Distribution of Parthenium hysterophorus L. in the Swabi District of Khyber Pakhtunkhwa
Distribution of Parthenium hysterophorus L. in the Swabi District of Khyber Pakhtunkhwa
Sadiq Ali* and Ijaz Ahmad Khan
Department of Weed Science, Faculty of Crop Protection Sciences, The University of Agriculture, Peshawar, Pakistan.
Abstract | Parthenium weed (Parthenium hysterophorus L.) is an annual, herbaceous invasive weed but it is perennial in Pakistan. Parthenium weed is a weed of a national significance in Pakistan. Although, parthenium weed is infesting many parts of Khyber Pakhtunkhwa province, but more affected regions are Peshawar valley and Hazara division where it has enormously invaded most of the open spaces like roadsides, wasteland and water ways resulting in loss of local biodiversity. Field survey of district Swabi (Peshawar Valley) of Khyber Pakhtunkhwa, was carried out during May-June, 2013-14 to study the distribution of Parthenium weed. Five villages were selected and thirty locations were randomly selected from each village. The data revealed that the flora is predominated by parthenium weed with the highest relative density of 63.4% among all weeds, followed by Cynodon dactylon (L.) Pers., Cannabis sativa L. and Chenopodium album L. with relative density of 11.37, 10.86 and 7.31%, respectively. Moreover, at different locations, the parthenium weed is competing with C. sativa which is not problematic like parthenium weed and replaced by the latter. Mean distribution of parthenium weed infestation was abundant and almost not uniform in all sites, because some sites were in hilly area. The computed data showed that the highest relative frequency of 28.71% was recorded for parthenium weed followed by C. sativa, C. dactylon and C. album having relative frequency of 13.33%, 12.71% and 10.16%, respectively. Comparatively, the other weeds were having a very low relative density and relative frequency at most of the locations studied. Importance value also shows that P. hysterophorus, C. sativa, C. dactylon and C. album were predominant weeds in district.
Received | March 03, 2017; Accepted | April 08, 2017; Published | June 09, 2017
*Correspondence | Sadiq Ali, Department of Weed Science, Faculty of Crop Protection Sciences, The University of Agriculture, Peshawar; Email: [email protected]
Citation | Ali, S., and I.A. Khan. Distribution of Parthenium hysterophorus L. in the Swabi District of Khyber Pakhtunkhwa. Sarhad Journal of Agriculture, 33(2): 269-275.
DOI | http://dx.doi.org/10.17582/journal.sja/2017/33.2.269.275
Keywords | Parthenium hysterophorus L., Relative density, Importance value, Swabi district
Introduction
Parthenium weed (Parthenium hysterophorus L.) is an alien invasive species and is an herbaceous annual plant but it is perennial in Pakistan (Hassan and Amin, 2009). It belongs to the family Asteraceae and is commonly known as parthenium weed (Navie et al., 1996). Parthenium weed is native to the Caribbean region, but has spread to the United States of America (USA), Australia, Pakistan, India, China, Pacific Islands and Africa (Adkins et at., 1996). Parthenium weed is spreading aggressively in the wasteland and roadsides of Pakistan. Its large and persistent soil seed bank, fast germination rate and ability to undergo dormancy make it well adapted to semi-arid environments (Khosla and Sobti, 1979). Allelopathic
Table 1: Absolute density (m-2) of various weed species in five villages of District Swabi.
| Weed Species | Ismaila | Nawaykale | Zaida | Swabi | Topi | Means |
| Parthenium hysterophorus L. | 33.56 | 34.62 | 24.71 | 30.48 | 17.48 | 28.17 |
| Cannabis sativa L. | 4.67 | 5.29 | 4.89 | 5.27 | 2.78 | 4.58 |
| Cynodon dactylon L. Pers. | 5.23 | 5.51 | 6.36 | 4.27 | 2.93 | 4.86 |
| Chenopodium album L. | 4.62 | 4.38 | 4.33 | 4.19 | 1.36 | 3.78 |
| Cyperus rotundus L. | 0.62 | 1.63 | 1.14 | 0.25 | 0.00 | 0.73 |
| Rumex crispus L. | 1.53 | 0.91 | 0.34 | 0.68 | 0.00 | 0.69 |
| Xanthium strumarium L. | 0.45 | 0.24 | 0.21 | 0.19 | 0.13 | 0.24 |
| Amaranthus viridis L. | 0.85 | 0.63 | 1.02 | 1.17 | 0.24 |
0.78 |
| Coronopus didymus (L.) Sm. | 1.54 | 1.23 | 1.22 | 1.83 | 0.00 | 1.16 |
| Euphorbia helioscopia L. | 0.33 | 0.14 | 0.14 | 0.11 | 0.00 |
0.14 |
potentials are present in virtually all plant parts. These chemicals may be released into the environment in sufficient quantities to affect the neighboring plants. Parthenium weed causes severe human health problems as well as agricultural losses. Parthenium weed and related genera contain sesquiterpene lactones (Picman and Towers, 1982) which induce severe allergic dermatitis and other symptoms. Agricultural losses can also be severe. In India, parthenium weed causes yield losses of up to 40% in several crops and it is reported to reduce forage production by up to 90% (Khosla and Sobti, 1979). In Australia, parthenium weed is a serious problem in perennial grasslands in central Queensland (Adkins et al., 1996; Navie et al., 1996), and reduce beef production by as much as AU$16.5 million annually (Chippendale and Panetta, 1994). Stock animals, especially horses, suffer from allergic skin reactions while grazing infested paddocks. Consumption of large amounts will produce taints in mutton (Tudor et al., 1982). Parthenium weed has been shown to reduce agricultural crop production by up to 40 % and reduce fodder production by 90% (Khosla and Sobti, 1979). Parthenium weed can also be a problem in healthy pastures. (Adkins et at., 1996). Parthenium weed causes severe human health problems as well as agricultural losses (Picman and Towers, 1982). Among the crops, maize has been perceived to be more severely affected by parthenium weed in the Khyber Pakhtunkhwa Province. They shrink the crop yield from 20-40% relying on weed species and density (Ashique et al., 1997). The degree of yield losses depends on nature, intensity, stage and duration of competition with weeds (Bosinc and Swanton, 1997). For this purpose the parthenium weed density was studied in the selected area of Khyber Pakhtunkhwa, Province.
Materials and Methods
The distribution of parthenium weed in the district Swabi was undertaken during the month of May-June 2013-14, to study the distribution of the invasive weed (Parthenium hysterophorus L.) along with the other vegetation. Five villages were randomly selected for the study; from each villages three sampling sites ranged from 3-5 Km. Ten randomly 1 m2 quadrate were taken from each site. Absolute density, relative density, absolute frequency, relative frequency and importance value were calculated by applying the following formulae.
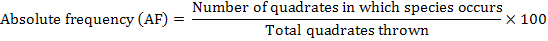
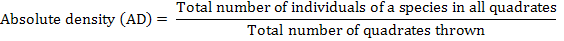
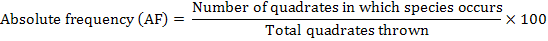
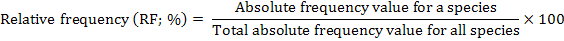

Results and Discussion
Absolute Density (m-2)
Enormous variability in weed dynamics were observed across the 5 locations studied (Table 1). Among the recorded weeds, the maximum absolute density of P. hysterophorus. 34.62 m-2 was recorded at village Nawaykale which was closely followed by village Ismaila 33.56 m-2, whereas the minimum absolute density were observed at village Topi having the absolute
Table 2: Relative density (%) of various weed species in five villages of District swabi.
| Weed Species | Ismaila | Nawaykale | Zaida | Swabi | Topi | Means |
| Parthenium hysterophorus L. | 62.85 | 63.43 | 62.92 | 55.70 | 70.14 | 63.01 |
| Cannabis sativa L. | 8.75 | 9.69 | 10.88 | 11.02 | 11.16 | 10.30 |
| Cynodon dactylon L. Pers. | 9.79 | 10.10 | 8.82 | 14.34 | 11.76 | 10.96 |
| Chenopodium album L. | 8.65 | 8.02 | 8.65 | 9.76 | 5.46 | 8.11 |
| Cyperus rotundus L. | 1.16 | 2.99 | 0.52 | 2.57 | 0.00 | 1.45 |
| Rumex crispus L. | 2.87 | 1.67 | 1.40 | 0.77 | 0.00 |
1.34 |
| Xanthium strumarium L. | 0.84 | 0.44 | 0.39 | 0.47 | 0.52 | 0.53 |
| Amaranthus viridis L. | 1.59 | 1.15 | 2.42 | 2.30 | 0.96 | 1.68 |
|
Coronopus didymus (L.) Sm. |
2.88 | 2.25 | 3.78 | 2.75 | 0.00 | 2.33 |
| Euphorbia helioscopia L. | 0.62 | 0.26 | 0.23 | 0.32 | 0.00 |
0.29 |
density of 17.48 m-2. Additionally, among the weeds C. dactylon. was the 2nd most dominant weed species across the roadside having absolute density of 6.36 m-2 and the mentioned species was found to be the predominant species, mostly in all villages 54.58, 53.40, 48.44, 44.36 and 24.92 plants m-2 at Nawaykale, Ismaila, Swabi, Zaida and Topi, respectively. Across all villages highest absolute density mean 28.17 m-2 were recorded for P. hysterophorus as followed by C. dactylon. 4.86 m-2 and C. sativa 4.58 m-2, C. album 3.78 m-2, Coronopus didymus (L.) Sm. 1.16 m-2, Amaranthus viridis L. 0.78 m-2, Cyperus rotendus L. 0.73 m-2, Rumex crispus L. 0.69 m-2, Xanthium strumarium L. 0.24 m-2 and Euphorbia helioscopia L. 0.14 m-2 were the least abundant species in the studied locations (Table 1).
The results indicated that mostly all the locations of District Swabi were highly infested with parthenium. It might be due to high adaptability of this species to this environment. Its rapidly spreading causes a serious threat to local biodiversity. Singh et al. (2005) reported that due to its allelopathic potential parthenium is considered a noxious weed. Similarly, in India, parthenuim has also been reported as a major threat to local vegetation (Yaduraju et al., 2005). Shabbir and Javaid (2010) showed parthenium and Conyza ambigua DC. as the most widespread species at Lahore District. In addition, Tamado et al. (2002) described various aspects of parthenium which contribute to its competitiveness are the high canopy, fast germination and maintenance of soil seed bank to regenerate. In another study Imponea carnea and P. hysterophorus were found toxic to animals causing many diseases and also suppressed the vegetative growth of native weeds species (Pandey et al., 2014). Moreover, due to its invasive nature, parthenium has infested many farms of East and Southern Africa (McConnachie et al., 2011), and now spreading more than 20 countries of Asia, Oceania and Africa (Dhileepan and Strathie, 2009). The spreading agencies of parthenium are vehicles, machinery, mud, water, livestock and grain seeds. These all contribute to spread of parthenium across many countries (Tamado, 2001).
Relative Density (%)
The data regarding relative density are shown in Table 2. The present survey showed that flora of different villages comprised of P. hysterophorus, C. sativa, C. dactylon, C. album, C. rotundus, R. crispus, X. strumarium, A. viridis, C. didymus and E. helioscopia. The results revealed that at all villages P. hysterophorus was found to be the most dominant species with relative density of 70.14, 63.43, 62.92, 62.85 and 55.70%, respectively. Among the villages, the maximum relative density was found in village Topi 70.14% and the minimum relative density was found for village Swabi 55.70%. The data further exhibit that C. dactylon was the next abundant weed species with a relative density of 14.34% observed at village Swabi and its minimum relative density 9.79% occurred in village Ismaila along the roadsides. Mean data revealed that P. hysterophorus was the predominant species with relative density of 63.01% followed by C. dactylon, C. sativa, C. album, C. didymus, A. viridis, C. rotundus, R. crispus, X. strumarium and E. helioscopia with relative densities of 10.96, 10.30, 8.11, 2.33, 1.68, 1.45, 1.34, 0.53 and 0.29 %, respectively.
At different locations, it was observed that parthenium expanding very fast and almost replaced C. sativa and other vegetation as well. It is supported by previous studies, that reported loss of native species, mostly due to rapid population growth and development
Table 3: Absolute frequency of various weed species in five villages of District Swabi.
| Weed Species | Ismaila | Nawaykale | Zaida | Swabi | Topi | Means |
| Parthenium hysterophorus L. | 100 | 100 | 100 | 100 | 60 | 92.00 |
| Cannabis sativa L. | 57 | 53 | 47 | 57 | 8 | 44.40 |
| Cynodon dactylon L. Pers. | 60 | 67 | 70 | 67 | 13 | 55.40 |
| Chenopodium album L. | 50 | 43 | 40 | 43 | 6 |
36.40 |
| Cyperus rotundus L. | 33 | 27 | 30 | 37 | 0 | 25.40 |
| Rumex crispus L. | 27 | 30 | 27 | 20 | 0 | 20.80 |
|
Xanthium strumarium L. |
30 | 33 | 30 | 37 | 8 | 27.60 |
| Amaranthus viridis L. | 20 | 20 | 23 | 27 | 4 | 18.80 |
| Coronopus didymus (L.) Sm. | 23 | 27 | 23 | 20 | 0 | 18.60 |
| Euphorbia helioscopia L. | 37 | 43 | 27 | 23 | 0 |
26.00 |
activities, climate change, deforestation, agricultural expansion and change in land use is contributing to rapid spreading of parthenium (Zuberi et al., 2014). Parthenium has invaded natural, disturbed and undisturbed areas and has the capability to adapt itself to various environments and habitats (Annapurna and Singh, 2003). A similar invasion of parthenium at National Wildlife Park in Southern India has also been reported (Evans, 1997). Khan et al. (2014) also found a heavy infestation of parthenium weed in Districts Mardan, Swabi and Charsadda. The above findings revealed that parthenium has the capability to disturb and change the local vegetation diversity. Evans, (1997) reported a significant variation in plant biodiversity by the invasion of parthenium. It has also been reported that being an invasive weed, parthenium weed is rapidly spreading on urban area, but spreading towards forest and agricultural lands (Tiwari et al., 2005; Shrestha, 2008). The most rapid spreading of parthenium has also been reported in NWFP and Kashmir (Javaid and Anjum, 2005).
Absolute Frequency
The data in Table 3 evidenced the composition of the studied sites in District Swabi. The results showed that among weeds species mean maximum absolute frequency 92.00% was observed for P. hysterophorus followed by C. dactylon 55.40% and C. sativa 44.40%, while minimum absolute frequency 18.60% was recorded for C. didymus and A. viridis 18.80%.
Among all the weeds P. hysterophorus got the highest absolute frequency 100, 100, 100, 100 and 60% at all villages i.e. Ismaila, Nawaykale, Zaida, Swabi and Topi, respectively. At all locations surveyed, C. dactylon was found as the 2nd most dominant species having absolute frequency 60, 67, 70, and 67% at village Ismaila, Nawaykale, Zaida and Swabi. However, its infestation was low at village Topi 13%. In addition, C. sativa occurred as the 3rd most abundant weed having absolute frequency 57, 53, 47 and 57 % at village Ismaila, Nawaykale, Zaida and Swabi. Likewise, its infestation was low at village Topi 8 %. In addition to, data regarding species showed that the lowest absolute frequency was recorded for C. didymus 23, 27, 23 and 20% at village Ismaila, Nawaykale, Zaida and Swabi and was totally absent at village Topi. The outcomes indicated that parthenium weed has high absolute frequency and during a very short time it become the most frequent and dense populated weed of Swabi villages. Due to its aggressive nature parthenium mostly reserve all areas of Swabi. Aggressive nature might be due to early maturity, rapid seed production, small size of the seeds and allelopathic effect which mostly suppress native species. In another study it has been reported that parthenium can easily grow at all types of soil and have considered the serious threat to other vegetation (Etana et al., 2015). Patil and Jadhav (2013) has been reported the invasiveness, spreading potential and environmental impacts of parthenium and recorded the worst weed. These results are linked with (Khan et al., 2014; Javaid et al., 2011; Zuberi et al., 2014; Kilewa and Rashid, 2014) they reported that parthenium weed spread along roadsides, residential, agricultural and pasture land as well.
Relative frequency (%)
The relative frequency of weeds is a good statistic showing the distribution of flora in an area. A reference to the data in Table 4 exhibits the dominance of P. hysterophorus at all 5 locations. The data table for species mean showed that among the recorded weeds species highest relative frequency 30.60% was found for P. hysterophorus followed by C. dactylon 15.00 %
Table 4: Relative frequency (%) of various weed species in five villages of District Swabi.
| Weed Species | Ismaila | Nawaykale | Zaida | Swabi | Topi | Means |
| Parthenium hysterophorus L. | 23 | 23 | 24 | 23 | 60 | 30.60 |
| Cannabis sativa L. | 13 | 12 | 11 | 13 | 8 |
11.50 |
| Cynodon dactylon L. Pers. | 14 | 15 | 17 | 16 | 13 | 15.00 |
| Chenopodium album L. | 11 | 10 | 10 | 10 | 6 | 9.40 |
|
Cyperus rotundus L. |
8 | 6 | 7 | 9 | 0 | 6.00 |
| Rumex crispus L. | 6 | 7 | 6 | 5 | 0 | 4.80 |
| Xanthium strumarium L. | 7 | 8 | 7 | 9 | 8 | 7.80 |
| Amaranthus viridis L. | 5 | 5 | 6 | 6 | 4 | 5.20 |
| Coronopus didymus (L.) Sm. | 5 | 6 | 6 | 5 | 0 | 4.40 |
| Euphorbia helioscopia L. | 8 | 10 | 6 | 5 | 0 |
5.80 |
Table 5: Importance Value of various weed species across five locations in District Swabi
| Weed Species | Ismaila | Nawaykale | Zaida | Swabi | Topi |
Means |
| Parthenium hysterophorus L. | 43 | 43 | 43 | 39 | 57 | 45.00 |
| Cannabis sativa L. | 11 | 11 | 11 | 12 | 9 | 10.80 |
|
Cynodon dactylon L. Pers. |
12 | 13 | 13 | 15 | 10 | 12.60 |
| Chenopodium album L. | 10 | 9 | 9 | 10 | 4 | 8.40 |
| Cyperus rotundus L. | 4 | 5 | 4 | 6 | 0 | 3.80 |
| Rumex crispus L. | 4 | 4 | 4 | 3 | 0 | 3.00 |
| Xanthium strumarium L. | 4 | 4 | 4 | 5 | 0 | 3.40 |
| Amaranthus viridis L. | 3 | 3 | 4 | 4 | 1 | 3.00 |
| Coronopus didymus (L.) Sm. | 4 | 4 | 5 | 4 | 0 | 3.40 |
| Euphorbia helioscopia L. | 5 | 5 | 3 | 3 | 0 |
3.20 |
and C. sativa 11.50%. Similarly, the results further showed that among the mentioned weed species lowest relative frequency 4.40% were recorded for C. didymus followed by R. crispus 4.80%. Villages wise the data showed that P. hysterophorus highly infested mostly all the villages. Highest relative frequency 60% of P. hysterophorus were recorded for village Topi followed by Zaida, Ismaila, Swabi and Nawaykale having relative frequency 24, 23, 23 and 23% respectively. However, with respect to village Topi the other villages are list infested with P. hysterophorus. Among weeds species C. dactylon was the 2nd dominant specie and recorded relative frequency 17, 16, 15, 14 and 13% for village Zaida, Swabi, Nawaykale, Ismaila and Topi correspondingly. Nevertheless, the data showed that among weeds less infestation was found for C. didymus mostly in all villages 6, 6, 5 and 5% as no relative frequency were recorded at village Topi 0%. Nonetheless, C. didymus and R. crispus possessed the smallest relative frequency at most of the sites examined thereby indicating them as unimportant among the weed flora of the target area.
The results indicated that native biodiversity is very important and it provides a lot of services in the surrounding. Loss of local biodiversity may cause many environmental changes such as habitat change, wildlife population, loss of species and other ecosystem disturbance. Kuensel (2007) reported that by increasing invasive weed like parthenium, it significantly decreases the native biodiversity. Marwat et al. (2008) described that due to invasive nature of parthenium it have the capability to replace the species through their allelopathic potential. In addition too, Navie et al. (2004) reported low diversity and richness of grassland community due to heavy infestation of parthenium. The presence of parthenium over a long time may result in decreasing species abundance, but also affect soil seed bank by reducing the regenerative ability of local species.
Importance value
The importance value determination is a good indicator for the floral distribution in the area. Among the weed species with the highest importance value of 45.00 was for P. hysterophorus followed by C. dactylon 12.60 and C. sativa 10.80. The lowest importance values were found for C. rotundus and A. viridis 3.00. Data regarding villages and species showed that maximum infestations were recorded for village Topi 57.00 followed by village Ismaila, Nawaykale, Zaida and Swabi 43, 43, 43 and 39, respectively. Parthenium however, ranked at the top emerging as the most prevalent weed at all the locations as well as in the mean values for all 5 locations (Table 5).
The perusal of data further exhibit that Parthenium-Cynodon-Cannabis community dominated at Topi and Swabi. At Zaida, Parthenium-Cynodon-Cannabis-Chenopodium community dominated, whereas at Ismaila Parthenium-Cynodon-Chenopodium-cannabis community was evidenced (Table 5). Parthenium was successful weed which has widely spread across all the villages of Swabi. Keeping in view the parthenium weed as most successful herb, it is necessary to effectively manage this menace and stop its further infestation rather minimize the existing stands of this useless and injurious plant. Our results are similar to Huy and Seghal (2004) who observed parthenuim dominancy with high IVI in the studied area. Similarly, Khan et al. (2014) showed parthenium as dominant species having high importance value at the locations studied. Furthermore, Belachew and Tessema (2015) confirmed parthenium as aggressive and competitive weed specie at different regions of Ethiopia.
Conclusions
The studied flora in the District Swabi revealed that the flora is dominated by parthenium with the highest relative density among all other weeds, followed by Cynodon dactylon (L.) Pers., Cannabis sativa L. and Chenopodium album L. Parthenium weed has spread rapid along roadsides, wastelands and also in agricultural fields of Swabi District of the Khyber Pakhtunkhwa Province. Its spreading needs control measures to safeguard the productivity of agro-ecosystems and ensure the sustainability of environment.
Authors’ Contribution
Mr. Sadiq Ali Ph.D Scholar Department of Weed Science carried out the study under the supervision of Dr. Ijaz Ahmad Khan, Associate Professor and Chairman Department of Weed Science, The University of Agriculture, Peshawar, Pakistan.
References
Adkins, S.W. and M.S. Sowerby. 1996. The allelopathic potential of parthenium weed (Parthenium hysterophourus L.) in Australia. Plant Prot. 11(1): 20-23.
Annapurna, C. and J.S. Singh. 2003. Variation of Parthenium hysterophorus in response to soil quality: Implications for invasiveness. Weed Res. 43(3): 190-198. https://doi.org/10.1046/j.1365-3180.2003.00332.x
Ashique. M., M.L.Shah and M. Shafi. 1997. Weeds of maize and their eradication. Zarat Nama 35: 89.
Belachew, K. and T. Tessema. 2015. Assessment of Weed Flora Composition in Parthenium Infested Area of East Shewa, Ethiopia. Malays. j. med. biol. res. 2: 63-70.
Bosnic, A.C. and C.J. Swanton. 1997. Influence of barnyard grass (Echinochloa crus-galli) time of emergence and density on corn (Zea mays). Weed Sci. 45: 276-282.
Chippendale, J.F. and F.D. Panetta. 1994. The cost parthenium weed to the Queensland cattle industry. Plant Prot. Quart. 9: 73-76.
Dhileepan, K. and L. Strathie. 2009. Parthenium hysterophorus L. (Asteraceae). In: Biological Control of Tropical Weeds using Arthropods (Muniappan, R, G.V.P. Reddy and Raman, A. (Eds). 274–318. Cambridge University Press, UK. https://doi.org/10.1017/CBO9780511576348.015
Etana. A., E. Kelbessa and T. Soromessa. 2015. Impact of Parthenium hysterophorus L. on soil chemical properties and its distribution in a reserve area: A case study in Awaish National Park (ANP) Ethiopia Academic J. 6(5): 116-124.
Evans, H.C. 1997. Parthenium hysterophorus L: A review of its weed status and the possibilities for biological control. Biocontrol News Inform. 18: 89-98.
Hassan, G. and A. Amin. 2009. First annual Tech. Report. HEC International Linkage Project with Univer. of Queensland, Australia, on Parthenium Weed.
Huy, L.Q. and R.N. Seghal. 2004. Invasion of Parthenium hysterophorus in Chire-Pine Forests and Its allelopathic effects. Abstracts of an Int. Workshop on protocols and Methodologies in Allelopathy, April 2-4, 2004 in Palampur (HP) India. CSK HP Agric. Univer. Palampur (HP) India: Inte. Allelo. Soc. pp. 52.
Javaid, A. and T. Anjum. 2005. Parthenium hysterophorus L. a noxious alien weed. Pak. J. Weed Sci. Res. 11: 1-6.
Javaid, A., K. Jabeen, S. Samad and A. Javaid. 2011. Management of parthenium weed by extracts and residue of wheat. Afr. J. Biotechol. Res. 10: 14399-14403. https://doi.org/10.5897/AJB10.1270
Khan, H., K.B. Marwat, G. Hassan, M.A. Khan and S. Hashim. 2014. Distribution of parthenium weed in Peshawar valley, Khyber Pakhtunkhwa-Pakistan. Pak. J. Bot. 46(1): 81-90.
Kilewa, K. and A. Rashid. 2014. Distribution of Invasive Weed Parthenium hysterophorus in natural and agroecosystems in Arusha Tanzania. Int. J. Sci. Res. 3(12): 1724-1727.
Khosla, S.N. and S.N. Sobti. 1979. Parthenium a national health hazard, its control and utility-a review. Pestic. 13: 121-127.
Kuensel Issue 7th July, 2007. National paper on biodiversity persistence and climate change 2011. http://www.nbc.gov.bt/wp-content/uploads/2010/06/National-Paper-on-Biodiversity-and-Climate-Change-_Bhutan1
Marwat, K.B., M.A. Khan, A. Nawaz and A. Amin. 2008. Parthenium hysterophorus L. A potential source of bioherbicide. Pak. J. Bot. 40(5): 1933-1942.
McConnachie, A. J., L. W. Strathie, W. Mersie, L. Gebrehiwot, K. Zewdie, A. Abdurehim, B. Abrha, T. Araya, F. Asaregew, F. Assefa, R. Gebre-Tsadik, L. Nigatu, B. Tadesse and T. Tana. 2011. Current and potential geographical distribution of the invasive plant Parthenium hysterophorus (Asteraceae) in eastern and southern Africa. Weed Res. 51(1): 71-84. https://doi.org/10.1111/j.1365-3180.2010.00820.x
Navie, S.C., F.D., Panetta, R.E. McFadyen and S.W. Adkins. 1996. The biology of Australian weeds, Parthenium hysterophorous L. Plant Prot. 11: 76-88.
Navie, S.C., F.D. Panetta, R.E. McFadyen and S.W. Adkins. 2004. Germinable soil seed banks of Central Queensland rangelands invaded by exotic weed Parthenium hysterophorus L. Weed Biol. Manag. 4: 154-167. https://doi.org/10.1111/j.1445-6664.2004.00132.x
Pandey, R., A. Bhandari and B. Pandey. 2014. Ecological status of weed flora found in Bhilai Nagar. Indian J. Sci. Res. 4 (1):115-120.
Patil, V.S. and P.S. Jadhav. 2013. A survey of weed flora in crop fields of Satara Tahsil (M.S.), India. Universal J. Environ. Res. Technol. 3(2): 233-241.
Picman, A.K. and G.H.N. Towers. 1982. Sesquiterpene lactones in various populations of Parthenium hysterophorus L. Biochem. Syst. Eco. 10:145-153. https://doi.org/10.1016/0305-1978(82)90021-7
Shabbir, A. and A. Javaid. 2010. Phytosociological survey and allelopathic effects of Parthenium weed in comparison to other weeds in Pakistan. Indian J. Agric. Res. 44(2): 119–124.
Singh, H.P., D.R. Batish, J.K. Pandher and R.K. Kohli. 2005. Phytotoxic effects of Parthenium hysterophorus residues on three Brassica species. Weed Biol. Manag. 5(3):105-109. https://doi.org/10.1111/j.1445-6664.2005.00172.x
Shrestha, B.B. 2008. Phailedai Hanikarak Banaspati [Nepali]. Kantipur national news 17th May 2008. p 10.
Tamado, T. 2001. Biology and management of Parthenium hysterophorus L. in Eastern Ethiopia. PhD Thesis Presented to the School of Graduate Studies of Swedish University of Agricultural Sciences Uppsala, Sweden. 75p.
Tamado, T., L. Ohlander and P. Milberg. 2002. Interference by the weed Parthenium hysterophorus L. with grain sorghum: Influence of weed density and duration of competition. Int. J. Pest Manag. 48(3): 183-188. https://doi.org/10.1080/09670870110101739
Tiwari, S., B. Adhikari, M. Siwakoti and K. Subedi. 2005. An Inventory and Assessment of Invasive Alien Plant Species of Nepal. IUCN – The World Conservation Union, Nepal. 115p.
Tudor, G.D., A.L. Ford, T.R. Armstrong and E.K. Bromage. 1982. Taints in meat from sheep grazing Parthenium hysterophorus. Aust. J. Exp. Agric. Ani Husban. 22:43-46. https://doi.org/10.1071/EA9820043
Yaduraju, N.T., M.B.B. Sushilkumar, P. Babu and A.K. Gogoi. 2005. Parthenium hysterophorus L. distribution, problems and management strategy in India. In: Proc. 2nd International conference on parthenium management (Bangalore, India, 5-7 December 2005). University of Agricultural Science, Bangalore, India. 6.10.
Zuberi, M.I., T. Gosaye and S. Hossain. 2014. Potential threat of allien invansive species: Parthenium hysterophorus L. to subsistence agriculture in Ethiopia. Sarhad. J. Agric. 30(1): 117-125.
To share on other social networks, click on any share button. What are these?








