Cytotoxic Potential of Fertility Booster Medicinal Plants as Evident through Brine Shrimp Toxicity Assay
Cytotoxic Potential of Fertility Booster Medicinal Plants as Evident through Brine Shrimp Toxicity Assay
Afsheen Noman Saddar1, Anila Naz Soomro2*, Sadaf Tabasum Qureshi1,
Syeda Saleha Hassaney1 and Mukhtiar Ahmed Mahar2
1Institute of Plant Sciences, Faculty of Natural Sciences, University of Sindh, Jamshoro
2Department of Fresh water biology and Fisheries, Faculty of Natural Sciences, University of Sindh, Jamshoro, Pakistan
ABSTRACT
The study was designed to check the toxicity of five fertility enhancing medicinal plants viz root of sweet flag, seed of radish, root of land-calotrops, leaves of peppermint and flower of red cabbage through brine shrimp toxicity assay. Brine shrimp eggs hatching was optimized by applying three temperature levels (25°C, 27°C and 29°C), three salinities (10 ppt, 30 ppt and 35 ppt), two food demands (0.8 g and 1.6 g) and two levels of shrimp egg (1 g/l and 1.5 g/l). Five concentrations (1200, 2500, 5000, 10000 and 15000 µg/ml) of aqueous extracts of five fertility enhancing plants were tested for 24 h incubation to check mortality of brine shrimp nauplii. Mean comparison of brine shrimp cytotoxicity assay revealed noticeable deviations from the both positive and negative controls. Alive percentage of nauplii observed in the aqueous extract of sweet flag root and radish seed ranged from 0 to 10%, land-calotrops root extract from 0 to 22.5%, peppermint leaves extract from 3 to 47.5% and for red cabbage extract ranged from 0 to 15%. Dose dependent death rate was only observed in red cabbage extract while other plant extracts given random death rate of nauplii as compare to +ve control (Ethyle methane sulphonate). Therefore, it can be finally concluded that all tested plants were toxic to brine shrimp, however comparatively higher toxicity was observed by flag root extract.
Article Information
Received 08 January 2020
Revised 15 November 2020
Accepted 12 January 2021
Available online 16 July 2021
(early access)
Published 07 March 2022
Authors’ Contribution
AN Soomro planned and managed the experiments. AN Saddar conducted the research. STQ helped in writing the manuscript. SSH helped in material identification. MAM supported for statistical analysis.
Key words
Cytotoxicity, Fertility booster, Brine shrimp, Mortality
DOI: https://dx.doi.org/10.17582/journal.pjz/20200108060108
* Corresponding author: anila.soomro@usindh.edu.pk
0030-9923/2022/0003-1497 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Approximately 48.5 million couples are affected with infertility worldwide (Mascarenhas et al., 2012; Agarwal et al., 2015). The frequency of infertility in Pakistan is approximately 22%, where primary infertility is 4% and secondary infertility is 18% (Ali et al., 2011). To overcome this major problem, both male and female use usually plant based medicines (Jaradat and Zaid, 2019). In Pakistan, especially in Sindh the mostly used fertility enhancers are sweet flag, radish, land-calotrops, peppermint and red cabbage (Rahman et al., 2011) . These plants are known to possess fertility enhancing substances, but some of their compounds like eugenol, sitosterol, saponin, menthol and glucosinolate are reported cytotoxic to normal and human cancer cell lines (Chauhan et al., 1992; Burel et al., 2001; https://www.poison.org/; Kijpornyongpan et al., 2014). Most often cytotoxicity of these therapeutic herbs is not evaluated before use. It is therefore, extremely essential to screen herbs for cytotoxicity.
Brine shrimp cytoxicity assay is preferred among other animal cytotoxicity assays because of its ease handling, quickness and inexpensiveness (Mayorga et al., 2010; Insanu et al., 2012; Veni and Pushpanathan, 2014; Elsyana et al., 2016; Kosasih et al., 2019). The most crucial hatching parameters of brine shrimp eggs are temperature, salinity, feed and shrimp egg quantities that drastically delay hatching process. Quantity of optimized parameters not only play major role during hatching stage but also during the assay (Vanhaecke and Sorgeloos, 1989; Wasonga and Olendi, 2014; https://www.oceannutrition.com/). Present study is designed to predict prospective cytotoxicity of five fertility enhancing medicinal plant parts viz. root of sweet flag, seed of radish, root of land-calotrops, leaves of peppermint and flower of red cabbage using brine shrimp toxicity assay.
Materials and methods
Three temperatures (25°C, 27°C and 29°C), three levels of salinity (10 ppt, 30 ppt and 35 ppt), two food demands (0.8 g and 1.6 g) and two shrimp egg quantities (1 g/l and 1.5 g/l) were tested. All the mentioned hatching parameters were applied in combinations.
Dried plant parts were purchased from local market of Hyderabad, dried in electric oven and then ground with pestle and mortar and electric grinder. Different concentrations (1200, 2500, 5000, 10000 and 15000 µg/ml) of plant aqueous extracts were prepared in vials. Ten alive nauplii was added and then the final volume was raised up to 5 ml by adding artificial sea water (35 ppt) according to the described procedure followed by Memita et al. (2018) and Braguini et al. (2018). Artificial sea water (5 ml) vial was used as negative control and 1000 µg/ml EMS (ethyl methane sulphonate) in sea water was used as positive control. After 24 h all vials were inspected with torch/ magnifying glass and surviving nauplii number were counted. Toxicity of plant aqueous extract was checked by mean percentage of alive and dead brine shrimp nauplii. Experiment was repeated thrice and the data represented as Mean±SD. Death and alive rate of brine shrimp nauplii was calculated by using following formulae (Arulvasu et al., 2015).
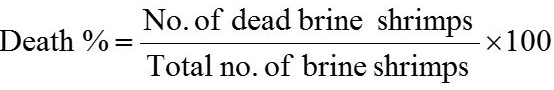
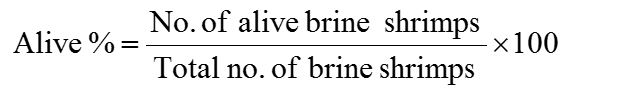
Results and discussion
In optimization highest hatching percentages (95 and 91) were found in 35ppt salinity with 27°C having 1and 1.5 g egg of brine shrimp with 0.8 g food, lowest hatching % (8 and 14) were observed in 35 and 10 ppt on 25ᵒC with 0.8 g and 1.6 g food, respectively though 35 ppt on 29°C hatched eggs moderately (Table I). Mean alive percentage of brine shrimp nauplii is compiled in Table II plant wise. Total alive nauplii observed in sweet flag root and radish seed extract were ranged from 0 to 10%, it means both plants induced nauplii death percentage from 90 to 100. Alive nauplii percentage witnessed in land-calotrops root aqueous extract ranged from 0 to 22.5% with death rate from 77.5 to 100%. In peppermint leaves aqueous extract 3 to 47.5% of alive nauplii observed with 52.5 to 97% death rate. In red cabbage flower aqueous extract alive percentage of brine shrimp nauplii was observed from 0 to 15 with 85 to 100% death rate. Out of all tested fertility enhancing plants sweet flag and radish seed were more cytotoxic. Dose dependent death effects were found in red cabbage, in its minimum concentration death rate was 85% and as the concentration increases the death percentage was about 90, 92.5 and 100%. While other tested plants effect was random, this may be due to high toxic potential of these extracts. Negligible death rate was observed in negative control indicating cytotoxic potential of different chemicals present in plant extracts.
Sweet flag root medium concentration (5000µg/ml) given lowest death rate, curve fluctuates randomly (Table II). Results supported by the work of number of researchers (Akeem et al., 2017). These scientists observed brine shrimp random mortality mediated by anti-malarial plants and random mitotic inhibition by anti typhoid in Allium cepa root tip cells, indicates low cytotoxic potentials of plant extracts. Sweet flag induced cytotoxicity may be because of eugenol and sitosterol as reported in independent studies on human cancer cell lines. Eugenol is proved hepatotoxic in previous studies as it exhausts cytoprotective thiols and interference in thiol-dependent processes to maintain lipid-based membrane integrity. Furthermore, the toxic effects of eugenol are more pronounced when it is converted to quinine methide metabolite (Thampson et al., 1998). Membrane damages by eugenol are also reported in human studies as a causative agent of stomatitis that is inflammatory process affecting oral mucous membranes sometimes conversion in to oral cancer (Deshpande et al., 2014). Exposure to 0.7 mmol l-1 of sitosterol for 72 h can cause contraction of the
Table I. Brine shrimp eggs hatching percentage by different temperatures, salinities, food levels and eggs concentration.
|
Temperature |
Salinity (ppt) |
Food (g) |
Brine shrimp eggs (g/l) |
Hatching % |
|
25oC |
10 ppt |
0.8 |
1 |
24 |
|
1.5 |
43 |
|||
|
1.6 |
1 |
14 |
||
|
1.5 |
32 |
|||
|
30 ppt |
0.8 |
1 |
41 |
|
|
1.5 |
52 |
|||
|
1.6 |
1 |
50 |
||
|
1.5 |
51 |
|||
|
35 ppt |
0.8 |
1 |
8 |
|
|
1.5 |
25 |
|||
|
1.6 |
1 |
60 |
||
|
1.5 |
63 |
|||
|
27oC |
10 ppt |
0.8 |
1 |
54 |
|
1.5 |
49 |
|||
|
1.6 |
1 |
28 |
||
|
1.5 |
19 |
|||
|
30 ppt |
0.8 |
1 |
55 |
|
|
1.5 |
47 |
|||
|
1.6 |
1 |
48 |
||
|
1.5 |
51 |
|||
|
35 ppt |
0.8 |
1 |
95 |
|
|
1.5 |
91 |
|||
|
1.6 |
1 |
60 |
||
|
1.5 |
50 |
|||
|
29oC |
10 ppt |
0.8 |
1 |
40 |
|
1.5 |
17 |
|||
|
1.6 |
1 |
44 |
||
|
1.5 |
32 |
|||
|
30 ppt |
0.8 |
1 |
31 |
|
|
1.5 |
45 |
|||
|
1.6 |
1 |
55 |
||
|
1.5 |
56 |
|||
|
35 ppt |
0.8 |
1 |
73 |
|
|
1.5 |
69 |
|||
|
1.6 |
1 |
65 |
||
|
1.5 |
55 |
endothelial cells and increase the release of intracellular lactate dehydrogenase type A (LDHA) in human being (Boberg et al., 1991). Elevated level of LDHA is molecular indicator of carcinogenesis as evident in many malignant tumors and a strong suspect behind tumor proliferation and malignant growth (Miao et al., 2013).
Radish seed aqueous extract induced toxicity pattern was low to high against brine shrimp nauplii but its two concentrations given similar death rate (Table II). It may be because of the presence of glucosinolates. Glucosinolate is natural compound found in radish and red cabbage. It is more toxicant after converting into secondary metabolites (isothiocyanate followed by thycinates, oxazolidinethiones and nitriles (Burel et al., 2001). Effect of land-calotrops aqueous extract on brine shrimp toxicity varies by different concentration; medium concentration (5000 µg/ml) was less toxic to brine shrimp. Saponin is main compound of land-calotrops, its direct and indirect action may be related with toxicity of different organisms. Toxicity of saponins to warm blooded animals varies with type of plant species as each contains different amounts, proportion of different saponins in mixture and of course frequency of intake (George, 1965; Okenfull and Sidhu, 1990). Fishes and cold-blooded animals also affected by saponins toxicity (Leistner and Drewke, 2010). Saponins (steroid glycosides) although are only absorbed in traces and their toxic effect is confined to the intestinal tract but its latent effects generate secondary metabolites with zinc and iron molecules, thus limiting their bioavailability (Chauhan et al., 1992). In addition, this plant extract also contains alkaloids like α and β-asarone that may contribute final cytotoxicity. Peppermint aqueous extract exerted indirect effect on brine shrimp toxicity, low concentration was more toxic and high concentration was less except (5000 µg/ml) concentration that given more lethal percent than 10000 µg/ml concentration (Table II). It may be due to activation of secondary metabolites. The cytotoxic effects of peppermint can be correlated with menthol that can cause cell death by unbalancing cytosolic Ca2+ levels (Kijpornyongpan et al., 2014). Menthol possesses ability to integrate and disrupt membrane structure and function through calcium channels according to a bacterial study (Landau and Shapira, 2012). It can cause systemic toxicity to humans after swallowing or inhaling concentrated amounts. Its serious effects include seizures, coma, and death (https://www.poison.org/).
Hepatotoxicity and nephrotoxicity in rats were described by Mentha piperita and mentha spicata due to imbalance hormone concentration; high level changes in germ cell captured meiosis (Akdogan et al., 2004). Cytotoxic effects of flavonoids found in peppermint in various cancer cells are well accepted (Park et al., 2015; Sak and Everaus, 2015).
Table II. Brine shrimp cytotoxicity induced by fertility booster medicinal plants aqueous extract.
|
Treatment with aqueus extract |
Concentration (µg/ml) |
% of alive nauplii (mean±SD) |
|
-ve Control (Artificial seawater) |
0 |
95±0.5 |
|
Sweet flag root |
1200 |
5±0.5 |
|
2500 |
3±0.5 |
|
|
5000 |
10±0.8 |
|
|
10000 |
00±0 |
|
|
15000 |
00±0 |
|
|
Radish seed |
1200 |
10±0 |
|
2500 |
7.5±0.9 |
|
|
5000 |
2.5±0.5 |
|
|
10000 |
2.5±0.5 |
|
|
15000 |
00±0 |
|
|
Land-calotrops root |
1200 |
5±0.5 |
|
2500 |
5±0.8 |
|
|
5000 |
22.5±2.87 |
|
|
10000 |
7.5±1.5 |
|
|
15000 |
00±0 |
|
|
Peppermint leaves |
1200 |
3±0.5 |
|
2500 |
10±1.41 |
|
|
5000 |
42.5±2.06 |
|
|
10000 |
47.5±0.95 |
|
|
15000 |
50±1.4 |
|
|
Red cabbage flower |
1200 |
15±1.29 |
|
2500 |
10±0.81 |
|
|
5000 |
7.5±1.5 |
|
|
10000 |
00±0 |
|
|
15000 |
00±0 |
|
|
+ve control (EMS) |
1000 |
00±0 |
No, Number; SD, Standard deviation.
Red cabbage aqueous extracts induced cytoxicity to test organism may be due to presence of cadmium common heavy metal of brasicaceae family that is reported to cause hepatotoxicity in animal tissue leading to nephrotoxicity. The probable reason of liver injury in rats was lipid peroxidation that consequently caused membrane damage altering permeability of membrane (Renugadevi and Prabu, 2010). Siddiqui (2010) revealed 30 days regular oral intake of CdCl2 solution (in drinking water) causes the secretion of low and high molecular proteins which cause damages in renal cortex, proximal tubules membrane, cell nuclei and blood vessels. Observed results are supported by previous test of ethanolic extract of Moringa olifera (drumstick tree) leaves, methanolic extract of Barringotina acutangula L. (Hijal) leaves (Assaduzaman et al., 2015).
Conclusion
Temperature is the most sensitive parameter for brine shrimp eggs hatching among others. Brine shrimp larvae were most sensitive to sweet flag root and least to peppermint. Red cabbage flower aqueous extracts induced concentration dependent cytotoxicity to test organism. All tested plant parts were highly toxic to brine shrimp larvae; therefore, it is essential to test plants at lower concentration by modern techniques to get semi lethal concentration.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Agarwal, A., Mulgund, A., Hamada, A. and Chyatte, M.R., 2015. Reprod. Biol. Endocrinol., 13: 1-9. https://doi.org/10.1186/s12958-015-0032-1
Akdogan, M., Ozguner, M., Kocak, A., Oncu, M. and Cicek, E., 2004. Urology, 64: 394-398. https://doi.org/10.1016/j.urology.2004.03.046
Akeem, A. Temitope, A.O., Omobolanle, F.F., Temidayo, O. and Opeyemi, O.O., 2017. Glob. J. Bio-Sci. BioTechnol., 6: 24-29.
Ali, S., Sophie, R., Imam, A.M., Khan, F.I., Ali, S.F., Shaikh A., and Farid-ul-Hasnain, S., 2011. BMC Publ. Hlth., 11: 4471-2458. https://doi.org/10.1186/1471-2458-11-760
Arulvasu, C., Jennifer, S.M., Prabbu, D. and Chandhiraseker, D., 2014. Sci. World J., 2014: 1-10. https://doi.org/10.1155/2014/256919
Asaduzzaman, M., Rana, S., Hassan, S., Hossain, M.M. and Das, N., 2015. Int. J. Pharm. Sci. Res., 6: 1179-1185.
Boberg, K.M., Pettersen, K.S. and Prydz, H., 1991. Scand. J. Clin. Lab. Invest., 51: 509–516. https://doi.org/10.3109/00365519109104559
Borght, V.M. and Wyns, C., 2018. Clin. Biochem., 62: 2-10. https://doi.org/10.1016/j.clinbiochem.2018.03.012
Braguini, W.L., Pires, N. V. and Alves., B. B., 2018. J. Young Pharm., 10: 159-163. https://doi.org/10.5530/jyp.2018.10.36
Burel, C., Boujard, T., Kaushik, S.J., Boeuf, G., Mol, K.A., Geyten, S.V.D., Darras, V.M., Kuhn, E.R., Balade, B.P., Querat, B., Quinsac, A., Krouti, M. and Ribaillier, D., 2001. Gen. Comp. Endocrinol., 124: 343-358. https://doi.org/10.1006/gcen.2001.7723
Chauhan, G.S., Eskin, N.A.M. and Tkachuk, R., 1992. Cereal Chem., 69: 85-88. https://doi.org/10.1021/ed069p88
Deshpande, A.N., Verma, S. and Macwan, C., 2014. Austin J. Dent., 1: 1007. https://doi.org/10.1136/bcr-2013-201989
Elsyana, V., Bintang, M. and Priosoeryanto, B.P., 2016. Adv. Pharmacol. Sci., 2016: 1-6. https://doi.org/10.1155/2016/3242698
George, A.J., 1965. Fd. Cosmet. Toxicol., 3: 85-91.
https://www.oceannutrition.com/uploads/pdf/How-to-Hatch-Brine-Shrimp.pdf https://www.poison.org/articles/what-happens-with-swallowing-or-inhaling-toomuch menthol--174
Insanu, M., Anggadiredja, J. and Kayser, O., 2012. Int. J. appl. Res. Nat. Prod., 5: 26-34.
Jaradat, N. and Zaid, A.N., 2019. BMC Complement. Altern. Med., 19: 1-12. https://doi.org/10.1186/s12906-019-2617-2
Kijpornyongpan, T., Sereemaspun, A. and Chanchao, C., 2014. Asian Pac. J. Cancer Prev., 15: 1551-1556. https://doi.org/10.7314/APJCP.2014.15.4.1551
Kosasih, K., Sumaryono, W., Supriono, A., Mudhakir, D., 2019. J. Pharmacogn. Phytochem., 8: 24-33.
Landu, E. and Shapira, R., 2012. Appl. environ. Microbiol., 78: 5361-5367. https://doi.org/10.1128/AEM.00894-12
Leistner, E. and Drewke, C., 2010. J. Nat. Prod., 73: 86-92. https://doi.org/10.1021/np9005019
Mascarenhas, M.N., Flaxman, S.R., Boerma, T., Vanderpoel, S. and Stevens, G.A., 2012. PLoS Med., 9: 1-12. https://doi.org/10.1371/journal.pmed.1001356
Mayorga, P., Parez, K., R., Cruz, S.M. and Caceres, A.Q., 2010. Rev. Bra. Farmacogn., 20: 897-903. https://doi.org/10.1590/S0102-695X2010005000029
Memita, A.Y.B.S., Macalinao, D., Reyes, L.E.G., Damian, Jr. E.E. and Dulay, R.M.R., 2018. Int. J. Pure appl. Sci., 7: 1591-1600.
Miao, P., Sheng, S., Sun, X., Liu J. and Huang, G., 2013. Int. Union Biochem. mol. Biol., 65: 904-910. https://doi.org/10.1002/iub.1216
Oakenfull, D. and Sidhu, G.S., 1990. Eur. J. clin. Nutr., 44: 79-88.
Park, C.L., Aldwin, C.M., Choun, S., George, L., Suresh, D.P. and Bliss, D., 2015. J. Hlth. Psychol., 35: 203-210. https://doi.org/10.1037/hea0000271
Rahman, A., U., Choudhary, M.I. and Wahab, A.U., 2011. Progress report district Shaheed Benazirabad., pp. 1-92.
Renugadevi, J. and Prabu, S.M., 2010. Exp. Toxicol. Pathol., 62: 171-181. https://doi.org/10.1016/j.etp.2009.03.010
Sak, K., Everaus, H., 2015. Asian Pac. J. Cancer Prev., 16: 4843-4847. https://doi.org/10.7314/APJCP.2015.16.12.4843
Siddiqui, M.F., 2010. East. J. Med., 15: 93-96. https://doi.org/10.1071/HI10015
Thampson, D.C., Barhoumi, R. and Burghardt, R.C., 1998. Toxicol. appl. Pharm., 149: 55-63.
Vanhaecke, P. and Sorgeloos, P., 1989. Annls Soc. R. Zool. Belg., 119: 7–23.
Veni, T. and Pushpanathan, T., 2014. J. Coast. Life Med., 2: 453-457.
Wasonga, A.G. and Olendi, R.J., 2014. Afr. J. environ. Sci. Technol., 2: 1-7.
To share on other social networks, click on any share button. What are these?










