Wheat (Triticum aestivum) and Maize (Zea mays L.) under Various Cropping Systems
Biochar Improves Viability of Arbuscular Mycorrhizal Fungi (Amf) in Soil and Roots of Wheat (Triticum aestivum) and Maize (Zea mays L.) under Various Cropping Systems
Zubaria Malik, Zahir Shah* and Muhammad Tariq
Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Soil amendment with biochar is considered as a means to improve soil fertility and other soil properties. However, the effects of biochar on soil biota specifically arbuscular mycorrhizal fungi (AMF) have received less attention. This study was therefore executed to assess the effect of biochar amendment on AMF in soil and roots of wheat and maize crops in a rotation experiment involving cereals {wheat (Triticum aestivum), maize (Zea mays L.)} and legumes {chickpea (Cicer arientinum), mungbean (Vigna radiata)} in a calcareous alkaline soil of Peshawar valley during 2015/16 and 2016/17. This study was conducted in an already established experiment which was in a randomized complete block design with split plot settings. Keeping cropping systems in main plots and biochar levels in subplots. Biochar treatments were no biochar (T1), 20 t biochar ha-1 in each season (T2), 40 t biochar ha-1 in winter (T3) and 40 t biochar ha-1 in summer season (T4). Root samples of wheat and maize were collected at harvest stages and assessed for AMF colonization. Soil samples were also collected at the time of root sampling and analyzed for AMF spores. The results showed that neither root colonization of AMF in wheat and maize nor AMF spores density in soil were significantly (P<0.05) affected by cropping systems in any year. The average AMF spores in soil ranged from 45.1 to 39.8 per 20 g soil in wheat phase and 41.2 to 37.0 per 20 g soil in maize phase suggesting no considerable variation with season. However, the root colonization of AMF in maize (42.7-26.0 %) was considerably greater than that in wheat (14.5-14.9 %). Biochar treatment significantly increased both the AMF spores density in soil and root colonization in wheat and maize crops relative to the control treatment. The maximum AMF spores in soil in both seasons (wheat and maize) were found in T2 where biochar was applied at 20 t ha-1 each in summer and winter. In each season, control treatment had the lowest spores density in soil. The same trend was observed for root colonization of AMF in wheat and maize with maximum colonization in T2 and lowest in T1. These results suggested that indigenous AMF can be increased in soil and roots of wheat and maize with biochar amendment in an alkaline calcareous soil with low fertility.
Received | December 16, 2018; Accepted | July 03, 2019; Published | August 21, 2019
*Correspondence | Zahir Shah, Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: zahirshah@aup.edu.pk
Citation | Malik, Z., Z. Shah and M. Tariq. 2019. Biochar improves viability of arbuscular mycorrhizal fungi (Amf) in soil and roots of wheat (Triticum aestivum) and maize (Zea mays L.) under various cropping systems. Sarhad Journal of Agriculture, 35(3): 834-846.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.3.834.846
Keywords | AMF, Biochar, Calcareous soil, Cropping systems, Soil properties
Introduction
Phosphorous (P) availability is one of the major yield limiting factors in alkaline calcareous soil. Although calcareous soils contain large amount of P but that is largely unavailable and the farmers have to apply chemical fertilizers to correct P deficiency. Chemical fertilizers are expensive and the farmers are mostly unable to purchase the required amount of P fertilizers. Thus the soils remain unfertilized and the crops suffer P deficiency. Arbuscular mycorrhizal fungi (AMF) is known to increase P availability to plants generally under stress conditions. Therefore, strategies are needed to improve the efficiency of AMF to increase the availability of P. Mycorrhizal fungi are frequently used as soil inoculums to improve P availability, soil properties and crop yields (Schwartz et al., 2006). There is increasing evidence that biochar may increase mycorrhizal fungi in soil and subsequently positively improve the soil quality. The application of biochar to soil often results in significant improvement in soil properties, plant growth as well as increase in mycorrhizal fungi. Several studies have shown that biochar amendment can alter soil nutrient availability, improve physical, chemical and biological properties of soil and in turn increase crop yields (Matsubara et al., 2002; Yamato et al., 2006; DeLuca et al., 2006; Gundale and DeLuca, 2006; Hamdani et al., 2017; Shah et al., 2017; Sara et al., 2018). Improvement in nutrient availability in soil through biochar can increase the performance of host plants which may help supporting colonization of AM fungi (Ishii and Kadoya, 1994). It has been observed that biochar enhanced the capacity of AM fungi to increase resistance of host plants to infection by plant pathogens (Matsubara et al., 2002). Biochar create microhabitat in soil where mycorrhizal fungi can extend its hyphae for exploration of resources to support the nearby plants (Saito, 1990; Warnock et al., 2007). Other studies have revealed that biochar increased colonization by mycorrhizal fungi that helped improve water supply and other resources to plants in drought risk environments (Blackwell et al., 2007). Biochar amendment also increased colonization of pother mycorrhizal fungi such as ectomycorrhizal fungi in soil (Mori and Marjenah, 1994). The activated C in biochar adsorbs signaling compounds such as strigolactones for arbuscular mycorrhizal fungi which then stimulate hyphal branching of fungi (Akiyama et al., 2005). Matsubara et al. (2002) reported that the application of biochar produced from coconut fiber had a suppressive effect on the soil borne pathogen Fusarium sp in asparagus field.
Lehmann et al. (2006) and Wornock et al. (2007) extensively reviewed the literature on responses of arbuscular mycorrhizal fungi and C sequestration to biochar and concluded that biochar improve the abundance and function of AMF. They proposed four mechanisms where biochar can improve the functioning of AMF. For example, biochar can improve the soil physico-chemical properties of soil, alter the functioning of other soil microorganisms which can mycorrhizae, provide shelter to AMF and production of certain compound which support the growth and functioning of AMF.
Arbuscular mycorrhizal fungi not only improve P availability in soil but also improve other soil characteristics which support plant growth. The growth of AMF in soil generate large of amount of organic matter which help improve the soil properties and plant growth. Jakobsen and Rosendahl (1990) reported that the hyphae of AM fungi can grow about 3% of root weight of host plant. The mycelium of AM fungi can grow from 10 to 100 m per cm of root (McGonigle and Miller, 1999). Moreover, the AM fungi can improve soil aggregation and help keep soil particles together that help better soil aeration and root growth (Miller and Jastrow, 2000). In addition, the AM fungal hyphae can enter into the microsites of biochar and other organic materials, and thus results in decomposition of organic materials by releasing of plant nutrients in soil (Hodge, 2003). The AM fungi also play important role in the availability of water in soil particularly at lower soil water potentials and thus support increased water uptake by plants (Mahdi et al., 2010).
Recent reports have revealed strong evidence of improvement in arbuscular mycorrihzal fungi in soil with biochar. Arbuscular mycorrhizal fungi have access to microsites in biochar where roots cannot enter and thus P present in microsites of biochar can be explored by AM fungi (Hammer et al., 2014). It has been reported that combined application of AM fungi and biochar increased P uptake and growth of maize (Mau and Utami, 2014). Yusif and Hayatu (2017) found that biochar application increased root colonization of arbuscular mycorrhizal fungi and available P relative to control treatment. The efficiency of AM fungi however changes with other factors. For example, Karaki et al. (2004) found low rate of roots colonization by AMF in winter when soil temperature was low. However, AM fungal colonization in wheat roots increased during spring when the temperature rises. The AM fungal spores in soil and roots also changes with soil properties. Sharif et al. (2006) observed greater spores of arbuscular mycorrhizal fungal spores in soil as well as the root colonization of wheat and maize crops by AMF in low fertile soils compared with soils which had greater organic matter and soil nutrients. They suggested that the incidence of AMF were more in marginal than in fertile soils. They further demonstrated that the arbuscular mycorrhizal spores were greater in neutral soils with low organic matter and the infection of wheat and maize roots were also higher probably because of higher spores density in same soil. Conversa et al. (2015) demonstrated that the AMF inoculation was more effective in improving plant growth when applied with 30% biochar. However, the role of biochar with respect to AM fungi and performance of plants may dependent on soil composition and prevailing environmental factors. Since limited information are available on the role of biochar in root colonization of AM fungi in calcareous soils, this experiment was therefore undertaken to determine the responses of AM fungi to biochar amendments under different cropping systems in the agro-climatic conditions of Peshawar valley.
Materials and Methods
Experimental procedure
This study was undertaken to determine the influence of a long-term application of different levels of biochar on arbuscular mycorrhizal spores density in soil and their colonization of roots of wheat and maize during winter 2015-16 and 2016-17 for wheat and summer 2015 and 2016 for maize seasons. The soil of the experimental site was silty clay loam, non-saline, alkaline in reaction and low in soil organic fertility (Shafi et al., 2007). The soil and root samples of wheat and maize were taken from field experiment where biochar has been in use in different treatments since 2014 at the university of agriculture, Peshawar. Soil (0-15 cm) and root samples were collected at harvest stages for both wheat and maize. In case of wheat, samples were collected after harvest of wheat from wheat-maize and wheat-mungbean cropping systems. In case of maize, samples were collected after harvest of maize from maize-wheat and maize-chickpea cropping systems. Both wheat and maize received the recommended doses of N, P, and K at 120:90:60 and 150:100:60 kg ha-1 respectively along with recommended cultural practices and irrigated when needed. Nitrogen in the form of urea was applied in two splits (viz., half at sowing and half at 3-4 leaf stage in case of wheat and at knee high stage in case of maize) whereas P in the form of single superphosphate and K in the form of potassium chloride were applied at sowing time.
The field experiment used in this study was a randomized complete block design and the arrangements of treatments were in split plot. Each treatment was replicated four times. The cropping systems were in main plots while biochar treatments were in subplots. There were four cropping systems viz., winter cereal- summer cereal, 2) winter cereal-summer legume, 3) winter legume-summer cereal and 4) winter legume- summer legume. Winter cereal was wheat and summer cereal was maize. Similarly, winter legume was chickpea and summer legume was mungbean. Biochar treatments were: 1) no biochar in any season (T1), 2) 20 t biochar to each winter and summer crops (T2), 3) 40 t biochar to each winter crop (T3), and 4) 40 t biochar to each summer crop (T4). In summer 2016, no biochar was applied to any crop.
The first wheat crop which was sown in November 2014 could not be sampled and not included in this study. In the following summer 2015, maize crop was planted on 17th July and raised to maturity. Four maize plants were randomly uprooted carefully in each treatment plot (on 12 Oct 2015) for mycorrhizal root colonization in maize roots. Each treatment plot was also sampled for soil from the surface at 0-15 cm depth at the time of root sampling for determination of spores density in rhizosphere soil. In the following winter 2015/16, wheat crop was planted on 19 Nov 2015 and raised to maturity. Six mature plants were carefully uprooted along with rhizosphere soil at crop maturity on 11 April 2016 in each treatment plot for AM root colonization of wheat and AM spores density in soil. In the second year, maize crop was planted on 21st July 2016 on same plots. At maturity (on 23rd Sep 2016), root and soil samples were collected for AM occurrence. In the following season, wheat crop was planted on 7 Nov 2016 on same plots. At maturity (on 21st April 2017), roots and soil samples were collected from each treatment plot for AMF root colonization and spores density in rhizosphere soil.
Characteristics of biochar
The biochar used in the experiment was made of pruned branches of Acasia trees at over 450 oC in the absence of oxygen. The characteristics of biochar used in different seasons during the course of the experiment are described in Table 1. The pH and EC were determined in 1:5 w/v biochar-to-distilled water suspension using pH and EC meters (Rhoades, 1996).
Table 1: Important characteristics of biochar used in the experiment.
| Characteristics | Biochar used for winter crops (2014/15) | Biochar used for summer crops (2015) | Biochar used for winter crops (2015/16 | Biochar used for winter crops (2016/17) | Average |
| pH | 7.42 | 7.15 | 6.80 | 6.91 | 7.07 |
|
EC (dS m-1) |
1.75 | 1.09 | 2.34 | 3.37 | 2.14 |
| Total N (%) | 2.10 | 3.70 | 2.30 | 3.10 | 2.80 |
| Organic C (%) | 63.5 | 70.4 | 66.8 | 67.4 | 67.0 |
| Total P (%) | 0.10 | 0.08 | 0.10 | 0.13 | 0.10 |
Total N was determined by the Kjeldhal method (Bremner and Mulvaney, 1982). Total P in biochar samples was determined spectrophotometerically after digestion (Jones and Case, 1990). Organic carbon (C) was determined by the loss on ignition method (Koide et al., 2011; Naeem et al., 2014).
The data showed that the pH of biochar varied from 6.80 to 7.42 with an average of 7.07. Similarly, the EC of biochar varied from 1.09 to 3.37 with an average of 2.14 dS m-1, total N ranged from 2.1 to 3.7 with an average of 2.80 %, organic C ranged from 63.54 to 70.37 with an average of 67 % and total P ranged from 0.08 to 0.13 with an average of 0.10 %. Arif et al. (2014) also reported in their study that biochar made of Acasia wood had pH 6.84, EC 3.0 dS m-1, N 2.25 % and P 0.14 %. Similarly, Naeem et al. (2014) found high organic C content (662 g kg-1) in wheat straw biochar formed at 500 oC. Batool et al. (2015) reported that biochar is generally rich in organic C (30 to 70%), and characterized with high mineral contents, high pH and EC values and low ash content.
Isolation and determination of Arbuscular Mycorrhizal fungal spores in soil
Arbuscular mycorrhizal spores were in soil were determined by the wet-sieving and decanting technique (Gerdeman and Nicolson, 1963; Brundrett, 1996). Fresh sieved rhizosphere soil sample of 20 g was shaken with 200 ml distilled water in a beaker with the help of hand stirrer to break up the clods. Then the beaker was transferred to magnetic stirrer for 15 minutes for stirring and then passed through sieves of three different sizes. The upper sieve was 2 mm, the middle was 250 um and the bottom was 50 um. The materials of second and third sieves were transferred to a 50 ml falcon tube by sprinkling distilled water on materials until volume become 25 ml. After that 25 ml of 70 % sugar solution was added in the falcon tube. Spores suspension was then be centrifuged with sugar solution in falcon tubes in centrifugation machine for three minutes at 2400 rpm. A syringe pipe was used to collect the spores layer from just above the layer of sugar solution. The spores density was counted using the procedure as described in Schenck and Pérez (1990).
Determination of AM fungal infection in wheat and maize roots
The AM fungal infection in the roots of maize and wheat were determined by the staining of fungal structures procedure as described in Philips and Hayman (1970) and Koske and Gemma (1989) with some modifications for non pigmented roots. The plants were uprooted at the maturity stage and the rhizosphere soil was washed with tape water. After thorough wash, the roots were cut in small pieces with sharp scissor. The small and thin pieces of root samples of about 2 cm were preserved in a plastic jar containing distilled water with 2 to 3 drops of formalin in it. The plastic jars were kept at 4 0C in a refrigerator until analyzed. For determination of AM fungal root infection, the root samples were washed with distilled water. The root samples were transferred to 10% KOH solution in a beaker in such a way that root samples were completely covered with KOH solution. The solution was heated for 10 minutes to eliminate the cytoplasm and most of the host nuclei of the roots path. Root samples were then washed with distilled water, heated in 0.01% tryphan blue solution in the similar way for 7 minutes and get tryphan blue stained root pieces. Spores and hyphae were turned blue due to their chitin content. Root pieces were then observed under the microscope with x40 magnification for morphological characteristics. Moreover, the technique presented in Giovannetti and Mosse (1980) was used for assessment of infection intensity in roots. Percent mycorrhizal infection in roots was determined using the following expression:
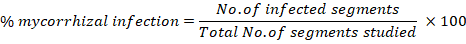
Statistical analysis
Data were analyzed statistically using statistical package Statistic 8.1. Least significance difference test was used to determine differences among means (Steel et al., 1997).
Results and Discussion
AMF spores density in soil in wheat phase
The results showed that the AMF spores density in soil were not significantly affected by the cropping system in any year (Table 2). Although non-significant, the AM fungal spores were slightly reduced in second year (2016/17) compared with first year (2015/16) of the experiment. However, the effect of biochar application was significant on AM fungal spores during both years. The pattern of response of AM fungal spores to biochar levels was similar in both years. During both years, the spores density was significantly greater in the biochar than in the control treatment. On average, the spore density ranged from 36.6 to 58.6 per 20 g soil in the biochar compared with 29.0 per 20 g soil in the control treatment. Moreover, in both years, the AM fungal spores were significantly highest for treatment receiving biochar at 20 t ha-1 each in winter and summer. The T3 and T4 treatments where biochar was applied (40 t biochar ha-1) to winter crops (T3) and summer crops (T4) exhibited statistically similar response on fungal spores during both years. On average, the maximum AM fungal spores of 58.6 per 20 g soil were observed in T3 treatment where biochar was applied at 20 t ha-1 in each season followed by 45.6 for T4 treatment where 40 t biochar ha-1 was applied to summer crops. Overall, the AMF spore density in soil was lowest in the control. Generally, the number of AM fungal spores in various treatments was lower than the desired levels in soil (Brundrett, 1996).
As far as variation among years are concerned, there were no statistical differences in AMF spores density between 2015/16 (45.1 per 20 g soil) and 2016/17 (39.8 per 20 g soil). The interactions between years, cropping systems and biochar levels were significant (Table 2; Figure 1 and 2). In first year, the highest spore density in soil was observed in treatment that has received 40 t biochar ha-1 only in wheat phase (T2) under both cropping systems (Figure 1). The number of spores however declined in treatment receiving no biochar in wheat phase (T3). The decline was drastic for wheat-mungbean than wheat-maize cropping systems. The spore density in soil under wheat-mungbean cropping system regained the momentum in T4 (in the maize phase) but that under wheat-maize remain static at same biochar treatment. In second year, the spores density in soil were similar for both cropping systems in the control (T1) and that gradually increased to almost similar extent for both cropping systems at treatment receiving biochar at 20 t ha-1 regularly in both seasons (T2) (Fig 2). However, where biochar was applied at 40 t ha-1 in the wheat phase only (T3), the spores density in soil remained the same as with the previous treatment for wheat-mungbean cropping system but drastically declined for wheat-maize system. On the other hand, the spores density increased in soil under wheat-maize and decreased in soil under wheat-mungbean T4 (receiving biochar only in summer season).
Table 2: Effect of cropping systems and biochar levels on arbuscular mycorrhizal fungal spores density in soil after harvest of wheat crop.
|
Treatment Cropping system |
2015/16 | 2016/17 | |
| soil spores density (no. per 20 g soil) | Mean | ||
| Wheat after maize | 45.1 | 38.3 | 41.7 |
| Wheat after mungbean | 45.0 | 41.4 | 43.2 |
| Grand mean | 45.1 | 39.8 | 42.5 |
| LSD (p ≤ 0.05) | ns | ns | ns |
|
Biochar in w-s$ (t ha-1) |
|||
|
T1. 0-0$ |
34.6b | 23.3c | 29.0c |
| T2. 20-20 | 62.8a | 54.3a | 58.6a |
| T3. 40-0 | 35.5b | 37.8b | 36.6c |
| T4. 0-40 | 47.5b | 43.8b | 45.6b |
| Grand mean | 45.1 | 39.8 | 42.5 |
| LSD (p ≤ 0.05) | 13.4 | 9.9 | 8.0 |
| Year means | 45.1 | 39.8 | ns |
Interactions significance/probability; CS X BC-**; Y X -; Y X BC; Y X CS X BC- - **; <27: deficient, 28 to 67: medium, > 67 high per 20 g soil (Brundrett et al., 1996). $” w” stands for winter, and “s” stands for summer; CS: cropping systems; BC: Biochar; Y: years.
This implies that the behavior of spore density in soil was consistent in the control and for treatment where biochar was regularly applied at 20 t ha-1 in each season (T2) under both cropping systems but it was inconsistent at other levels of biochar. It was however evident that biochar did improve the AM spore density in soil. It could be due to the facts that activated C of biochar adsorbs AMF signaling compounds strigolactones which stimulate hyphal branching of AMF (Akiyama et al., 2005). Yusif and Hayatu (2017) found that biochar application increased root colonization of arbuscular mycorrhizal fungi and available P relative to control treatment. Moreover, biochar application at 20 t ha-1 improved soil properties compared to other biochar rates (Yusif and Hayatu, 2017). Hussain et al. (2016) found maximum spores density of 58 and 46 with mycorrhiza inoculation in the presence of half and full level of vermicompost, respectively. Both the two most commonly occurring types of mycorrhizal fungi were increased with application of biochar amendments (Warnock et al., 2007).
AMF root colonization of wheat
The data revealed that the effect of cropping was on AMF root colonization intensity non-significant, but that of biochar treatments was significant (Table 3). Overall, the AMF root colonization in wheat was higher in second year than first year. Although non-significant, the year mean of 2016/17 was higher (14.9 %) than (14.5 %) 2015/16. Moreover, the AMF root colonization in wheat was higher for wheat-mungbean than wheat-maize cropping system in both the years. The data described that root colonization of AMF was significantly higher for biochar than in control treatment in both years. The maximum AMF root colonization in year 1 was obtained in T2 and this was statistically at par with T4. However, the colonization of AM fungi in wheat in T3 was statistically similar that in the control treatment in first year. In second year, the root colonization of AMF in wheat was greater significantly with biochar treatments relative to the control treatment. However, no significant (P<0.05) differences in root colonization of AMF in wheat were found among the biochar treatments. On average, the AMF root colonization in wheat was significantly greater in biochar than in the control treatment. However, the dirrences in AMF root colonization were non-significant among biochar treatments. Overall the AMF root colonization in wheat was lower than the desired level. Similar results were reported by Sharif et al. (2006) who observed 28-43 % AM fungal infection in wheat roots in soil containing relatively more organic matter in northern areas of Pakistan. On the other hand Karaki et al. (2004) found negligible AM fungal colonization during winter months when the soil temperature was low but during the spring, AM fungal colonization increased gradually in wheat crop.
Makoto et al. (2010) reported that ecto-mycorrhizal infection of roots in larch seedling significantly enhanced by 19-157% with biochar amendments. The roots colonization of wheat by AM fungi also increased by 20-40% two years after biochar amendments from 0.6 to 6 t ha-1, relative to colonization rate of 5-20% in the control treatment (Solaiman et al., 2010). This indicates the residual effect at least for two years after biochar application. The residual effect of biochar on soil properties and crop yields were also recently reported in Sara et al. (2018). Rillig et al. (2010) found that biochar stimulated spore germination of AM fungi.
Table 3: Effect of cropping systems and biochar levels on root colonization of arbuscular mycorrhizal fungi in wheat crop.
|
Treatment Cropping system |
2015/16 | 2016/17 | |
| AMF root colonization (%) | Mean | ||
| Wheat after maize | 14.2 | 13.8 | 14.0 |
| Wheat after mungbean | 14.9 | 16.0 | 15.4 |
| Grand mean | 14.5 | 14.9 | 14.7 |
| LSD (p ≤ 0.05) | ns | ns | ns |
|
Biochar in w-s$ (t ha-1) |
|||
|
T1. 0-0$ |
10.0b | 8.1b | 9.1b |
| T2. 20-20 | 18.3a | 16.5a | 17.4a |
| T3. 40-0 | 12.9b | 17.1a | 15.0a |
| T4. 0-40 | 16.8a | 17.9a | 17.4a |
| Grand mean | 14.5 | 14.9 | 14.7 |
| LSD (p ≤ 0.05) | 3.2 | 6.1 | 3.3 |
| Year means | 14.5 | 14.9 | |
Interactions significance/probability; CS X BC; **-; Y X CS; Y X BC; Y X CS X BC; $ “w” stands for winter and “s” for summer; CS: cropping systems; BC: Biochar; Y: years.
Table 4: Effect of cropping systems and biochar levels on arbuscular mycorrhizal fungal spores density in soil after harvest of maize crop.
|
Treatment Cropping system |
2015 | 2016 | |
| AM spores density in soil (no.per 20 g soil) | Mean | ||
| Maize after wheat | 39.0 | 35.0 | 37.0 |
| Maize after chickpea | 43.5 | 39.1 | 41.3 |
| Grand mean | 41.2 | 37.0 | 39.1 |
| LSD (p ≤ 0.05) | ns | ns | ns |
|
Biochar in w-s$ (t ha-1) |
|||
|
T1. 0-0$ |
20.5c | 20.7c | 20.6c |
| T2. 20-20 | 60.1a | 58.1a | 59.1a |
| T3. 40-0 | 50.2a | 31.5bc | 40.8b |
| T4. 0-40 | 34.2b | 38.0b | 36.1b |
| Grand mean | 41.2 | 37.0 | 39.1 |
| LSD (p ≤ 0.05) | 12.0 | 11.6 | 8.0 |
| Year means | 41.2 | 37.0 | ns |
Interactions significance/probability; CS X BC; Y X CS; Y X BC; *; Y X CS X BC; $ “w” stands for winter and “s” for summer; ; CS = cropping systems; BC = Biochar; Y = years.
The effect of biochar on AM mycorrhizae is not positive in every case and can have negative influence too. There is evidence where biochar amendment reduced the abundance of AMF spores in soil and their infection in roots (Gaur and Adholeya, 2000; Birk et al., 2009; Warnock et al., 2010). The possible reasons for such negative effect is not clear but could be due to one or more of the following reasons; (i) biochar application usually increase P and water availability to plants thus the need for mycorrhizal symbiosis with the plants is reduced (Corbin et al., 2003; Covacevich et al., 2006; Gryndler et al., 2006); (ii) biochar can improve the soil conditions, e.g., improvement in soil pH or water relations, or (iii) direct negative effects from high salt or heavy metal contents that are detrimental to AM fungi (Killham and Firestone, 1984).
AMF spores density in soil in maize phase
The data regarding AMF spores density in soil at harvest stage of summer crops (maize, mungbean) as influenced by cropping systems and biochar treatment are presented in Table 4. The data showed that there were no significant (P<0.05) differences in AMF spores density in soil between maize after wheat and maize after chickpea cropping systems in both years. However, AMF fungal spores in soil were significantly (P<0.05) higher in biochar than in the control treatment in both years. In first year, the maximum AM fungal spores in soil were found in T2 which were applied with biochar at 20 t ha-1 each in winter and summer (T2) and this was statistically at par with T3. Among biochar treatments, the lowest number of AM fungal spores in soil were found T4 where biochar was applied at 40 t ha-1 in summer phase only. In second year, the maximum AM fungal spores in soil were found in T2 where 20 t biochar ha-1 applied each in winter and summer followed by T4 which received biochar at 40 t ha-1 in summer phase only. However, differences between T3 and T1 (control) were statistically non-significant (P<0.05). On average, the maximum AM fungal spores in soil were found in T2 followed by T3 and T4, respectively. The lowest number of AM spores in soil were found in the control where no biochar was applied in any season to any crop. Among interactions, interactions between biochar and years were found significant (P<0.05) for AM fungal spores in soil after harvest of maize crop (Figure 3). It was observed that AM fungal spores increased linearly in T2 in both years. The spores density decreased in T3 in both years but the decrease was more drastic in second compared with first year. In T4, the decrease in spores density continued in first year but not in second year. Haerida and Karmadibrata (2002) found varied types of spores in the treatment receiving 7.5 g biochar kg-1 soil with no AMF inoculation.
AMF root colonization of maize
The results showed that root colonization by AMF in maize was not influenced significantly (P<0.05) in first year but was significantly influenced in second year (Table 5). The root colonization of AMF in maize was significantly greater following chickpea than wheat cropping. However, overall the root colonization of AM fungi in maize decreased in second year. As far as the effect of biochar was concerned, the root colonization of AMF in maize was significantly (P<0.05) greater in biochar than in the control treatment. In first year, the maximum root colonization of AMF was found in T2 (receiving biochar at 20 t ha-1 each in winter and summer) which was statistically at par with T3 (receiving biochar at 40 t ha-1 by winter crops only). Among biochar treatments, the lowest root colonization of AMF was observed in T4 (receiving biochar at 40 t ha-1 by summer crops only). The lowest root colonization was obtained in the control treatment where no biochar was applied to any crop in any season.
In second year, the maximum root colonization of AMF in maize was observed in T2 (receiving biochar at 20 t ha-1 each in winter and summer) and T4 (receiving 40 t ha-1 biochar in summer only) and differences between these two treatments were statistically non-significant. Moreover, differences in root colonization of AMF in maize were statistically non-significant (P<0.05) between T4 (receiving biochar at 40 t ha-1 in summer only) and T1 (control treatment with no biochar amendment). On average, the maximum root colonization of AMF in maize was obtained in T2 (receiving biochar at 20 t ha-1 each in winter and summer). The data further showed that the root colonization of AMF in T3 (receiving biochar at 40 t ha-1 in winter only) and T4 (receiving biochar at 40 t ha-1 in summer only) were significantly lower than in T2 but greater than in T1. Differences between T3 and T4 were statistically non-significant.
Table 5: Effect of cropping systems and biochar levels on AMF root colonization of maize crop.
|
Treatment Cropping system |
2015 | 2016 | ||
| AMF root colonization of maize (%) | Mean | |||
| Maize after wheat | 45.3 | 22.0b | 33.6 | |
| Maize after chickpea | 40.1 | 30.0a | 35.1 | |
| Grand mean | 42.7 | 26.0 | 34.3 | |
| LSD (p ≤ 0.05) | ns | * | ns | |
|
Biochar in w-s$ (t ha-1) |
||||
|
T1. 0-0$ |
20.1c | 11.0b | 15.5c | |
| T2. 20-20 | 61.9a | 40.2a | 51.1a | |
| T3. 40-0 | 53.4ab | 11.2b | 32.3b | |
| T4. 0-40 | 35.5bc | 41.6a | 38.5b | |
| Grand mean | 42.7 | 26.0 | 34.3 | |
| LSD (p ≤ 0.05) | 19.5 | 5.2 | 9.7 | |
| Year means | 42.7a | 26b | 7.23 | |
Interactions significance/probability; CS X BC; Y X CS; *; Y X BC; ** Y X CS X BC; $“w” stands for winter and “s” for summer; CS: cropping systems; BC: Biochar; Y: years.
The interactions between years and cropping systems and between years and biochar levels were significant (P<0.05). The AMF root colonization of maize was higher in first year than in second year for both the cropping systems (Figure 4). The root colonization of AMF decreased in second year for both cropping systems but the decrease was more drastic for maize after wheat than for maize after chickpea. Figure 5 also described that the root colonization of AMF in maize was generally higher in first year than in second year in all biochar treatments except T4 (receiving biochar at 40 t ha-1 in summer only). The effect of biochar varied for T3 (receiving biochar at 40 t ha-1 in winter only) where the root colonization of AMF in maize was significantly lower in second year than in first year. However, variation for T4 was non-signficant between first and second year. Mau and Utami (2014) reported a successful AM fungi and biochar combination which resulted better uptake of P and growth of maize. Sharif et al. (2006) suggested that AM fungal spores in soil and roots colonization of wheat and maize crops by AM fungi were higher in low fertile soil compared with fertile soil. In fertile soils of Hazara division, 32 to 42 % AM fungal infection were observed in roots of maize crop whereas 40 to 59 % infection rates by mycorrhizal fungi were observed in low fertile soils in the same area.
Conclusions and Recommendations
This experiment has shown that root colonization of AM fungi in wheat and maize and AM fungal spores in soil in both seasons were significantly increased with biochar amendment but were not influenced significantly by the cropping systems. The effect of continuous application biochar was more pronounced than application of biochar only in one season. Thus biochar application may be considered as a strategy to improve the soil biota including AM fungi in area deficiency in soil organic matter.
Acknowledgements
This study was undertaken in the Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar-Pakistan with the financial support of the Higher Education Commission, Islamabad under indigenous 5000 PhD Scholarship Programme.
Authors Contribution
Zubaria Malik: Conducted the experiments, collected the data and wrote the article.
Zahir Shah: Major supervisor of the first author, assisted in planning experiments, analysed the data and finalized the article.
Muhammad Tariq: Co-supervisor of the first author, helped in experiments, collection of data and writing the article.
Novelty Statement
The current research highlights novel information about long term application of different levels of biochar on Arbuscular Mycorrhizal Fungi (AMF) in soil and roots of wheat (rotated with maize and mungbean crop in summer) and maize (rotated with wheat and chickpea crop in winter) for two years.
References
Akiyama, K., K. Matsuzaki and H. Hayashi. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nat. 435 (7043): 824-827. https://doi.org/10.1038/nature03608
Angelini, J., S. Castro and A. Fabra. 2003. Alterations in root colonization and nod C gene induction in the peanut-rhizobia interaction under acidic conditions. Plant Physiol. Biochem. 41 (3): 289-94. https://doi.org/10.1016/S0981-9428(03)00021-4
Arif, M., F. Jalal, M.T. Jan and D. Muhammad. 2014. Integration of biochar and legumes in summer gap for enhancing productivity of cereal based cropping system. Sarhad J. Agric. 30 (4): 393-403. https://doi.org/10.1080/21683565.2014.996696
Bais, H.P., T.L. Weir, L.G. Perry, S. Gilroy and J.M. Vivanco. 2006. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann. Rev. Plant Biol. 57: 233-66. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Batool, A., S. Taj, A. Rashid, A. Khalid, S. Qadeer, A.R. Saleem and M.A. Ghufran. 2015. Potential of soil amendments (biochar and gypsum) in increasing water use efficiency of Abelmoschus esculentus L. Moench. Front. Plant Sci. Vol. 6 Article 733. https://doi.org/10.3389/fpls.2015.00733
Birk, J.J., C. Steiner, W.C. Teixeira, W. Zech and B. Glaser. 2009. Microbial response to charcoal amendments and fertilization of a highly weathered tropical soil. In: Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., WinklerPrins, A.M.G.A., Rebellato, L. (Eds.), Amazonian Dark Earths: Wim Sombroek’s Vision. Springer, Berlin, pp. 309-324. https://doi.org/10.1007/978-1-4020-9031-8_16
Bremner, J.M. and C.S. Mulvaney. 1982. Nitrogen-Total. In: A.L. Page, R.H. Miller and D.R. Keeney (eds), methods of soil analysis. Part 2. Soil Sci. Soc. Am. Inc. Madison, WI. pp. 595–624.
Brundrett, C.M. 1996. Mycorrhizas in natural ecosystems. Adv. Ecol. Res. 21: 171-313. https://doi.org/10.1016/S0065-2504(08)60099-9
Conversa, G., A. Bonasia, C. Lazzizera and A. Elia. 2015. Influence of biochar, mycorrhizal inoculation and fertilizer rate on growth and flowering of Pelargonium (Pelargonium zonale L.) plants. Front. Plant Sci. 6: pp. 429. https://doi.org/10.3389/fpls.2015.00429
Corbin, J.D., P.G. Avis and R.B. Wilbur. 2003. The role of phosphorus availability in the response of soil nitrogen cycling, understory vegetation and arbuscular mycorrhizal inoculum potential to elevated nitrogen inputs. Water, Air and Soil Poll. 147: 141-161. https://doi.org/10.1023/A:1024569615325
Covacevich, F., M.A. Marino and H.E. Echeverrica. 2006. The phosphorus source determines the arbuscular mycorrhizal potential and the native mycorrhizal colonization of tall fescue and wheatgrass. Eur. J. Soil Biol. 42: 127-138. https://doi.org/10.1016/j.ejsobi.2005.12.002
DeLuca, T.H., M.D. MacKenzie, M.J. Gundale and W.E. Holben. 2006. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci. Soc. Am. J. 70: 448–453. https://doi.org/10.2136/sssaj2005.0096
Elmer, W.H. and J.J. Pignatello. 2011. Effect of biochar amendments on mycorrhizal associations and Fusarium crown and root rot of asparagus in replant soils. APS Publ. publ. online: 14 Jul 2011. https://doi.org/10.1094/PDIS-10-10-0741
Gaur, A. and A. Adholeya. 2000. Effects of the particle size of soil-less substrates upon AM fungus inoculum production. Mycorrhiza. 10: 43-48. https://doi.org/10.1007/s005720050286
Gerdeman, J.W. and T.H. Nicolson. 1963. Spores of mycorrhiza, Endogone species extracted from soil by wet sieving and decanting. Mycol. Soc. 46 (2): 235-244. https://doi.org/10.1016/S0007-1536(63)80079-0
Giovannetti, M. and B. Mosse. 1980. Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 84: 489-500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Gryndler, M., J. Larsen, H. Hrselovac, V. Rezaccovac, H. Gryndlerovac and J. Kubact. 2006. Organic and mineral fertilization, respectively, increase and decrease the development of external mycelium of arbuscular mycorrhizal fungi in a long-term field experiment. Mycorrhiza. 16: 159-166. https://doi.org/10.1007/s00572-005-0027-4
Gundale, M.J. and T.H. DeLuca. 2006. Temperature and source material influence ecological attributes of ponderosa pine and douglas-fir charcoal. For. Ecol. Manage. 231: 86–93. https://doi.org/10.1016/j.foreco.2006.05.004
Hamdani, S.A.F., M. Aon, L. Ali, Z. Aslam, M. Khalid and M. Naveed. 2017. Application of Dalbergia sissoo biochar enhanced wheat growth, yield and nutrient recovery under reduced fertilizer doses in calcareous soil. Pak. J. Agric. Sci. 54 (1): 107-115. https://doi.org/10.21162/PAKJAS/17.5102
Hammer, E.C., Z. Balogh-Brunstad, I. Jakobsen, P.A. Olsson, S.L.S. Stipp and M.C. Rilling. 2014. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 77: 252-60. https://doi.org/10.1016/j.soilbio.2014.06.012
Herrmann, S., R. Oelmüller and F. Buscot. 2004. Manipulation of the onset of ectomycorrhiza formation by indole-3-acetic acid, activated charcoal or relative humidity in the association between oak microcuttings and Piloderma croceum: influence on plant development and photosynthesis. J. Plant Physiol. 161 (5): 509-17. https://doi.org/10.1078/0176-1617-01208
Hodge, A. 2003. Plant nitrogen capture from organic matter as affected by spatial dispersion, interspecific competition and mycorrhizal colonization. New Phytol. 157 (2): 303-14. https://doi.org/10.1046/j.1469-8137.2003.00662.x
Hussain, S., M. Sharif, S. Khan, F. Wahid, H. Nihar, W. Ahmad, I. Khan, N. Haider and T. Yaseen. 2016. Vermicompost and mycorrhiza effect on yield and phosphorus uptake of wheat crop. Sarhad J. Agric. 32 (4): 372-381. https://doi.org/10.17582/journal.sja/2016.32.4.372.381
Ishii, T. and K. Kadoya. 1994. Effects of charcoal as a soil conditioner on citrus growth and vesicular–arbuscular mycorrhizal development. J. Jpn. Soc. Hort. Sci. 63: 529–535. https://doi.org/10.2503/jjshs.63.529
Jakobsen, I. and L. Rosendahl. 1990. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol. 115 (1): 77-83. https://doi.org/10.1111/j.1469-8137.1990.tb00924.x
Karaki, G.A., B. McMicheal and J.C. Zac. 2004. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza. 14 (4): 263-269. https://doi.org/10.1007/s00572-003-0265-2
Killham, K. 1985. A physiological determination of the impact of environmental stress on the activity of microbial biomass. Environ. Poll. Ser. A. 38: 283-294. https://doi.org/10.1016/0143-1471(85)90133-3
Koide, R.T., P. Krittika and P. Matthew. 2011. Quantitative analysis of biochar in field soil. Soil Biol. Biochem. 43: 1563-1568. https://doi.org/10.1016/j.soilbio.2011.04.006
Koske, R.E. and J.N. Gemma. 1989. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 92 (4): 486-488. https://doi.org/10.1016/S0953-7562(89)80195-9
Lehmann, J., J. Gaunt and M. Rondon. 2006. Biochar sequestration in terrestrial ecosystems a review. Mitigation Adaptation Strategies Global Change. 11: 403-427. https://doi.org/10.1007/s11027-005-9006-5
Makoto, K., Y. Tamai, Y. Kim and T. Koike. 2010. Buried charcoal layer and ectomycorrhizae cooperatively promote the growth of Larix gmelinii seedlings. Plant Soil. 327(1): 143-152.
Matsubara, Y., N. Hasegawa and H. Fukui. 2002. Incidence of Fusarium root rot in asparagus seedlings infected with arbuscular mycorrhizal fungus as affected by several soil amendments. J. Jpn. Soc. Hort. Sci. 71: 370–374. https://doi.org/10.2503/jjshs.71.370
McGonigle, T.P. and M.H. Miller. 1999. Winter survival of extraradical hyphae and spores of arbuscular mycorrhizal fungi in the field. Appl. Soil Ecol. 12 (1): 41-50. https://doi.org/10.1016/S0929-1393(98)00165-6
Mori, S. and Marjenah. 1994. Effect of charcoaled rice husks on the growth of Dipterocarpaceae seedlings in East Kalimantan with special reference to Ectomycorrhiza formation. J. Jpn. For. Soc. 76 (5): 462-464.
Naeem, M.A., M. Khalid, M. Arshad and R. Ahmad. 2014. Yield and nutrient composition of biochar produced from different feedstocks at varying pyrolytic temperatures. Pak. J. Agric. Sci. 51 (1): 75-82.
Phillips, J.M. and D.S. Hayman. 1970. Improved procedures for clearing roots and staining parasitic and AM fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55: 158. https://doi.org/10.1016/S0007-1536(70)80110-3
Rillig, M.C., M. Wagner, M. Salem, P.M. Antunes, C. George, H.G. Ramke, M.M. Titirici and M. Antonietti. 2010. Material derived from hydrothermal carbonization: effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 45: 238-242. https://doi.org/10.1016/j.apsoil.2010.04.011
Saito, M. 1990. Charcoal as a micro-habitat for VA mycorrhizal fungi, and its practical implication. Agric. Eco. Environ. 29: 341-344. https://doi.org/10.1016/0167-8809(90)90298-R
Sara, K., Z. Shah and T. Shah. 2018. Residual effect of biochar on soil properties and yield of maize (Zea mays L.) under different cropping systems. Open J. Soil Sci. 8: 16-35. https://doi.org/10.4236/ojss.2018.81002
Schwartz, M.W., J.D. Hoeksema, C.A. Gehring, N.C. Johnson, J.N. Klironomos, L.K. Abbott and A. Pringle. 2006. The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecol. Lett. 9: 501–515. https://doi.org/10.1111/j.1461-0248.2006.00910.x
Shah, T., Sara and Z. Shah. 2017. Soil respiration, pH and EC as influenced by biochar. Soil Environ. 36(1): 77-83. https://doi.org/10.25252/SE/17/51184
Shafi, M., J. Bakht, M.T. Jan and Z. Shah. 2007. Soil C and N dynamics and maize (Zea mays L.) yield as affected by cropping systems and residue management in North-western Pakistan. Soil Till. Res. 94: 520–529. https://doi.org/10.1016/j.still.2006.10.002
Sharif, M., M.S. Sarir and Nasrullah. 2006. Field evaluation of arbuscular mycorrhizal fungi in wheat-maize cropping system in Hazara division of North West Frontier Province. Pak. J. Biol. Sci. 9 (3): 487-492. https://doi.org/10.3923/pjbs.2006.487.492
Solaiman, Z.M., P. Blackwell, L.K. Abbott and P. Storer. 2010. Direct and residual effect of biochar application on mycorrhizal colonization, growth and nutrition of wheat. Aus. J. Soil Res. 48: 546-554. https://doi.org/10.1071/SR10002
Soltanpour, P.N. and A.P. Schawab. 1977. A new soil test for simultaneous extraction of macro- and micronutrients in alkaline soil. Commun. Soil Sci. Plant Anal. 8:195–207. https://doi.org/10.1080/00103627709366714
Warnock, D.D., D.L. Mummey, B. McBride, J. Major, J. Lehmann and M.C. Rillig. 2010. Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: results from growth-chamber and field experiments. Appl. Soil Ecol. 46: 450-456. https://doi.org/10.1016/j.apsoil.2010.09.002
Warnock, D.D., J. Lehmann, T.W. Kuyper and M.C. Rilling. 2007. Mycorrhizal responses to biochar in soil– concepts and mechanisms. Plant Soil, 9-20.c 2010 19th World congress of soil science, soil solutions for a changing world, 1–6 August 2010, Brisbane, Australia. Published on DVD. https://doi.org/10.1007/s11104-007-9391-5
Yamato, M., Y. Okimori, I.F. Wibowo, S. Anshori and M. Ogawa. 2006. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 52 (4): 489-495. https://doi.org/10.1111/j.1747-0765.2006.00065.x








