Dynamics and Potential of Commercially Harvested Shrimps by Estuarine Set Bagnet in the Indus River Estuary, Sindh, Pakistan
Dynamics and Potential of Commercially Harvested Shrimps by Estuarine Set Bagnet in the Indus River Estuary, Sindh, Pakistan
Nazia Qamar1,2*, Sher Khan Panhwar2 and Wang Ping1
1Key Laboratory of Pharmaceutical Engineering, Ministry of Education, College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou, 310014 China
2Fishery Biology Laboratory, Center of Excellence in Marine Biology, University of Karachi, 75270, Sindh
ABSTRACT
In this study, periodic changes of population dynamics of high valued shrimp species Exopalaemon styliferus (112), Metapenaeus monoceros (265), Parapenaeopsis stylifera (688) collected from September 2017 to April 2018 by Estuarine Set Bagnet are discussed. High linearity (>0.8) among parameters was estimated by fitting a liner model and a negative allometric growth pattern in E. styliferus, P. stylifera and slight positive growth was calculated for M. monoceros. A moderate growth rate for E. styliferus whereas fast growth rate for M. monoceros and P. stylifera was calculated by applying von Bertalanffy model. Higher natural mortalities (M) for E. styliferus and M. monoceros and fishing mortalities for P. stylifera was estimated. Biological reference points or Fishing limitations (Flimitation) for three species was beyond proposed limit of exploitation rate of 0.5 indicates that resource have been reached maximum sustainable yield. A persistent recruitment pattern for three species recorded with prominent peaks (82% in June-July) for E. styliferus, (70% June-October for M. Monoceros and (47% April-May) estimated for P. stylifera. Based on the data it is recommended that use of Estuarine Set Bagnet ESBN should be banned especially in the Indus River Estuary IRE and it is hoped that data can be used for shrimp fishery management in Pakistan.
Article Information
Received 02 September 2016
Revised 11 May 2018
Accepted 22 November 2019
Available online 24 March 2020
Authors’ Contribution
NQ collected and analysis the data and wrote first draft of the manuscript. SKP guided the research and helped in manuscript write-up. PW helped in manuscript preparation.
Key words
Estuarine set bagnet, Population dynamics, Commercially valued shrimps, Indus River Estuary-Sindh
DOI: https://dx.doi.org/10.17582/journal.pjz/20160902150951
* Corresponding author: [email protected]; [email protected]
0030-9923/2020/0004-1255 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Penaeid shrimps inhabiting in tropical and sub-tropical waters (Fischer and Bianchi, 1984) are highly valued marine stuff with size varying from 2.5‒35 TLcm (Carpenter and Niem, 1998). Indus River Estuary a versatile ecosystem provide seems vulnerable owing to use of Estuarine Set Bagnet (ESBN) in the river mouth that ultimately harm stock and that does not allow recruits to attain size of maturity and play role in reproduction and expansion. Along with a number of fish and shellfish species of high commercial importance for instance Exopalaemon styliferus, Parapenaeopsis stylifera and Metapenaeus monoceros have been caught. The frequent use of ESBN at the mouth of the Rive has snarled-up entrance of marine and freshwater animals vice versa that has collectively damaged estuarine biodiversity and ecosystem. These shrimps are also catch with cast net, gill net and beach seines at the depth of 50‒60 meters depth (Fischer and Bianchi, 1984). Hence E. styliferus (Roshna prawn) is uncommon species inhabits in shallow coastal water and occasionally found in fresh water.
The population abundance of fisheries resources depends on the rate of natality and mortality whereas total biomass depends on fish growth. Population dynamics supposed to be the best management strategies which conveniently predicting future yields and stock biomass at different fishing levels. Length weight relationships of these species was reported from other part of the world (Rao, 1990; Nandakumar, 1997; Safaie, 2017; Sarda, 2002) but information on growth, mortality, exploitation and age of these species is scarce in literature. According to ten year statistics data compiled by Marine Fisheries Department, Government of Pakistan M. monoceros and P. stylifera composed 17-29% and 50-65% of the total shrimp landing catches from the coastal waters of northern Arabian Sea. Present study was aimed to discuss population dynamics of three highly commercially important shrimps species sampled from Indus River Estuary for the sake of conservation and management of most important fishery resource.
MATERIALs AND METHODS
Sampling method and data analysis
Data of three shrimp species Exopalaemon styliferus, Metapenaeus monoceros, Parapenaeopsis stylifera were collected from Indus River Estuary IRE from September 2017 to April 2018 using Estuarine Set Bagnet ESBN. The net was fixed for the period of at least sex hours (one high and one low tide) during day time. A total of 1065 individuals (E. styliferus,112, M. monoceros, 265, P. stylifera, 688) were sampled and kept in deep freezer for further analyses. Species was clearly identified by using FAO field guide (Fischer and Bianchi, 1984). Each individual shrimp was measured in centimeter and weighed in grams. The entire data were pooled as one-year and analyzed from FAO-ICLARM stock Assessment Tools (FiSAT).
Length weight relationship
Length and weight relationship was used for estimating the weight corresponding to a given length. It is the building block of stock assessment. The length weight relationship was estimated with the equation, W=aLb, W= weight, L= length, a and b are intercept and slope, the data were converted on natural log to fit linear straight line of log length and log weight. The equation can be demonstrated logarithmically logw=loga+blogL. The linear regression LW relationship estimated using Microsoft excel (Data analysis tool).
Estimation of growth parameters
The von Bertalanffy growth equation (VBGF) was used to describe the growth.
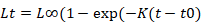
Where Lt= predicated length at age t, L∞= asymptotic length, K= growth coefficient, t0= the age when the fish at zero length. Using input data from length frequencies and ELEFAN 1 program, asymptotic length (L∞) and growth coefficient (K) were estimated. Growth performance index GPI was applied to compare growth of similar species from different geographical distributions (Pauly and Munro, 1980).
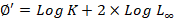
From the estimate of growth parameters (K, L∞), the instantaneous rate of annual total mortality (Z) was estimated using the length converted catch equation (Pauly, 1983). The instantaneous rate of natural mortality (M) was obtained using Pauly’s empirical formula (1980):
LnM=-0.0152-0.279×In L∞+0.6543×K+0.463×In T where L∞ is an asymptotic length, K is the growth coefficient (Yr-1) and T is the annual average of water temperature (oC); in the distribution area these species, it was 26.5℃. From estimate of the growth parameters (K, L∞), the instantaneous rate of total mortality (Z) was estimated using a length converted catch equation (Pauly, 1983), subtracting the estimate of M and Z, the instantaneous rate of fishing mortality (F) is given by F=Z-M. The exploitation rate E=F/Z is obtained with the formula: U= F (1−exp (−Z))/Z (Beaverton and Holt, 1957).
RESULTS
Catch composition and size structure
The declining trends of shrimp catches have been identified from annual catch records documented from (1999−2009) by M.F.D for Penaeus spp. whereas Metapenaeus spp. catch composition recorded 17−24% among overall shrimp catches. Parapenaeopsis stylifera found to be most abundant (65−68%) being landed at Karachi Harbour (MFD, 2012) (Fig. 1).
About 74% of the E. styliferus was sampled between 5.5−7.4 cm TL. This size is below marketable size and landing recorded only in October 2017. Most of the individual of Metapenaeus monoceros (43.7%) lies within the size class of (9−10.9 cm) TL. For P. stylifera (90.2%) of the individual were in the size class 6−9.9cm TL. The highest individual was caught in September with marketable size 6−9.9cm TL (Fig. 2).
Table I. Summary of linear model parameters estimated for Exopalaemon styliferus, Metapenaeus monoceros, Parapenaeopsis stylifera sampled from Indus Estuary.
|
Species name |
TL |
BW |
a |
b |
r2 |
CI (a) |
CI(b) |
SE(a) |
SE(b) |
||
|
Min-max |
Mean±SD |
Min-max |
Mean±SD |
||||||||
|
E. styliferus |
4.8-15 |
6.83±2.05 |
0.48-18.4 |
2.93±3.30 |
0.015 |
2.69 |
0.93 |
-1.93_-1.72 |
2.51-2.76 |
0.053 |
0.064 |
|
M. monoceros |
5.2-16.2 |
9.20±1.70 |
0.72-30.3 |
5.34±3.89 |
0.004 |
3.15 |
0.96 |
-2.45_-2.30 |
3.08-3.23 |
0.036 |
0.037 |
|
P. stylifera |
4.6-13 |
8.02±1.25 |
1.11-12.5 |
3.09±1.66 |
0.007 |
2.86 |
0.87 |
-2.21_-2.07 |
2.77-2.94 |
0.037 |
0.041 |
Table II. Population parameter estimated for Exopalaemon styliferus, Metapenaeus Monoceros and Parapenaeopsis stylifera [number pf individuals (n), asymptotic length (L∞), growth coefficient (K), natural (M), fishing (F), total (Z) mortalities, Fishing limitations (Flimit), optimum fishing (Fopt), age at length zero (t0), growth performance index / phi (ø), exploitation ratio (U) and exploitation rate (E)].
|
Species name |
n |
L∞ |
K |
M |
F |
Z |
Flimits |
Fopt |
t0 |
ø |
U |
E |
Lc/Linf |
|
E. styliferus |
112 |
13.65 |
0.72 |
1.75 |
1.27 |
2.23 |
0.85 |
0.63 |
-0.09 |
2.12 |
0.53 |
0.57 |
0.34 |
|
M. monoceros |
265 |
15.75 |
1.20 |
2.34 |
1.43 |
3.35 |
0.95 |
0.75 |
-0.14 |
2.47 |
0.20 |
0.43 |
0.33 |
|
P. stylifera |
698 |
18.90 |
1.10 |
2.10 |
4.05 |
8.52 |
2.69 |
2.02 |
-0.28 |
2.60 |
0.40 |
0.48 |
0.24 |
Length−weight relationships
The size and weight ranges of each species are given in in Table I. The coefficient of determination R2 values (0.87−0.96), slopes were lower than 3.0 (p<0.05) defines negative allometric growth pattern (Table I).
The growth parameters obtained from the maximum length by ELEFAN1 in the FiSAT which was superimposed on structure of length frequency data (Fig. 2). The values obtained for L∞ and K showed that P. stylifera attain less asymptotic length and slow growth than M. monoceros and E.styliferus. The growth performance parameters were obtained from ELEFAN 1. The growth performance index (ø) during the present investigations showed low value in P. stylifera and highest in E. styliferus.
Growth, Mortalities, Recruitment and Age assessment
Total mortalities (Z), natural (M) and Fishing (F) and exploitation rate (E) was low in M. monoceros (Table II). The growth parameters obtained from ELEFAN 1 (L∞, K, t0) were used as input values to estimate the instantaneous rate of total mortality from the length converted curve method (Fig. 3). The total mortality coefficients from length-converted catch curves indicate an annual estimatefor 1 year.
The recruitment pattern was continued throughout the year with one major peak, about (82.4%) in June to July in E. styliferus (69.84%) from June to October in M. monoceros and (46.68) % in April−May in P. stylifera (Fig. 4). The dynamics of the stock influenced by the number of survivors and portion of population subjected to natural mortality and fishing mortality are very much reflected in (Fig. 5). In E. styliferus, fishing mortality is more than 1 with the size class (5.5−6.5) TLcm and highest for the larger group greater than the length of 12.5 TLcm. In M. monoceros the fishing mortality rises when the shrimps attain 5.0 TLcm and shrimps remain susceptible till the attain 13.0 TLcm. In P. stylifera, only small individual less than 6.9 TLcm have can survive, others are susceptible to fishing mortality. Here, fishing mortality increases towards higher length group as it followed fishing down the size, that’s why. Larger size group in the population were subsequently from the stock by the fishing gears. Initial terminal fishing mortality was kept as one for estimation and terminal fishing mortality one lies to removal of all adults from the population. The number of individual in the population reduces towards the higher classes but biomass values were more in higher length groups. This is mainly due to growth and resultant increases in weight of individuals. The results of Relative yield and biomass per recruit analysis of three species with current Lc is shown in (Fig. 5). The Lc/L∞ plotted against exploitation rate. Fitting von Bertalanffy growth curve age of each species was estimated using length frequency cohort data. The growth curves are depicted (Fig. 6).
DISCUSSION
Lack of organized fishery is fundamentally deteriorating costal, estuarine and oceanic ecosystems. In this study we highlight use of ESMB can vastly damage shrimp industry is further remained unmanaged. Marine Fisheries Department document separate landing data including data of Monoceros spp. P. stylifera (kiddy shrimps). Most of the shrimps have been exported due to high export earning and lest local consumption. Total length is a common biometry to detect morphometric traits and to be used to determine functional relationship between size and weight of animals. A negative slope value determines that length increase inversely increases but weight does not. Similarly, a negative slope value for P. stylifera was calculated by Safaie (2017) from Persian Gulf. Agreeing with Dineshbabu (2006), Nandakumar and Srinath (1999) and Lalithedevi (1987) slopes for M. monoceros this study reconfirms isometric growth of the species in Pakistan.
A quick, reliable and frequently method of estimating growth, mortality and asymptotic length ELEFAN-1 approach was adopted to understand dynamics of single and multi species (Pauly, 1980, 1982). Previously, no information regarding asymptotic length (L∞) and growth coefficient (K) of E. styliferus was seen in published form. However, annual estimation of L∞ and K of M. monoceros was found lower than previously reported (Nandakumar, 2000; Dineshbabu, 2006) from India. Similar results obtained from the comparison of above parameters of P. stylifera stated by Safaie (2017). The lifespan of shrimps is very short (Etim and Sankare, 1998) and they attain their asymptotic length in the first or second year and are measured by a high K-value (Beverton and Holt, 1957; Garcia and Le Reste, 1981). For growth mortality and exploitation pooled data was used therefore male and female comparison is not possible although value found to be in range (Safaie, 2017).
A comparison of growth performance index GPI (Munro and Pauly, 1983) is suitable for computation of overall growth performance of similar species from geographically distributed regions. Lower GPI was estimated for P. stylifera than reported (Methews et al., 1987; Suseelen and Rajan, 1989; Sarada, 2002; Dineshbabu, 2005; Safaie, 2017). However, GPI values can be vary within the related taxa and have narrow normal distributions (Spare and Venema, 1992).
Short life span and high mortality rate can easily damage shrimp stocks and bad ecosystem health of Indus River Estuary might be another factor. We observed relatively fast growth in E. styliferus followed by M. monoceros than P. stylifera. Moreover, higher natural mortalities than fishing was found in two species except in E. stylifera. These results are in agreement with our result. Our results also agreed with those of Beverton and Holt (1959) who observed that fast growing species have high K-value and high natural mortality rate. For P. stylifera, our values also lie within the range as described by Safaie (2017) from Persian Gulf of Iran. It might be due to short lived species who are exposed to strong fluctuation in growth due to variability in environmental condition (Sparre, 1990). Low fishing mortality may be subjected to younger age groups of shrimps because they are either not recruited fully and are more vulnerable to the fishing gear used (King, 1995). Values estimated in this study are consistent with those reported earlier within the range for other penaeids (Garcia and Von Zalinge, 1982; Safaie, 2015; Niamaimandi et al., 2007).
The fishing limitations (Flim) and optimum fishing (Fopt) level demonstrates that stock have been exploited beyond the optimum fishing level of 0.5 / year-1 Gulland (1971). Estimates of relative yield and biomass per recruit demonstrates that constant increase of fishing effort will ruin stock over 90% of the biomass and reduction yield by 25 percentage.
Recruitment pattern in these three species showed consistent recruitment with marked peaks round the year. In this context Garcia (1984) added that shrimp annual recruitments loosely related to the parental stock size and only depends on environmental factors and restrictions caused by anthropogenic activities rather than fishing pressure (Garcia and Le Raste, 1981). According to their prediction, recruitment based on environmental factor such as rainfall, temperature, river outflow, estuarine hydrology etc. It is concluded that due to high commercial value of these shrimps management measurement are unavoidable. Therefore, strict ban must be imposed on the ESBN especially in the Indus River Estuary and creek areas. Moreover, these short lived and fast growth of aquatic animals is more susceptible to environmental degradation and low freshwater flow into estuarine area.
Statement of conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
Beverton, R.J.H. and Holt, S.J., 1957. On the dynamics of exploited fish populations. Fish. Investig. Ser., II. 19: 1–533.
Beverton, R.J.H. and Holt, S.J., 1959. A review of the life spans and mortality rates of fish in nature and their relation to growth and other physiological characteristics. Ciba Founda. Colloq. Age., 5: 142–180. https://doi.org/10.1002/9780470715253.ch10
Carpenter, K.E. and Niem, V.H., 1998. FAO species identification guide for fishery purposes. The livingmarine resources of the Western Central Pacific. Vol. 2. Cephalopods, crustaceans, holothurians and sharks. FAO, Rome. pp. 687–1396.
Dineshbabu, A.P., 2005. Growth of kiddy shrimp. Parapenaeopsis stylifera (H. Milne Edwards,1837) along Saurashtra coast. Indian J. Fish., 52: 165–170
Dineshbabu, A.P., 2006. Length-weight relationship and growth of the speckled shrimp Metapenaeus monoceros (Fabricus) off Sauashtra. J. mar. biol. Assoc. India, 48: 180-184.
Etim, L. and Sankare, Y., 1998. Growth and mortality, recruitment and yield of the fresh- water shrimp, Macrobrachium vollenhovenii, Herklots, 1851 (Custacea: Palaemonidae) in the Fahe reservoir, Cote d’ivoire, West Africa. Fisher. Res., 38: 211-223. https://doi.org/10.1016/S0165-7836(98)00161-1
Fischer, W. and Bianchi, G., 1984. FAO species identification sheets for fishery purposes. Western Indian Ocean (Fishing Area 51). Prepared and printed with the support of the Danish International Development Agency (DANIDA). FAO, Rome. pp. 1–6.
Garcia, S.M. and Le Reste, L. 1981. Life cycles, dynamics, exploitation and management of coastal penaeid shrimp stocks. FAO Fish. Tech. Pap., 203: 1–215.
Garcia, S.M. and van Zalinge, N.P., 1982. Simple fishing in Kuwait: methodology for a joint analysis of the artisanal and industrial fisheries. In: Report on the workshop on assessment of the shrimp stocks of the west coast of the Gulf between Iran and the Arabian Peninsula. Fisheries development in the Gulf, FAO Rome. pp. 119–142.
Garcia, S.M., 1984. A note on environmental aspects of penaeid shrimp biology and dynamics. In: Penaeid shrimp – their biology and dynamics (eds. J.A. Gulland and B.J. Rothschild). Fishing News Books Ltd. Farnham. pp. 268–271.
GoP, WWF, IUCN, 2000. Biodiversity action plan for Pakistan: A framework for conserving our natural Wealth. Government of Pakistan, World Wildlife Fund forNature, and International Union for Conservation of Nature, Pakistan, https://portals.iucn.org/library/efiles/documents/2000-081.pdf>, accessed 20 July 2015.
Gulland, J.A., 1971. The fish resources of the Ocean. Fishing News (Books), Ltd. for FAO Surrey. pp. 1–255.
King, M., 1995. Fisheries biology, assessment and management. Fishing News Books, Blackwell Science, Oxford. pp. 342.
Lalithadevi, S., 1987. Growth and population dynamics of three penaeid prawns in trawling grounds off Kakinada. Indian J. Fish., 34: 245-263.
Le Cren, E.D., 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol., 20: 201. https://doi.org/10.2307/1540
Mathews, C.P., Al-Hossaini, M., Abdul Ghaffar, A.R. and Al-Shoushni, M., 1987. Assessment of short-lived stocks with special reference to Kuwait’s shrimp fisheries: a contrast of results obtained from traditional and recent size-based techniques. In: Length-based methods in fisheries research (eds. D Pauly and GR Morgan). ICLARM Conference Proceeding 13, Manila/Safat: International Center for Living Aquatic Resources Management/Kuwait Institute for Scientific Research. pp.147–166.
MFD, 2012. Hand book of fisheries statistics of Pakistan Vol. 20. Marine Fisheries Department, Govt. of Pakistan, Fish harbour, West wharf Karachi.
Munro, J.L. and Pauly, D., 1983. A simple method for comparing growth of fishes and invertebrates. ICLARM Fishbyte, 1: 5-6.
Nandakumar, 1997. Biology, population characteristics and fishery of the speckled shrimp Metapenaeus monoceros (Fabricius, 1798) along the Kerala coast. Thesis submitted to the cochin University of Science and Technology. pp. 340.
Nandakumar, G. and Krishnamoorthi, B., 1990. Age and growth of Metapenaeus monoceros (Fabricus) along Kakinada coast. J. mar. biol. Assoc. India, 32: 156-161.
Nandakumar, G. and Srinath, R., 1999. Stock assessment of Metapenaeus monoceros (Fabricius) from Cochin waters. Indian J. Fish., 46: 221-226
Nandakumar, G., 2000. Age and growth of the speckled shrimp Metapenaeus monoceros (Fabricius) along the Cochin coast. J. mar. biol. Assoc. India, 42: 101–111.
Niamaimandi, N., Arshad, A., Daud, S.K., Saed, R.C. and Kiabi, B., 2007. Population dynamic of green tiger prawn, Penaeus semisulcatus (De Haan) in Bushehr coastal waters. Persian Gulf Fish. Res., 86: 105–112. https://doi.org/10.1016/j.fishres.2007.05.007
Pauly D., 1983. Some simple methods for the assessment of tropical fish stocks. FAO Fish. Tech. Pap., 234: 52.
Pauly, D. and Munro, J.L., 1984. Once more on the comparison of growth infish and invertebrates. Fishbyte, 2: 21.
Pauly, D., 1980. A selection of simple methods for assessment of tropical fish stocks. FAO. Fish. Circul., 729: 1-54.
Pauly, D., 1980. On the interrelationships between natural mortality, growth parameters and meanenvironmental temperature in 175 fish stocks. J. Cons. Int. Expl. Mer., 39:175–192. https://doi.org/10.1093/icesjms/39.2.175
Pauly, D., 1982. Studying single - species dynamics in a tropical multispecies context, In: Theory and management of tropical fisheries (eds. D. Pauly and G.I. Murphy). ICLARM Conference Proceedings 9: 33-70.
Rao, K., 1990. Age and growth of Metapenaeus monoceros (Fabricius) along Kakinada coast. J. mar. biol. Assoc. India, 32: 156-161.
Safaie M., 2015. Population dynamics for banana prawns, Penaeus merguiensisde Man, 1888 in coastal waters off the northern part of the Persian Gulf, Iran. Trop. Zool., 28:1–14. https://doi.org/10.1080/03946975.2015.1006459
Safaie, M., 2017. Population dynamics of kiddy shrimp, Parapenaeopsis stylifera (H. Milne Edwards, 1837) in the north-west of Qeshm Island, Iran. Trop. Zool., 30: 13-27. https://doi.org/10.1080/03946975.2017.1278662
Sarada, P.T., 2002. Fishery, biology and population dynamics of Parapenaeopsis stylifera at Calicut. Indian J. Fish., 49: 351–360.
Sparre, P. and Venema, S.C., 1992. Introduction to tropical fish stock assessment Part 1. Manual. FAO Fish. Tech. Pap., Rome, 306: 407.
Sparre, P., 1990. Can we use traditional length-based fish stock assessment when growth is seasonal? ICLARM, Fishbyte, 8: 29–32.
Suseelan, C. and Rajan, K.N., 1989. Stock assessment of the Kiddi shrimp (Parapenaeopsis stylifera) off Cochin. In: Contributions to tropical fish stock assessment in India (eds. SC Venema and NP Zalinge). FAO Rome. pp. 15–30.
To share on other social networks, click on any share button. What are these?










