Biological Degradation of some Synthetic and Bio-Pesticides Sprayed on Cauliflower Crop
Biological Degradation of some Synthetic and Bio-Pesticides Sprayed on Cauliflower Crop
Muhammad Faheem Akbar1,*, Muhammad Abdul Haq2, Imtiaz Ahmad1, Vasileva Viliana3 and Amjad Sultan1
1Department of Agriculture and Agribusiness Management, University of Karachi
2Department of Food Science and Technology, University of Karachi
3Institute of Forage Crops, 89 Genearl Vladmire Vazov Str, Pleven 5800, Bulgaria
ABSTRACT
To prevent adverse effects of pesticides on human, it is a must to establish control measures in order to ensure maximum residue limits (MRLs) to be respected. Considering the concerns about pesticide residues in food and their environmental impacts, this study designed to find out the biological degradation of conventional and bio pesticides sprayed on cauliflower crop. Bio pesticides are environment-friendly products and highly biodegradable with low persistency and residual effects. Imidacloprid, endosulfan and profenofos were selected as convectional, while spinosad and biosal (neem compound) as bio pesticides, and were sprayed at the rates of 49.4, 642.2, 988, 35.5 and 158 g a.i. ha-1 respectively. The pesticide residues were analyzed after 0, 1, 3 and 7 days of application in the leaves and cauliflower curd using high performance liquid chromatography. First order degradation kinetics was fitted on this data and degradation rate constants and half-life were calculated. Conventional pesticides were found to be more persistent in the crop (Average half-life: 2.94, 4.06 and 2.74 days for imidacloprid, endosulfan and profenofos respectively) than bio pesticides (Average half-life: 3.87 and 1.81 days for spinosad and biosal respectively). The crops treated with bio pesticides were found safer for human consumption even after few hours of spray compared with codex and EU MRLs. Whereas, endosulfan and profenofos treated crops were not found to be fit for consumption even after 7 days of application, as they were not degraded down to the EU and Codex MRL within usual pre-harvest interval (PHI). Imidacloprid being bio-rational (low risk) pesticide degraded quickly and the crop was also safe for consumption on the next day of application. This study also endorsed the hazardous impacts of endosulfan as its worldwide use is recommended to be phased out globally.
Article Information
Received 04 July 2018
Revised 10 March 2019
Accepted 18 May 2019
Available online 11 March 2020
Authors’ Contribution
MFA conducted the experiments and wrote the article. MAH and AS analyzed the data. IA, VV and AS helped in preparation of the manuscript.
Key words
Biological degradation, Synthetic pesticides, Bio pesticides, MRLs, Cauliflower
DOI: https://dx.doi.org/10.17582/journal.pjz/20180704120727
* Corresponding author: [email protected]
0030-9923/2020/0003-1121 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Insect pests are responsible for potential damage to human by devastating food, commodities and crops (Pogačnik and Franko, 1999). Pesticides are important for boosting agricultural production as an essential tool in Integrated Pest Management (IPM), but their injudicious use causes negative effects on human health (Soomro et al., 2008) and non-target organisms in the environment, with increased resistance to insect pests (Kranthi et al., 2001; Saleem and Akbar, 2019). High reliance on pesticides by untrained farmers has augmented health hazards to the farm workers (Ahmad and Soomro, 2001) and the ultimate consumers due to residual effects (Ahmad et al., 2013).
Additionally, they have severe disadvantages like development of pesticide resistance, pest resurgence, pest upsets and secondary pest outbreak and incidence of undesirable residue (Gurusubramanian et al., 2005; Sarnaik et al., 2006).
The intense practice of pesticides usage has created a great concern particularly in case of vegetables, as these are consumed within few days after their harvest. Masud and Hasan (1992) reported higher level of insecticide residues in vegetables above maximum residue limits. Pesticides residues on fruits and vegetables may result in countless damage to male fertility; as 30 out of 37 crop chemicals tested impeded with the action of testosterone, an important hormone (Ahmad, 2004). It is quite an alarming situation that highly toxic organophosphate and organochlorine pesticides are most frequently being used on various vegetables including okra, brinjal, cauliflower, tomato, peas and onion etc. (Khan et al., 2006). Bio-pesticides provide a much safer alternative to their synthetic counterparts. Plant derivatives have good potential (Isman, 2000; Dhingra et al., 2008) as insect control agent, as they are believed to be environment friendly, safe to human and other non-target organisms. The dissipation of neem compound in the environment is supposed to be quick, but literature on its degradation rates in different crops is scare. Spinosad®, composed of spinosyns A and D, substances derived from naturally occurring soil bacterium Saccharopolyspora spinosa by aerobic fermentation of this actinomycete species. Its degradation has extensively been studied by Tomkins et al. (1999) on different crops, but not on cauliflower. It is classified as a reduced risk pesticide by US Environmental protection agency (Cleveland et al., 2002).
Keeping in view the environment-toxicological problems due to the residues of synthetic insecticides; present study was designed to evaluate the effectiveness of bio-insecticides as compared to conventional insecticides with focus on their degradation kinetics. The practical expediency of insecticide depends on its effectiveness against insects as well as its degradation rate. The dissipation rate of insecticides selected in this study, except biosal (azadirachtin) has been previously reported under different agro-ecological conditions on different crops, but not in conditions of Karachi region. Since the degradation rate depends on nature of compound, environmental factors and the type of crop, therefore one of the objectives of this study was to find out the degradation rate of synthetic insecticides in comparison with bio-insecticides in consumable and non consumable parts of the vegetable crops. The modeling of degradation was performed to determine the safe pre-harvest intervals (PHIs) as per codex and EU MRLs.
Materials and Methods
Chemicals
HPLC grade solvents were purchased from Fisher Scientific (USA). Pesticide standards viz, Endosulfan, Imidacloprid, Profenofos and Spinosad were purchased from Dr. Ehrenstorfer GmbH, Germany. Azadirachtin standard was obtained from Sigma Aldrich (Steinheim, Germany). All other chemicals used for analysis were of analytical grade.
Sampling for residual studies
For the purpose of residual studies, representative samples of cauliflower leaves and curd were taken from each treated plot after insecticides’ application. One hundred g cauliflower leaves and 500 g of cauliflower curd were collected after 1 h (0 Day), 1, 3 and 7 days of insecticide treatment. From these samples, sub samples were made for extraction, clean up and further analysis.
Extraction of conventional insecticides (imidacloprid, endosulfan, profenofos)
Conventional insecticides were extracted and cleaned up by the method of Shahida and Hassan (2002) with modification. Thirty gram sample was homogenized in a high speed blender with 100 mL mixture of n-hexane, acetone (3:1, v/v). The homogenate was shaken on electric shaker (Orbital Shaker Model QSM-747, Digitek Instrument China) at 200 RPM for 4 h and then left at -20ºC for 24 h. The clear supernatant was decanted and concentrated to 2 ml on rotary vacuum evaporator (Buchi Rotavapor® R-210) at 30ºC. The concentrated extract was then passed through a mini-column. The mini column was prepared by loading Florisil (1 g) + activated charcoal (0.1 g) into a pasture pipette with a layer of anhydrous sodium sulphate on both sides of the column. The column was plugged with glass wool. The eluent was evaporated completely and re-dissolved in 1 mL respective mobile phase (Table I).
Extraction of bio-insecticides (spinosad and biosal)
Spinosad was extracted and cleaned up by the method of Sharma et al. (2007) with modifications. Thirty gram sample was blended with 100 mL acetonitrile and water mixture (8:2, v/v) for 2 min. The homogenate was shaken on electric shaker at 200 RPM for 30 min. The homogenate was filtered through a Buchner funnel and the residues were rinsed with 20 mL acetonitrile and water mixture (8:2, v/v). The combined filtrate was partitioned with dichloromethane in a separating funnel. After phase separation, the organic phase was collected. Ten mL methanol and 1 mL (1N) sodium hydroxide along with additional dichloromethane was added to the aqueous phase. The mixture was again partitioned. The pooled organic phase was evaporated to dryness in a rotary vacuum evaporator at 30ºC and the residue was finally dissolved in respective mobile phase (Table I).
Biosal (a. i. Azadirachtin) was extracted with a modified procedure of Caboni et al. (2002). Thirty gram sample was blended with 50 mL acetonitrile in the presence of 10 g Na2SO4. The homogenate was shaken on electric shaker at 200 RPM for 30 min and then filtered. The filtrate was dried under nitrogen flux. The residue was dissolved in 10 mL n-hexane. The hexane extract was purified by the method of Ramesh and Balasubramanian (1999) using graphitized carbon black containing SPE (Solid Phase Extraction) cartridge. The cartridge was preconditioned with n-hexane.
The extract was added to the cartridge and completely eluted. The cartridge was then eluted with acetonitrile, eluent was collected and dried in rotary vacuum evaporator and the residue was reconstituted with 1 mL mobile phase (Table I).
HPLC determination
All the insecticides were determined by High Performance Liquid Chromatography (HPLC). Perkin Elmer HPLC system equipped with series 200 binary
Table I.- HPLC conditions for insecticides residue determination.
|
S.No. |
Insecticides |
Mobile phase |
Flow rate |
Detector |
|
1 |
Imidacloprid |
H2O 70% + ACN* 30% |
1.0 |
254 |
|
2 |
Endosulfan |
ACN 70% + H2O 30% |
2.0 |
214 |
|
3 |
Prophenofos |
ACN 65% +H2O 35% |
2.0 |
282 |
|
4 |
Spinosad |
MeOH** 35% (containing 0.5% ammonium acetate) + 65% ACN |
2.0 |
250 |
|
5 |
Azadirachtin |
MeOH 35 % + ACN 15 % + H2O 50% |
1.2 |
220 |
*ACN, Acetonitrile; **MeOH, Methanol.
pump and series 200 UV visible detector was used. Brownlee column Spheri-5 ODS 5m, 250 x 4.6 mm was used for all insecticides. All solvents were properly degassed using sonicator and filtered through 0.45 µ nylon filter medium before use. Twenty µL of the filtered (0.45 µ nylon filter) sample was injected. HPLC conditions are given in Table 1. Data was recorded and analyzed using TotalCrom Workstation version 6.3.
Statistical analysis
Generalized exponential decay curve of below type was fitted on the residual data using regression analysis.
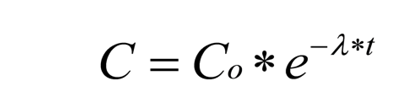
Where Co= amount of insecticide recovered at time (t) =0, λ = the degradation constant and, t = time in days, e = the base of natural logarithm.
Half life was calculated using these fitted curves.
Results
Residue levels in cauliflower crop are presented in Table II and are compared with codex and EU MRLs. Residue less than practical limit of quantification are not presented and are indicated as ND (not detected).
The initial concentration of imidacloprid at time 0 days was found to be high in cauliflower leaves (2.694 mg kg-1), while it was low in cauliflower curd (0.975 mg kg-1). The residue levels of endosulfan were found higher in leaves (5.188 mg kg-1), while it was comparatively low in cauliflower curd (2.205 mg kg-1). Initial concentration of profenofos in leaves was somewhat comparable to endosulfan (3.958 mg kg-1), whereas in cauliflower curd it was 2.557 mg kg-1. For spinosad these initial values in leaves were 3.222 mgkg-1 and in cauliflower curd 2.540 mg kg-1.
After 7 days of spray the residues of endosulfan and profenofos were higher than the codex and EU MRLs in consumable part i.e. cauliflower curd as 0.820 mg kg-1, whereas for profenofos these levels were 0.538 mg kg-1. The residues for imidacloprid and spinosad were below the prescribed levels of codex and EU MRLs.
Half life (Table II) of imidacloprid in cauliflower was 2.53 and 3.35 days in leaves and curd, respectively. For endosulfan half life in the leaves of cauliflower was 2.91 days, whereas for cauliflower curd half life was 5.21 days. Half life of profenfos in the leaves was observed as 2.49 days, whereas in the consumable parts of cauliflower, it was 2.99 days. For spinosad half life observation was 3.87 days both in the leaves and cauliflower curd.
Since azadirachtin was not detected at the dose used in field trials, hence, a separate experiment was conducted using ten times more from recommended dose i.e. 158 g. a. i. ha-1 (Table III). The initial concentration of azadirachtin was found to be 0.413 mg kg-1 in cauliflower leaves, while it was 0.178 mg kg-1 in cauliflower curd. At 7 days post- application azadirachtin was not detected in the leaves and cauliflower curd. Half life of azadirachtin was observed as 1.75 days in cauliflower leaves, whereas for the consumable parts it was recorded as 1.87 days.
Discussion
Several methods have been developed for the estimation of insecticides residue; most of them rely on gas chromatography (GC) (Guerin et al., 1992; Polese et al., 1996; Brito et al., 2002; Siddique et al., 2003). High performance liquid chromatography (HPLC) provides broad spectrum alternatives, because it can simultaneously handle acidic, basic, neutral, ionic, and thermally unstable insecticides (Siddique et al., 2003).
Analysis of insecticide residues by HPLC
All the insecticides were successfully separated under the employed chromatographic conditions without interfering impurities. This also support that the applied clean up techniques were adequate. Endosulfan, spinosad and azadiractin were resolved to their constituents, endosulfan α and β, spinosad A and D and azadirachtin A and B. A diminutive peak broad banding was observed in the samples for endosulfan, this might be due to initially high impurities in the purified extract. However, the peaks were still detectable and quantifiable. Comparatively noisy base line was observed in case of azadirachtin samples;
Table II.- Residues and degradation kinetics of insecticides crop*.
|
Time (Day) |
Imidacloprid |
Endosulfan leaf |
Prophenofos |
Spinosad |
|
Residue (mg kg-1) |
||||
|
0 |
2.694+0.103 |
5.188+0.047 |
3.958+0.201 |
3.222+0.223 |
|
1 |
1.884+0.039 |
4.071+0.190 |
2.959+0.167 |
2.658+0.104 |
|
3 |
1.225+0.047 |
2.439+0.034 |
1.740+0.083 |
1.949+0.017 |
|
7 |
0.387+0.024 |
1.075+0.011 |
0.547+0.009 |
0.876+0.029 |
|
λ** |
0.274+ 0.018 |
0.238+0.011 |
0.278+0.021 |
0.179+0.016 |
|
Half Life |
2.53+ 0.18 |
2.91+0.15 |
2.49+0.21 |
3.87+0.39 |
|
R2*** |
0.982 |
0.99 |
0.977 |
0.959 |
|
Cauliflower curd |
||||
|
0 |
0.975+0.043 |
2.205+0.036 |
2.557+0.140 |
2.540+0.132 |
|
1 |
0.818+0.008 |
2.054+0.116 |
2.094+0.083 |
2.188+0.122 |
|
3 |
0.516+0.025 |
1.609+0.017 |
1.230+0.019 |
1.455+0.069 |
|
7 |
0.233+0.009 |
7 0.820+0.027 |
0.538+0.002 |
0.745+0.023 |
|
λ |
0.207+ 0.012 |
0.133+0.011 |
0.232+0.017 |
0.179+0.015 |
|
Half Life |
3.35+0.23 |
5.21+0.51 |
2.99+0.24 |
3.87+0.38 |
|
R2 |
0.982 |
0.955 |
0.975 |
0.96 |
|
EU-MRLs |
0.5 |
0.05 |
0.05 |
2 |
|
Codex- |
0.5 |
N/A**** |
N/A |
N/A |
|
MRLsPHI-EU**** |
3.24+0.19 |
28.67+2.37 |
17.05+1.25 |
1.34+0.11 |
|
PHICodex***** |
3.24+0.19 |
N/A |
N/A |
N/A |
*Data is displayed as mean + SEM, **λ , Decay constant (Days-1); ***R2, Coefficient of determination; ****N/A, Not Available; ****PHI-EU, Pre-harvest interval based on EU MRLs; *****PHI-Codex, Pre-harvest interval based on Codex MRLs.
this is possibly due to the fact that the applied insecticide biosal is a mixture of very closely related compounds.
Degradation kinetics of insecticides
The insecticide residue levels are shown in Table II. Just after application the residues were detected in both leaves and curd. The initial residual level of same insecticide was found different in both the parts. This can be explained by differences in surfaces which received these insecticides. Especially the surface area and surface characteristics e.g. presence or absence of wax and spines affect the amount of insecticide which retain after spray (Freeman and Beattie, 2008).
The initial level of imidacloprid was found to be in the range of 0.97 mg kg-1 to 2.69 mg kg-1, which is in consistence with the findings of (Mansoor-ul-Hassan et al., 2005; Arora, 2009). The initial level of endosulfan was found to be in the range of 2.20 mg kg-1 to 5.18 mg kg-1. Mukherjee (2003) analyzed 44 samples of different vegetables, sprayed with endosulfan and found all samples of brinjal, cabbage, tomato, cauliflower, chilli, okra and mustard were contaminated with endosulfan as compared to maximum residue limits 2 mg kg-1. The initial level of profenofos was found to be in the range of 2.55 mg kg-1 to 3.95 mg kg-1. While, for spinosad this level was found to be in the range of 2.54 mg kg-1 to 3.22 mg kg-1. This is in the line with Sharma et al. (2007) who applied spinosad at 17.5 and 35.0 g ha-1 a.i. respectively and found the initial level of 3.8 mg kg-1 to 5.8 mg kg-1. In the initial trials the azadirachtin containing biosal was not detected at the recommended usage level. Therefore, a separate study at 10 time’s higher level i.e 158 g a.i ha-1. Table III was constructed to assist the degradation kinetics of azadirachtin (Caboni et al., 2002).
The degradation rate and half life of same insecticide were found different in cauliflower leaves and curd. This could be due to difference in crop morphology as the leaves of different crops have different cuticle and other environmental factors such as temperature and humidity.
Endosulfan was found to be most persistent followed by profenofos and imidacloprid. Conventional insecticides showed comparatively higher degradation rate in leaf than in fruit. The leaves have a great rate of metabolism due to a large number of varieties of enzymes as compared to the fruits. This difference of the phenomenon exhibited by the endosulfan as compared to the others could be due to the fact that endosulfan is a non-polar compound, whereas, rest of the two are polar ones. Being highly soluble in water polar compounds are absorbed readily and are degraded. Contrarily, endosulfan being non-polar penetrates slowly and degrades gradually as well.
Table III: Residues and degradation kinetics of Azadirachtin (@158 grams a.i ha-1) in cauliflower crop*.
|
Time (Day) |
Residue (mg kg-1) cauliflower leaves |
Residue (mg kg-1) cauliflower curd |
|
0 |
0.413+0.018 |
0.178+0.009 |
|
1 |
0.266+0.014 |
0.131+0.001 |
|
3 |
0.108+0.001 |
0.055+0.002 |
|
7 |
ND** |
ND |
|
λ *** |
0.396+0.027 |
0.371+0.024 |
|
Half Life |
1.75+0.11 |
1.87+0.14 |
|
R2**** |
0.988 |
0.986 |
|
EU-MRLs |
1 |
1 |
|
Codex-MRLs |
N/A***** |
N/A |
|
PHI-EU****** |
N/Ap******* |
N/Ap |
|
PHI-Codex******** |
N/A |
N/A |
*Data is displayed as mean + SEM, **ND, Not detectable; ***λ, Decay constant (Days-1); ****R2, Coefficient of determination; *****N/A, Not available; ******PHI-EU, Pre-harvest interval based on EU MRLs; *******N/Ap, Not applicable; ********PHI-Codex, Pre-harvest interval based on Codex MRLs
Half life of spinosad was found to be comparable to conventional insecticide. The half life was found to be same in both the parts as 3.87 days. This is in agreement with Sharma et al. (2008) as they worked on dissipation behavior of spinosad in cabbage and cauliflower. They reported its half life 1.43 and 0.99 days for lower rate (17.5 g a.i. ha-1) and 1.66 and 1.52 for higher rate (35.0 g a.i. ha-1) for cabbage and cauliflower respectively, which are winter season crops. Akbar et al. (2010) and Abar et al. (2012) reported the half life of spinosad as1.20 and 1.31 days in the leaves and fruits of summer season crop-okra, while the half life of spinosad in the winter season crop-cabbage, leaves and head was reported as 3.45 and 3.50 days, respectively. The lower half life in summer season crops can be explained by the fact that spinosyns are to be broken down by sunlight or high temperature (Cleveland et al., 2002).The half-life for spinosyn- A, has also been reported as 1.6 to 16 days, which mostly depends on the amount of sunlight received.
The half life of azadirathnin was found to be lowest among all the insecticides studied. Caboni et al. (2002) pointed out that the major cause of degradation of azadirathnin is sunlight.
Pre-harvest interval (PHI)
Residue data was successfully fitted on exponential decay kinetics, this is shown by the high coefficient of determination R2>0.9 (Sharma et al., 2007). Using this prediction equations pre-harvest interval (PHI) was calculated considering EU/Codex MRLs.
The residue data (Tables II) revealed that the crops treated with endosulfan were not consumable in usual practices of harvesting because the insecticide was not degraded up to the required EU/Codex MRLs before 28 days for cauliflower. Similarly, the crops treated with profenofos cannot be consumed even after 17 days of spray, because the edible parts were found to contain higher than maximum residue limit set by the European Union (EU- MRLs, 0.05 mg kg-1) and codex (No, 2004). The pre-harvest interval (PHI) obtained in this study is in consistence with persistent nature of endosulfan and profenofos, which has been reported in several studies (Mukherjee, 2003; Chowdhury et al., 2007; Baig et al., 2009). Since these crops cannot be retained in the field for such a long time (Talukder et al., 2003) after last spray that is why these two insecticides should not be used on vegetable crops, as they are consumed after very short time of harvest. Our conclusion is supported by Mukherjee (2003) who analyzed 44 samples of different vegetables (sprayed with endosulfan) for residues and found all samples of egg plant, cabbage, tomato, cauliflower, chilli, okra and mustard were contaminated with endosulfan as compared to maximum residue limits 2 mg kg -1. Similarly, for the case of profenofos, Baig et al. (2009), analyzed 36 samples collected from fields of egg plant and okra, and found 1.45 and 1.6 mg kg -1 residues, respectively.
Imidacloprid treated crops were safe to consume after 3 days of spray (EU-MRL 0.5 mg kg -1), as already reported safe PHI by (Akbar et al., 2010; Abar et al., 2012) for okra and cabbage. This is in agreement with Santharam et al. (2003) who did not found the residues of imidacloprid in chilli fruits collected in the first harvest when applied at 250, 375 and 500 ml ha-1. In the case of bio-insecticides the EU MRLs are 1 mg kg -1 for azadirachtin and 1 mg kg -1 for spinosad. This level was not achieved even just after the spray, in this study. This is in consistence with the findings of Mossler and Dunn (2005). They suggested 4 h pre-harvest interval (PHI) for spinosad and the restricted entry interval (REI) for azadirachtin and spinosad under the Worker Protection Standard is 4 h. We concluded that both bio-insecticides can be applied up to the day of harvest (PHI=0 day).
The low level of azadirachtin found at time zero indicates that high level of it can be applied. The degradation rate given in Table II can be used to approximate the level of application. For example, if we consider usual PHI of 3 days (Talukder et al., 2003), based on field conditions, then the regression equation reveals that application dosage of azadirachtin can be increased up to 15 times to initial dosage we used, i.e. 15.8 g ha-1. However, a separate study is required to find out the impact of high dose on environment, insects and plant physiology, with their economic feasibility.
Conclusion
The present study led to the conclusion that bio-insecticides; biosal and spionsad, and bio-rational insecticide; imidacloprid can successfully be incorporated as a part of insect pest management program; reducing the sole dependency on conventional insecticides. Additionally, the HPLC technique can be successfully applied to follow the residues of conventional and bio-insecticides. It was observed that bio-insecticides degraded rapidly as compared to synthetic insecticides, which is good for the environment and human health. Due to very low degradation rate endosulfan and profenofos were not found to be safe for application on vegetable crops, as they were not found to be degraded down to the EU and Codex MRL within usual pre-harvest interval (PHI). Hence both the insecticides should not be used due to their long persistent nature, particularly on vegetable crops. Considering the health and environmental hazards of organochlorine insecticides, the worldwide use of endosulfan is recommended to be phased out globally.
Statement of conflict of interest
The authors declared that there is no conflict of interests regarding the publication of this article.
References
Ahmad, I., 2004. Pesticide residues in fortified water, soil, food, fruits and vegetable samples in Pakistan. J. exp. Zool. India, 7: 67-72.
Ahmad, I., Anwar, T. and Tahir, S., 2013. Pesticide residues in agricultural commodities in Pakistan’s perspective: With reference to safe limits. Int. J. biol. Res., 1: 31-37.
Ahmad, I. and Soomro, M., 2001. Policy and strategy for rational use of pesticides in pakistan: Building consensus for action. Report to the UNDP/FAO Global IPM Facility. Government of Pakistan, 137pp.
Akbar, M.F., Haq, M.A., Parveen, F., Yasmin, N. and Sayeed, S.A., 2010. Determination of synthetic and bio-insecticides residues during aphid, Myzus persicae (Sulzer) control on cabbage crop through high performance liquid chromatography. Pak. Entomol., 32: 155-162.
Akbar, M., Haq, M.A.,Yasmin, N. and Khan, M., 2012. Degradation analysis of some synthetic and bio-insecticides sprayed on okra crop using hplc. J. chem. Soc. Pak., 34: 306-311.
Arora, S., 2009. Analysis of insecticides in okra and brinjal from ipm and non-ipm fields. Environ. Monit. Assess., 151: 311-315. https://doi.org/10.1007/s10661-008-0272-z
Baig, S.A., Akhtera, N.A., Ashfaq, M. and Asi, M.R., 2009. Determination of the organophosphorus pesticide in vegetables by high-performance liquid chromatography. Am-Eura. J. Agric. environ. Sci., 6: 513-519.
Brito, N., Navickiene, S., Polese, L., Jardim, E., Abakerli, R. and Ribeiro, M., 2002. Determination of pesticide residues in coconut water by liquid–liquid extraction and gas chromatography with electron-capture plus thermionic specific detection and solid-phase extraction and high-performance liquid chromatography with ultraviolet detection. J. Chromatogr, A., 957: 201-209. https://doi.org/10.1016/S0021-9673(02)00351-5
Caboni, P., Cabras, M., Angioni,A., Russo, M. and Cabras, P., 2002. Persistence of azadirachtin residues on olives after field treatment. J. Agric. Fd. Chem., 50: 3491-3494. https://doi.org/10.1021/jf020076+
Chowdhury, A.G., Das, C., Kole, R.K., Banerjee, H. and Bhattacharyya, A., 2007. Residual fate and persistence of endosulfan (50 wdg) in bengal gram (Cicer arietinum). Environ. Monit. Assess., 132: 467-473. https://doi.org/10.1007/s10661-006-9549-2
Cleveland, C.B., Bormett, G.A., Saunders, D.G., Powers, F.L., McGibbon, A.S., Reeves, G.L., Rutherford, L. and Balcer, J.L., 2002. Environmental fate of spinosad. 1. Dissipation and degradation in aqueous systems. J. Agric. Fd. Chem. 50: 3244-3256. https://doi.org/10.1021/jf011663i
Dhingra, S., Walia, S., Kumar, J., Singh, S., Singh, G. and Parmar, B.S., 2008. Field efficacy of azadirachtin-a, tetrahydroazadirachtin-a, neemazal® and endosulfan against key pests of okra (Abelmoschus esculentus). Pest Manage. Sci., 64: 1187-1194. https://doi.org/10.1002/ps.1615
Freeman, B.C. and Beattie, G.A., 2008. An overview of plant defenses against pathogens and herbivores. The Plant Health Instructor. https://doi.org/10.1094/PHI-I-2008-0226-01
Guerin, T.F., Kimber, S.W., and Kennedy, I.R., 1992. Efficient one-step method for the extraction of cyclodiene pesticides from aqueous media and the analysis of their metabolites. J. Agric. Fd. Chem., 40: 2309-2309. https://doi.org/10.1021/jf00023a051
Gurusubramanian, G., Borthakur, M., Sarmah, M. and Rahman, A., 2005. Pesticide selection, precautions, regulatory measures and usage: Plant protection in tea. Tocklai Experimental Station, Assam Printing Works Private Limited, Jorhat, Assam, India. pp. 81-91.
Isman, M.B., 2000. Plant essential oils for pest and disease management. Crop Protec., 19: 603-608. https://doi.org/10.1016/S0261-2194(00)00079-X
Khan, B.A., Farid, A., Khan,N., Rasul, K. and Perveen, K., 2006. Survey of pesticide use on fruits and vegetables in district peshawar. Sarhad J. Agric., 22: 497-page
Kranthi, K.R., Jadhav, D., Wanjari, R., Kranthi, S. and Russell, D., 2001. Pyrethroid resistance and mechanisms of resistance in field strains of Helicoverpa armigera (lepidoptera: Noctuidae). J. econ. Ent., 94: 253-263. https://doi.org/10.1603/0022-0493-94.1.253
Mansoor-ul-Hassan, F.A., Sagheer, M., Iqbal, M.F. and Tariq, M., 2005. Residual persistence of chlorpyrifos, imidacloprid and acephate in brinjal fruit. Pak. Entomol., 27: 53-55.
Masud, S. and Hasan, N., 1992. Pesticide residues in foodstuffs in pakistan-organochlorine, organophosphorus and pyrethroid insecticides in fruits and vegetables. Pak. J. scient. indust. Res., 35: 499-499.
Mossler, M.A. and Dunn, E., 2005. Florida crop/pest management profile: Okra. Institute of Food and Agricultural Sciences, University of Florida, Florida.
Mukherjee, I., 2003. Pesticides residues in vegetables in and around delhi. Environ. Monit. Assess., 86: 265-271. https://doi.org/10.1023/A:1024057420937
EU, MRLs No, E., 2004. 852., 2004. Corrigendum to regulation (ec) no 852/2004 of the european parliament and of the council of 29 april 2004 on the hygiene of foodstuffs. Off. J. Eur. Union, 226: 3-21.
Pogačnik, L. and Franko, M., 1999. Determination of organophosphate and carbamate pesticides in spiked samples of tap water and fruit juices by a biosensor with photothermal detection. Biosens. Bioelectr., 14: 569-578. https://doi.org/10.1016/S0956-5663(99)00029-9
Polese, L., Minelli, E., Jardim, E.F. and Ribeiro, M., 1996. Small-scale method for the determination of selected organochlorine pesticides in soil. Fresenius Anal. Chem., 354: 474-476. https://doi.org/10.1007/s0021663540474
Ramesh, A. and Balasubramanian, M., 1999. Rapid preconcentration method for the determination of azadirachtin-a and-b, nimbin and salannin in neem oil samples by using graphitised carbon solid phase extraction. Analyst, 124: 19-21. https://doi.org/10.1039/a806527f
Saleem, M.S. and Akbar, M.F., 2019. Bio efficacy of neonicotinoids and Insect Growth Regulators (IGRs) against BemiciatabaciGenn. on Solanum melongena L. 2019. Pakistan J. Zool., 51:1615-1620.
Santharam, G., Kumar, K., Chandrasekaran, S. and Kuttalam S., 2003. Bioefficacy and residues of imidacloprid in chillies used against chilli thrips. Madras Agric. J., 90: 395-399.
Sarnaik, S., Kanekar, P. Raut, V., Taware, S., Chavan, K. and Bhadbhade, B., 2006. Effect of application of different pesticides to soybean on the soil microflora. J. envir. Biol., 37: 423-426.
Shahida, A. and Hassan, N., 2002. Efficiency of mini-column for the detection of multiple insecticide residues in vegetables. Phillipine J. Sci., 131: 149-153.
Sharma, A., Srivastava, A., Ram, B. and Srivastava, P.C., 2007. Dissipation behaviour of spinosad insecticide in soil, cabbage and cauliflower under subtropical conditions. Pest Manage. Sci., 63: 1141-1145. https://doi.org/10.1002/ps.1437
Siddique, T., Okeke, B.C., Arshad, M. and Frankenberger, W.T. 2003. Enrichment and isolation of endosulfan-degrading microorganisms. J. environ. Qual., 32: 47-54. https://doi.org/10.2134/jeq2003.0047
Siddique, T., Zahir, Z.A. and Frankenberger, W.T. Jr., 2003. Reversed-phase liquid chromatographic method for analysis of endosulfan and its major metabolites. J. Liq. Chromatogr. Relat. Technol., 26: 1069-1082. https://doi.org/10.1081/JLC-120020094
Soomro, A., Seehar, G., Bhanger, M. and Channa, N., 2008. Pesticides in the blood samples of spray-workers at agriculture environment: The toxicological evaluation. Pak. J. anal. environ. Chem., 9: 32 – 37.
Talukder, M., Munnaf, M., Alam, M., Salammn, M. and Amin, M., 2003. Influence of sowing time, plant spacing and picking interval on the growth and yield of okra. Pak. J. biol. Sci., 6: 1626-1630. https://doi.org/10.3923/pjbs.2003.1626.1630
Tomkins, A., Holland, P., Thomson, C., Wilson, D. and Malcolm, C., 1999. Residual life of spinosad on kiwifruit-biological and chemical studies. In: Proceedings of The New Zealand Plant Protection Conference. New Zealand Plant Protection Society; 1998, pp. 94-97.
To share on other social networks, click on any share button. What are these?










