Carotenoids in Pearl Oyster Pinctada fucata: The Tissue Distribution and Correlation to Color Parameters
Carotenoids in Pearl Oyster Pinctada fucata: The Tissue Distribution and Correlation to Color Parameters
Bo Zhang1, Changbo Zhu1, Zihao Meng1,4, Baosuo Liu1, Lian Zhong3, Guiju Huang1, Jiaqi Su1, Sigang Fan1 and Dahui Yu1,2,*
1Key Laboratory of South China Sea Fishery Resources Exploitation and Utilization, Ministry of Agriculture, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou 510300, China
2Guangxi Key Laboratory of Beibu Gulf Marine Biodiversity Conservation, Qinzhou University, Qinzhou 535011, China
3Yanzhou Center for Product Quality Control, Yanzhou 272000, Shandong, China
4College of Fisheries and Life Science, Shanghai Ocean University, Shanghai, China
ABSTRACT
The pearl oyster, Pinctada fucata, is an economically important and carotenoid-containing bivalve shellfish that is cultured for pearls and acts as a source of seafood. To investigate the distribution of carotenoids in P. fucata and establish a more efficient method to assess carotenoid contents, we measured the carotenoid levels in selectively bred P. fucata individuals of different colors and analyzed the correlations between TCC (total carotenoid content) and color parameters. The percentage of total carotenoids in the adductor, gill, mantle, and visceral mass was 6.50%, 10.79%, 15.11% and 67.61%, respectively. Generally, the tissue-specific carotenoid distribution is ranked in the order being adductor < mantle < gill < visceral mass. Significant correlations were found between TCC and the color parameters, with the highest fit (r = 0.908) in the Pearson’s correlation between the color parameter, a* (red degree), and TCC of the adductor. Measuring the a* is, therefore, likely to be an appropriate, rapid, reliable, and nondestructive method to estimate TCC in bivalves.
Article Information
Received 15 April 2018
Revised 22 May 2018
Accepted 31 May 2018
Available online 12 June 2019
Authors’ Contribution
BZ and ZM did the experitments. BZ wrote the manuscript. CZ, BL, LZ, GH, JS, and SF assisted in the experitments. DY designed the experiments, provided financial support and revised the manuscript.
Key words
Carotenoids, Color parameter, Pearl oyster, Pinctada fucata, Tissues.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.5.1655.1661
* Corresponding author: [email protected]
0030-9923/2019/0005-1655 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Carotenoids are photosynthetic pigments that animals are deficient of de novo synthesis. Carotenoids, also as a nutrient, play multiple biological roles in morphology, behavior, immunology and fitness (Diler and Dilek, 2002; Maoka, 2011), which attracts great interests of the aquatic animal researchers and further deepens our understanding of the bioactivity of natural pigments (Rowe et al., 2012). In particular, dietary intake of carotenoids contributes to avoiding skin oxidative damage (Palombo et al., 2007) and macular degeneration (Chew et al., 2013). It’s reported that carotenoids ameliorates the increased serum phospholipid oxidation in Alzheimer’s disease patients (Ademowo et al., 2017). Besides, carotenoids are considered as an important dietary supplement for the skin pigmentation or body color (Meilisza et al., 2017; Liu et al., 2014), improve the anti-oxidative capabilities, hepatic HSP70 levels, and resistance to acute crowding stress (Liu et al., 2016), and higher levels of carotenoids in eggs associated with improved survival to zoea III (Palacios et al., 2001).
Carotenoids from Marine animals show different structures and many can be derived from β-carotene, fucoxanthin, peridinin, diatoxanthin, alloxanthin, and astaxanthin, etc. (Maoka, 2011). Carotenoids can give rise to bright color in the skin or muscle of some fish species and in the soft parts of some edible mollusks (Choubert et al., 2009; Li et al., 2010; Meyers, 1994; Tejera et al., 2007), which influences the quality, acceptance, taste, and flavor of these aquatic products, as well as the purchasing desire of consumers (Tunio et al., 2013; Yanar et al., 2007). Previous studies of carotenoids in mollusks were focused on the localization, sources, functions, and prospective biotechnological usage of these pigments, and concluded that the carotene amount in mollusks depends on factors such as sexual maturity, seasonal variation, sources of dietary algal, and whether the animal is artificially reared or not (Kantha, 1989). However, the carotenoid contents and its distribution patterns in edible mollusks have gained great attention recently since the benefits of carotenoids are not limited in shell and the soft parts coloration (Ji et al., 2013; Zheng et al., 2010), e.g. their capability of anti-oxidation (El-Agamey et al., 2004; Gostyukhina et al., 2013; Soldatov et al., 2007; Ji et al., 2013) and even gene expression regulation (Li et al., 2014; Wang et al., 2014; Zheng et al., 2012a). The industry application is also proposed as carotenoids are inclined for pearl color and bleaching (Han et al., 2011; Zhang et al., 2001).
The current methods used to evaluate carotenoid contents are overpriced and time-consuming, making it difficult to assess the carotenoid level of mollusks in a high-throughput way. Furthermore, an understanding of the level change and distribution of carotenoid in mollusks during their growth may facilitate the selective breeding. Hence, the establishment of a rapid and reliable method to quantify the carotenoids in bivalves will not only considerably optimize the commercial effectiveness in aquaculture but also advance shellfish genetics and breeding for research. Until now, a few studies have correlated reflectance color measurements with pigment content in different fruits and vegetables (Humphries et al., 2004; Fratianni et al., 2005; Meléndez-Martínez et al., 2003, 2007). Color values and carotenoid concentrations have been shown to be correlated (Arias et al., 2000; Ruiz et al., 2005, 2008). Color measurement has, therefore, been considered as a potential and effective method for the rapid estimation of carotenoid content, but the method has never been translated into metazoans, like bivalves.
The pearl oyster, Pinctada fucata, is one of the main oyster species that are cultivated in south China to produce seawater pearls. In recent years, an increasing number of cultivators have also turned to farming this edible marine bivalve as a source of food. Recently, it has been reported that some individuals of this species contain a high concentration of carotenoids (Wu et al., 2016; Meng et al., 2017), which in turn have been found to play a beneficial role in the survival rates of P. fucata exposed to high temperature stress (Meng et al., 2017). Thus, carotenoid content could serve as a preferred index to breed stress-resistant lines in selective breeding practices. In this study, we investigated the tissue distribution of carotenoids and the relationship between the color parameters and total carotenoid content (TCC) in P. fucata, with aim of establishing a rapid and simple method to estimate TCC in bivalves.
Materials and Methods
Animal collection
Pearl oysters were collected from Lingshui, Hainan Province, China, and were estimated to be approximately 18 months old. To ensure that the sample population covered a wide range of colors, individuals were classified based on the coloration of the soft parts. Adductor, gill, mantle, and visceral mass were sampled and immediately frozen at -20°C.
Determination of color parameters
The adductor of each individual was sectioned laterally with a special slicer to obtain slices of the same thickness. Each adductor slice was then placed into a groove cushioned by a tin foil. The depth of the groove was a little less than the thickness of the adductor slice. The groove and slice were covered with a thin, square piece of colorless glass, and the edge of the glass was sealed by foils. Care was taken to ensure that was no air was trapped between the adductor slice and the glass, and that their contact area had a diameter greater than 4 mm. Color parameters were measured using a chroma meter tristimulus colorimeter (NS-820, 3NH, China) that was calibrated with a white porcelain reference plate. The visible reflectance spectra were obtained through a silicone photocell and a Pulsed Xenon Lamp as a source of illumination (illuminant D65, 2° view angle, illumination area diameter 4 mm). The apparatus calculated and returned the color parameters from the spectra. The color coordinates of the uniform color space CIELAB L*, a*, b*, H, E, and chroma (C*) were determined for the slice-glass contact area.
Determination of TCC
All samples were dried in a vacuum freeze-dryer and then ground to a fine powder with a pestle and mortar. TCC was determined following the method of Li et al. (2010) and Zheng et al. (2012b). The extraction solutions contain acetone and anhydrous sodium sulfate. To avoid degradation and isomerization of carotenoids, dark brown tubes were used and the extractions were performed in an aphotic environment. Approximately 0.1 g of homogenized sample was mixed with 0.1 g anhydrous sodium sulfate, diluted with 2 mL acetone, and incubated at 20°C in a dark room for 3 h at 200 rpm/min. Then the mixtures were centrifuged at 5000 rpm for 5 min. The resulting supernatant was separated, and the insoluble residue was treated as the way described above. TCC was determined by spectrophotometry (at 450 nm) and calculated using the following formula:
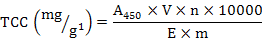
Where, A450 is the optical density value at 450 nm, 10000 is a constant, V is the volume of the extracting solution (ml), n is the number of dilution times, E is the extinction coefficient (mean value  2500 of colored carotenoids), and m is the mass (g) of the sample.
2500 of colored carotenoids), and m is the mass (g) of the sample.
Data analysis
The percentage of the total carotenoid (TC) quantity, the percentage of fresh weight (FW), and the TCC of the collective soft parts (marked as TCCS) were calculated for each individual using the formulas below:
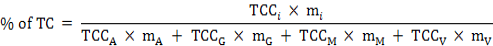

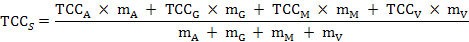
Where, TCCi and mi are the carotenoid content and weight of one of the four tissues (i = adductor, gill, mantle, or visceral mass); TCCA, TCCG, TCCM, and TCCV represent the TCC of the adductor, gill, mantle, or visceral mass; and mA, mG, mM, and mV represent the mass of them, respectively.
It was difficult to directly analyze and compare the distribution characteristics of carotenoids in different parts of the collected soft parts. So the relative TCC was employed according to the following formula:

Statistical analysis
The results were subjected to a one-way analysis of variance (ANOVA). Correlations between TCC and the color values were analyzed using Pearson’s correlation. All statistical analyses were conducted using SPSS Software for Windows (SPSS, 19.0, IBM, USA). Statistical significance was set at P < 0.05 for all analyses unless noted otherwise.
Results
Distribution of carotenoids in P. fucata
The percentage of carotenoids in the adductor, gill, mantle, and visceral mass was 6.50%, 10.79%, 15.11% and 67.61%, respectively, with significant differences (P < 0.05) between the tissues (Table I). The percentage of fresh weight in different tissues were ranked as adductor (17.38%), gill (17.29%), mantle (25.84%) and visceral mass (39.49%) with significant differences except the adductor-gill pairwise comparison. Carotenoids were unevenly distributed in the different parts of soft tissues. The relative TCC of the visceral mass, gill, mantle, and adductor were 1.73, 0.64, 0.59, and 0.36, respectively (Table I). The average TCC of the visceral mass was 1.73 times than that of the collective soft parts. The TCC in P. fucata can be generally summarized in the order of visceral mass > gill > mantle > adductor, with significant differences in pairwise comparisons, except the gill-mantle pairing.
Table I.- Mean (standard error) of percentage of total carotenoid amount, percentage of fresh weight and relative TCC (Mean ± SD) in different tissues.
|
Combination |
Tissues |
|||
|
Adductor |
Gill |
Mantle |
Visceral mass |
|
|
Percentage of total carotenoid amount |
6.50 ± 4.01d |
10.79± 3.46c |
15.11± 4.25b |
67.61± 9.49a |
|
Percentage of fresh weight |
17.38± 3.08c |
17.30± 4.48c |
25.84± 3.58b |
39.49± 5.79a |
|
Relative TCC |
0.36± 0.15c |
0.64± 0.21b |
0.59± 0.17b |
1.73± 0.26a |
Means with different letters indicate significant difference (P < 0.05).
Table II.- Pearson correlation between TCC of the four tissues and soft part.
|
TCCA |
TCCG |
TCCM |
TCCV |
TCCS |
|
|
TCCA |
1 |
0.795** |
0.959** |
0.790** |
0.891** |
|
TCCG |
1 |
0.728** |
0.652** |
0.759** |
|
|
TCCM |
1 |
0.791** |
0.886** |
||
|
TCCV |
1 |
0.972** |
|||
|
TCCS |
1 |
**, P < 0.01; A, adductor; G, gill; M, mantle; V, visceral mass; S, softpart.
Pairwise correlation of TCC among tissues
The Pearson’s correlation coefficients of TCC among the four tissues (TCCA, TCCG, TCCM and TCCV) and the collective soft parts(TCCS) are listed in Table II. The correlation coefficient between each pair of TCCA, TCCG, TCCM, TCCV and TCCS were found to be significant (P < 0.01). The TCC of all tissues had a high correlation with the TCC of the collective soft parts, especially that of the visceral mass (r = 0.972) and the adductor (r = 0.891). Moreover, the correlation coefficients between the TCC of the adductor and of other three tissues were also found to be relatively high, with the highest being between the TCC of the adductor and the mantle (r = 0.959).
The linear correlation between the TCC of these four tissues and the TCC of the collective soft parts were found to be different (Fig. 1), and the linear correlation between the TCC of the visceral mass and that of the collective soft parts was found to be the highest (R2 = 0.9453). The second highest correlation of TCC was seen between the adductor and the collective soft parts (R2 = 0.7938), and the lowest was between the gill and the collective soft parts (R2 = 0.5755).
Table III.- Pearson correlation between TCC and color measurements of the adductor.
|
L* |
a* |
b* |
C* |
H |
E |
a*/b* |
|
|
r |
-0.645** |
0.908** |
0.692** |
0.728** |
-0.773** |
0.747** |
0.817** |
**, P < 0.01.
Relationship between TCC of the adductor and the color parameter
Correlation coefficients between the TCCA and the color parameters were assessed (Table III). Significant correlations were observed between TCCA and all color parameters (P < 0.01). The color parameter, a*, showed the highest correlation coefficient with TCCA (r = 0.908), indicating a highly positive correlation, which is also in agreement with previous studies (Meléndez-Martínez et al., 2003, 2007). The linear correlation between TCCA and a* (R2 = 0.8251) is shown in Figure 2.
Discussion
Pinctada fucata is a multipurpose bivalve that is cultured in the south of China for pearl production and as a source of food. The soft part tissues of this species are rich in carotenoid pigments and exhibit a wide range of coloration. Carotenoids showed potential in improving the survival rates of P. fucata under high-temperature stress by enhancing the antioxidant system (Meng et al., 2017). Investigations into the genetic features of carotenoids in this bivalve may provide basic support and guidance for genetic and breeding practices. Attempts to correlate color parameters and the TCC began in plant-based research many years ago in order to develop more effective and efficient methods of carotenoid content quantification (Arias et al., 2000; Ruiz et al., 2005, 2008), but similar research has never been carried out in animals.
The present study found a significant correlation between the TCC and the color parameter, a*, in P. fucata, suggesting that the measurement of this color parameter may offer the most appropriate, rapid, reliable, and nondestructive method to estimate the carotenoid content in bivalves. This linear correlation may, however, be improved to some extent in further research by improving the accuracy of the TCC determination in the adductor.
The distribution of carotenoids in P. fucata was found to be uneven and higher in specific tissues, which has similarly been reported in other mollusks (Zheng et al., 2010, 2012a). In our study, the visceral mass was found to have the most total carotenoids (67.61%) and fresh weight (39.49%), and had the highest relative TCC (1.73 times as much as the TCC of the collective soft parts). The relative TCC of the adductor was found to be the lowest (0.36) with 6.50% of the total carotenoid amount and 17.38% of the fresh weight. This variation in the TCC among the tissues is consistent with the findings reported for other marine mollusks (Zheng et al., 2010, 2012a; Wu et al., 2016). For example, TCC followed the order of gonad > mantle > adductor > gill in the noble scallop (Zheng et al., 2010), and Wu et al. (2016) found the TCC in Pinctada martensii to be significantly different among the adductor, gill, hepatopancreas, mantle edge, and mantle center of different cultured groups.
In these studies, the highest correlation coefficient between the TCC of the four tissues and that of the collective soft parts was obtained for the visceral mass, which may be due to there being high-TCC tissue in the visceral mass. For example, the gonad is reported have the highest TCC in noble scallop (Zheng et al., 2010), and hepatopancreas is likely to be an important tissue in the transformation and accumulation of carotenoids in P. martensii (Wu et al., 2016). In addition, the present study found the correlation coefficient between each pair of the TCC of the different tissues to be significantly different, and that the high linear correlations among them revealed the possibility that the TCC of the collective soft parts could be evaluated by that of other tissues, such as the adductor and visceral mass. To our knowledge, our results are the first of their kind reported for mollusks.
This difference in the correlation coefficient may due to the metabolism of carotenoids: a result of the absorption, conversion, transfer, and/or storage of these pigments. Previously, the work of Li et al. (2010) revealed that the main pigment isolated from the muscle tissues of the Yesso scallop (Patinopecten yessoensis) was identified as the carotenoid, pectenolone, implying a certain level of selectivity in the absorption or conversion of carotenoids in bivalves. Another study showed that the TCC in the noble scallop (Chlamys nobilis) was related to body tissue, shell color, and gender (Zheng et al., 2012b). This latter study demonstrated that carotenoids might be transferred from other tissues to gonad during maturation in mollusks, particularly in the female individuals (Zheng et al., 2012b). In fact, earlier report of Kantha (1989) had indicated that sexual maturity, seasonal variation, sources of dietary algal, and whether the animal is artificially reared or not may all impact on the carotene amount in mollusks. And similar research in wild marine shrimps demonstrated that the carotenoid contents were considerably higher during spring and summer than that in winter and autumn seasons (Yanar et al., 2004), too. What’s more, recent research on the expression pattern of PySCD in two types of Yesso scallops detected a significantly greater number of PySCD transcripts in the carotenoid-rich scallops, implying that PySCD might be involved in carotenoid accumulation (Li et al., 2015). So, the high-level TCC in pearl oysters in our research probability due to the sample season and the expression of some genes which need further studies.
Although our findings indicate that the high level of correlation among the TCC of different tissues makes it possible to estimate the TCC of every individual just by evaluating the TCC of one tissue, further research is required to better understand the correlations among the different tissue types in P. fucata.
Acknowledgements
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (2015TS08), Guangdong Provincial Science and Technology Project (2015A020209045), Special Fund by Sanya Government (2014KS04), Special Fund for Marine Fisheries Research and Extension of Guangdong Province (Z2014006, Z2015006, Z2015013, Z2015009, B201601-Z03 and Z2014003) and the Key Science and Technology Plan Projects of Hainan Province (ZDYF2016086), and Earmarked Fund for China Agriculture Research System (Grant No. CARS-48).
Statement of conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
Ademowo, O.S., Dias, H.K.I., Milic, I., Devitt, A., Moran, R., Mulcahy, R., Howard, A.N., Nolan, J.M. and Griffiths, H.R., 2017. Phospholipid oxidation and carotenoid supplementation in Alzheimer’s disease patients. Free Rad. Biol. Med., 108: 77-85. https://doi.org/10.1016/j.freeradbiomed.2017.03.008
Chew, E.Y., Clemons, T.E., San-Giovanni, J.P., Danis, R., Ferris, F.L., Elman, M., Antoszyk, A., Ruby, A., Orth, D., Bressler, S., Fish, G., Hubbard, B., Klein, M., Chandra, S., Blodi, B., Domalpally, A., Friberg, T., Wong, W., Rosenfeld, P., Agron, E., Toth, C., Bernstein, P. and Sperduto, R., 2013. Lutein+ zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The age-related eye disease study 2 (AREDS2) randomized clinical trial. J. Am. med. Assoc., 309: 2005-2015. https://doi.org/10.1001/jama.2013.4997
Arias, R., Lee, T.C., Legendre, L. and Janes, H., 2000. Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J. Agric. Fd. Chem., 48: 1697-1702. https://doi.org/10.1021/jf990974e
Baker, R. and Günther, C., 2004. The role of carotenoids in consumer choice and the likely benefits from their inclusion into products for human consumption. Trends Fd. Sci. Tech., 15: 484-488. https://doi.org/10.1016/j.tifs.2004.04.0094
Choubert, G., Cravedi, J.P. and Laurentie, M., 2009. Effect of alternate distribution of astaxanthin on rainbow trout (Oncorhynchus mykiss) muscle pigmentation. Aquaculture, 286: 100-104. https://doi.org/10.1016/j.aquaculture.2008.09.001
Diler, İ. and Dilek, K., 2002. Significance of pigmentation and use in aquaculture. Turk. J. Fish. aquat. Sci., 2: 97-99.
El-Agamey, A., Lowe, G.M., McGarvey, D.J., Mortensen, A., Phillip, D.M., Truscott, T. G. and Young, A.J., 2004. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys., 430: 37-48. https://doi.org/10.1016/j.abb.2004.03.007
Fratianni, A., Irano, M., Panfili, G. and Acquistucci, R., 2005. Estimation of color of durum wheat. Comparison of WSB, HPLC, and reflectance colorimeter measurements. J. Agric. Fd. Chem., 53: 2373-2378. https://doi.org/10.1021/jf040351n
Gostyukhina, O.L., Soldatov, A.A., Golovina, I.V. and Borodina, A.V., 2013. Content of carotenoids and the state of tissue antioxidant enzymatic complex in bivalve mollusc Anadara inaequivalvis. Br. J. Evol. Biochem. Physiol., 49: 309-315. https://doi.org/10.1134/S0022093013030055
Han, J.W., Luo, W., Zheng, D.H. and Zhang, Y.M., 2011. The effect of different carotenoids of β-carotene on early pearl color and luster degrees in Hyriopsis cumingii. Ocean. Limnol. Sin., 42 573-579.
Humphries, J.M., Graham, R.D. and Mares, D.J., 2004. Application of reflectance colour measurement to the estimation of carotene and lutein content in wheat and triticale. J. Cereal Sci., 40: 151-159. https://doi.org/10.1016/j.jcs.2004.07.005
Ji, L., Liao, J., Song, W., Liu, J. and Ma, X., 2013. Influence of astaxanthin on pearl oyster Pinctada Martensii. Adv. Mater. Res., 781-784: 889-894.
Kantha, S.S., 1989. Carotenoids of edible molluscs: A review. J. Fd. Biochem., 13: 429-442. https://doi.org/10.1111/j.1745-4514.1989.tb00410.x
Li, N., Hu, J., Wang, S., Cheng, J., Hu, X., Lu, Z., Lin, Z., Zhu, W. and Bao, Z., 2010. Isolation and identification of the main carotenoid pigment from the rare orange muscle of the Yesso scallop. Fd. Chem., 118: 616-619. https://doi.org/10.1016/j.foodchem.2009.05.043
Li, X., Bai, Z., Luo, H., Liu, Y., Wang, G. and Li, J., 2014. Cloning, differential tissue expression of a novel hcApo gene, and its correlation with total carotenoid content in purple and white inner-shell color pearl mussel Hyriopsis cumingii. Gene, 538: 258-265. https://doi.org/10.1016/j.gene.2014.01.046
Li, X., Ning, X., Dou, J., Yu, Q., Wang, S., Zhang, L., Wang, S., Hu, X. and Bao, Z., 2015. An SCD gene from the Mollusca and its upregulation in carotenoid-enriched scallops. Gene, 564: 101-108. https://doi.org/10.1016/j.gene.2015.02.071
Liu, X., Wang, H. and Chen, Z., 2014. Effect of carotenoids on body colour of discus fish (Symphysodon aequifasciatus axelrodi Schultz, 1960). Aquacul. Res., 47: 1309-1314. https://doi.org/10.1111/are.12591
Liu, F., Shi, H., Guo, Q., Yu, Y., Wang, A., Lv, F. and Shen, W., 2016. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellf. Immunol., 51: 125-135.
Maoka, T., 2011. Carotenoids in marine animals. Mar. Drugs, 9: 278-293. https://doi.org/10.3390/md9020278
Meilisza, N., Jusadi, D., Zairin, M., Artika, I., Utomo, N.B.P., Kadarini, T. and Suprayudi, M.A., 2017. Digestibility, growth and pigmentation of astaxanthin, canthaxanthin or lutein diets in Lake Kurumoi rainbowfish, Melanotaenia parva (Allen) cultured species. Aquacul. Res., 48: 1-9. https://doi.org/10.1111/are.13372
Meléndez-Martínez, A.J., Vicario, I.M. and Heredia, F.J., 2003. Application of tristimulus colorimetry to estimate the carotenoids content in ultrafrozen orange juices. J. Agric. Fd. Chem., 51: 7266-7270. https://doi.org/10.1021/jf034873z
Meléndez-Martínez, A.J., Vicario, I.M. and Heredia, F.J., 2007. Rapid assessment of vitamin A activity through objective color measurements for the quality control of orange juices with diverse carotenoid profiles. J. Agric. Fd. Chem., 55: 2808-2815. https://doi.org/10.1021/jf0635412
Meng, Z., Zhang, B., Liu, B., Li, H., Fan, S. and Yu, D., 2017. High carotenoids content can enhance resistance of selected Pinctada fucata families to high temperature stress. Fish Shellf. Immunol., 61: 211-218. https://doi.org/10.1111/imm.12724
Meyers, P.S., 1994. Developments in world aquaculture, feed formulation and role of carotenoids. Pure appl. Chem., 66: 1069-1076. https://doi.org/10.1351/pac199466051069
Palacios E., Racotta, I.S., Heras, H., Marty, Y., Moal, J. and Samain, J., 2002. Relation between lipid and fatty acid composition of eggs and larval survival in white pacific shrimp (Penaeus vannamei, Boone, 1931). Aquacul. Int., 9: 531-543. https://doi.org/10.1023/A:1020589924810
Palombo P., Fabrizi, G., Ruocco, V., Ruocco, E., Fluhr, J., Roberts, R. and Morganti, P., 2007. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol., 20: 199-210. https://doi.org/10.1159/000101807
Rowe, M., Tourville, E.A. and McGraw, K.J., 2012. Carotenoids in bird testes: Links to body carotenoid supplies, plumage coloration, body mass and testes mass in house finches (Carpodacus mexicanus). Comp. Biochem. Physiol. B, 163: 285-291. https://doi.org/10.1016/j.cbpb.2012.06.005
Ruiz, D., Egea, J., Tomás-Barberán, F.A. and Gil, M.I., 2005. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J. Agric. Fd. Chem., 53: 6368-6374. https://doi.org/10.1021/jf0480703
Ruiz, D., Reich, M., Bureau, S., Renard, C.M.G.C. and Audergon, J.M., 2008. Application of reflectance colorimeter measurements and infrared spectroscopy methods to rapid and nondestructive evaluation of carotenoids content in apricot (Prunus armeniaca L.). J. Agric. Fd. Chem., 56: 4916-4922. https://doi.org/10.1021/jf7036032
Soldatov, A.A., Gostyukhina, O.L. and Golovina, I.V., 2007. Antioxidant enzyme complex of tissues of the bivalve Mytilus galloprovincialis Lam. under normal and oxidative-stress conditions: A review. Appl. Biochem. Microbiol., 43: 556-562. https://doi.org/10.1134/S0003683807050092
Tejera, N., Cejas, J.R., Rodríguez, C., Bjerkeng, B., Jerez, S., Bolaños, A. and Lorenzo, A., 2007. Pigmentation, carotenoids, lipid peroxides and lipid composition of skin of red porgy (Pagrus pagrus) fed diets supplemented with different astaxanthin sources. Aquaculture, 270: 218-230. https://doi.org/10.1016/j.aquaculture.2007.01.019
Tunio, M.T., Yang, S., Chen, Z., Zubair, M., Qiu, J., Zhao, Y., Chen, G., Chow, Y. and Chen, A., 2013. Effect of pigments with different origins on pigmentation and performance of broilers. Pakistan J. Zool., 45: 1715-1725.
Wang, Y.J., Zheng, H.P., Zhang, B., Liu, H.L., Deng, H.J. and Deng, L.H., 2014. Cloning and respond of a cold shock domain protein (CnCSDP) gene to cold stress in noble scallop Chlamys nobilis (Bivalve: Pectinidae). Mol. Biol. Rep., 41: 7985-7994. https://doi.org/10.1007/s11033-014-3694-4
Wu, X.F., Lei, C., Chen, S.M., Li, J.H., Wang, Q.H. and Jiao, Y., 2016. Total carotenoid content in soft tissues of two-sized groups in pearl oyster Pinctada martensii stock. J. Guangdong Ocean Univ., 36: 31-34.
Yanar, Y., Celik, M. and Yanar, M., 2004. Seasonal changes in total carotenoid contents of wild marine shrimps (Penaeus semisulcatus and Metapenaeus monoceros) inhabiting the eastern Mediterranean. Fd. Chem., 88: 267–269. https://doi.org/10.1016/j.foodchem.2004.01.037
Yanar, Y., Büyükçapar, H. and Laurentie, M., 2007. Effect of carotenoids from red pepper and marigold flower on pigmentation, sensory properties and fatty acid composition of rainbow trout. Fd. Chem., 100: 326-330. https://doi.org/10.1016/j.foodchem.2005.09.056
Zhang, G., Xie, X. and Wang, Y., 2001. Raman spectra of nacre from shells of main pearl-culturing mollusk in China. Spectrosci. Spect. Anal., 21: 193-196.
Zheng, H., Liu, H., Zhang, T., Wang, S., Sun, Z. and Liu, W., 2010. Total carotenoid differences in scallop tissues of Chlamys nobilis (Bivalve: Pectinidae) with regard to gender and shell colour. Fd. Chem., 122: 1164-1167. https://doi.org/10.1016/j.foodchem.2010.03.109
Zheng, H., Zhang, Q., Liu, H., Liu, W., Sun, Z., Li, S. and Zhang, T., 2012a. Cloning and expression of vitellogenin (Vg) gene and its correlations with total carotenoids content and total antioxidant capacity in noble scallop Chlamys nobilis (Bivalve: Pectinidae). Aquaculture, 366-367: 46-53. https://doi.org/10.1016/j.aquaculture.2012.08.031
Zheng, H., Liu, H., Liu, W., Sun, Z. and Zhang, Q., 2012b. Changes of total carotenoid and lipid content in scallop tissues of Chlamys nobilis (Bivalve: Pectinidae) during gonad maturation. Aquaculture, 342-343: 7-12.
To share on other social networks, click on any share button. What are these?










