Effect of Citric Acid Acidified Moringa oleifera Seed Meal based Diet on Minerals Absorption, Carcass Composition and Hematological Indices of Cirrhinus mrigala Fingerlings
Effect of Citric Acid Acidified Moringa oleifera Seed Meal based Diet on Minerals Absorption, Carcass Composition and Hematological Indices of Cirrhinus mrigala Fingerlings
Majid Hussain1, Syed Makhdoom Hussain2, Razia Iqbal3, M. Mudassar Shahzad4,*, Syed Zakir Hussain Shah3, Afia Muhammad Akram4, Nisar Ahmad5 and Muhammad Zubair ul Hassan Arsalan2
1Institute of Molecular Biology and Biotechnology, The University of Lahore, Lahore
2Department of Zoology, Government College University, Faisalabad
3Department of Zoology, University of Gujrat, Gujrat
4Department of Zoology, Division of Science and Technology, University of Education, Lahore
5Department of Zoology, University of Education, Lahore, DG Khan Campus
ABSTRACT
This study was conducted to evaluate the effect of citric acid (CA) treated Moringa oleifera seed meal (MOSM) based diet on mineral absorption, carcass composition and hematological indices in Cirrhinus mrigala fingerlings. Basal diet was supplemented with 0%, 1%, 2%, 3%, 4% and 5% CA resulting in the formulation of six experimental diets. Ten fingerlings were stocked in tanks in triplicate for each treatment. Feed was given at 5% live wet body weight of fingerlings for 90 days. Results showed that diet acidification with CA significantly (p< 0.05) improved the mineral absorption, carcass composition and hematological indices of C. mrigala fingerlings compared to control diet. Data shows that mineral absorption was higher (p˂0.05) at medium levels of CA supplementation (2%, 3% or 4% levels) compared to extreme levels (1% and 5%). Maximum body crude protein and crude fat contents were observed in C. mrigala fed 2 % and 3% CA supplemented diets, respectively. Moreover, fingerlings fed CA acidified diets showed significant improvement (p< 0.05) in hematological parameters compared to control diet. Comparison of treatments showed maximum values of RBCs (2.83×106mm-3), WBCs (7.76×103mm-3), PLT (65.96), Hb (8.47 g/100ml), PCV (24.51 %), and MCV (187.11 fl) in fingerlings fed 3% CA supplemented diet. In conclusion, 3% CA acidified MOSM based diet performed better regarding mineral absorption, carcass composition and hematological indices C. mrigala fingerlings.
Article Information
Received 31 May 2019
Revised 30 June 2020
Accepted 01 April 2021
Available online 04 August 2021
(early access)
Published 20 April 2022
Authors’ Contribution
MH conducted the trial and did analysis. SMH planned, supervised, provided research materials and prepared manuscript. RI co-supervised the research. MMS helped in statistical analysis. RI and MMS preparation of manuscript. SZHS helped in compiling the results. SH helped in chemical analysis. NA helped in collection of results and analysis. MZHA formulated fish feed and helped in data collection.
Key words
Citric acid, Minerals absorption, Carcass, Hematological indices, Fingerlings.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190531050527
* Corresponding author: drmudassarshahzad@gmail.com
0030-9923/2022/0004-1737 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Cirrhinus mrigala is one of major Indian carps commonly consumed throughout Pakistan because of its good meat quality and taste. It has wide distribution in freshwater reservoirs of Pakistan of substantial economic importance and market value (Rauf, 2015; Hussain et al., 2017). Supplementary feed constitutes more than 50% expenditure in carp. To meet the future requirements of food production through aquaculture, economically viablefeeds of good quality are necessary (FAO, 2012). Fishmeal is being used as a major component in the formulation of domestic livestock and aquaculture diets and also serves as a taste attractant for herbivorous and omnivorous fish species (Davis and Arnold, 2000; FAO, 2007). In the previous three decades fish meal prices have increased in real terms and are expected to be increased further with continuous growth in demand (FAO, 2016). Due to the limited supply and increased cost of fishmeal, aquaculture feed industry and research institutions have conducted a large number of studies to reduce the dependency of the aquaculture industry on fishmeal (Rana et al., 2009; Tacon et al., 2006). In order to obtain economically sustainable, environment friendly and viable production researchers are evaluating unconventional protein sources predominantly from plant products such as leaves, seeds and other agricultural byproducts due to their high protein contents (Richter et al., 2003; Abo-State et al., 2014).
Moringa oleifera plant is one of the potential plant protein sources for inclusion in aquaculture diets (Chuks et al., 2013). The leaves and pods of plant contain high profile minerals like magnesium (Mg), zinc (Zn), phosphorus (P), manganese (Mn), calcium (Ca) in trace amount, and are a good source of vitamins, amino acids, protein, beta-carotene and various phenolics (Majhi, 2013). Moringa kernel and the fat free kernel meals have 36.7% and 61.4% of crude protein, respectively.
These plant ingredients contain anti-nutritional compounds which are very bitter in taste and result in their poor acceptability to fish (Francis et al., 2001). Phytic acid chelates with minerals in plant seeds (Jorquera et al., 2008) which practically become non-available for agastric and monogastric fishes (Baruah et al., 2007). Phytate forms mineral-phytate complexes leading to reduced mineral bioavailability from the digestive tract and an adverse impact on carcass composition and retention of nutrients (Greiner and Konietzny, 2006).
The problem can be solved by supplementing organic acids in plant-based diets (Reda et al., 2016; Hussain et al., 2017). Citric acid (CA) is one of the organic acids with high buffering capacity and unique flavor, which has been widely used in diets of fish (Hossain et al., 2007). It also increases the efficacy of exogenous as well as endogenous phytases by providing an optimum gut pH. Besides, it acts as an antimicrobial agent and stimulates feeding in fish (Shah et al., 2015a). Significantly higher mineral absorption has been revealed in broiler chicks (Boling-Frankenbach et al., 2011), L. rohita fingerlings (Baruah et al., 2007) and C. mrigala fingerlings (Hussain et al., 2018) fed CA acidified diets. The present study was designed to study the impact of CA supplementation in Moringa oleifera seed meal (MOSM) based diets on minerals absorption, carcass composition and hematological indices of C. mrigala fingerlings.
Materials and methods
Procurement of fish and experimental conditions
C. mrigala fingerlings were procured from Government Fish Seed Hatchery, Faisalabad. Before the start of experiment, fingerlings were bathed in NaCl (5g/L) solution for specific time period to disinfect them. V-shape like water tanks were designed especially for the collection of fish fecal material. Fingerlings were acclimatized in these tanks for two weeks during which they were fed basal diet once in a day to apparent satiation (Allan and Rowland, 1992). Water quality parameters like pH, dissolved oxygen and temperature were recorded on daily basis. Tap water was used throughout the experiment.
Processing of feed ingredients and experimental diets
Feed ingredients were purchased from local commercial feed market. Before the formulation of the experimental diets standard methods (AOAC, 1995) were used to analyze ingredient chemical composition (Table I). After fine grinding, feed ingredients were passed through (0.5mm) mesh size, mixed in a food-mixer for 5 min and fish oil was added gradually. One control diet (0% CA) and five test diets with 1%, 2%, 3%, 4% and 5% CA were prepared, respectively, using MOSM as main test ingredient. Diets were blended with water in food-mixer to form suitable dough and subsequently pellets (Lovell, 1989).
Plan of feeding and sample collection
Ten fingerlings of C. mrigala stocked in each tank were fed first at 8:00 am and then at 2:00 pm daily on their prescribed diet as of 5 % live wet body weight. The whole experiment was triplicated. After the completion of two hours feeding session, the unutilized diet was collected through the valves from each tank. The tanks were washed thoroughly to remove remaining diet particles and then water was refilled in each tank. Three hours after tanks washing, fecal material was collected through valves of fecal collection pipes. Total of 5 g fecal material was collected from each tank until the completion of 90 days feeding period. Breakdown of fecal strings was minimized by extreme care during fecal collection to avoid nutrient
Table I.- Ingredients composition (%) of test diets.
|
Ingredients |
Test diet-I (control) |
Test diet-II |
Test diet-III |
Test diet-IV |
Test diet-V |
Test diet-VI |
|
MOSM |
35 |
35 |
35 |
35 |
35 |
35 |
|
Fish meal |
15 |
15 |
15 |
15 |
15 |
15 |
|
Soybean meal |
15 |
15 |
15 |
15 |
15 |
15 |
|
Wheat flour |
17 |
16 |
15 |
14 |
13 |
12 |
|
Rice polish |
8 |
8 |
8 |
8 |
8 |
8 |
|
Fish oil |
6 |
6 |
6 |
6 |
6 |
6 |
|
Vitamin premix |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
|
Mineral premix |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
|
Ascorbic acid |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
|
Chromic oxide |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
|
Citric acid level |
0 % |
1 % |
2 % |
3 % |
4 % |
5 % |
|
Total |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100 |
MOSM, Moringa oleifera seed meal; Test diet-I, with 0% CA; Test diet-II-VI, with 1%, 2%, 3%, 4% and 5% CA.
Table II.- Analyzed minerals composition in MOSM based diets.
|
Diets/ Minerals |
Citric acid levels |
PSE |
P |
|||||
|
Test diet-I (control) |
Test diet-II |
Test diet-III |
Test diet-IV |
Test diet-V |
Test diet-VI |
|||
|
Ca |
0.87 |
0.88 |
0.89 |
0.87 |
0.88 |
0.88 |
0.0384 |
0.9994 |
|
Na |
0.0085 |
0.0085 |
0.0084 |
0.0085 |
0.0086 |
0.0084 |
0.0367 |
0.9952 |
|
K |
1.4 |
1.39 |
1.4 |
1.39 |
1.4 |
1.39 |
0.0267 |
0.9976 |
|
P |
2.02 |
2.03 |
2.01 |
2.02 |
2.01 |
2.02 |
0.0274 |
0.9981 |
|
Fe |
0.046 |
0.047 |
0.047 |
0.048 |
0.047 |
0.048 |
0.0018 |
0.9735 |
|
Cu |
0.0054 |
0.0056 |
0.0055 |
0.0055 |
0.0055 |
0.0056 |
0.0002 |
0.9954 |
|
Zn |
0.042 |
0.041 |
0.042 |
0.042 |
0.043 |
0.043 |
0.0022 |
0.9912 |
|
Mn |
0.024 |
0.024 |
0.025 |
0.025 |
0.024 |
0.025 |
0.0025 |
0.9964 |
|
Mg |
0.0093 |
0.0092 |
0.0093 |
0.0094 |
0.0093 |
0.0094 |
0.0003 |
0.9973 |
|
Cr |
0.028 |
0.028 |
0.027 |
0.028 |
0.027 |
0.027 |
0.0021 |
0.9929 |
|
Al |
0.00064 |
0.00063 |
0.00062 |
0.00063 |
0.00063 |
0.00062 |
2.24024 |
0.9857 |
For diet treatment, see Table I.
leaching. For chemical analysis, fecal material was oven dried at 60°C, grinded completely and stored in laboratory.
Chemical analysis of feed and feces
Analyzed minerals composition in MOSM based diets is presented in Table II. Feed ingredients, experimental diets and feces samples were homogenized separately by motor and pestle and standard procedures (AOAC, 1995) were applied for analysis. For minerals estimation, diets and feces samples were digested separately in a perchloric acid and boiling nitric acid (1:2) mixture (AOAC, 1995). Distilled water used for appropriate dilution of samples and minerals contents were estimated by atomic absorption (Hitachi Polarized Atomic Absorption Spectrometer, Z-8200). Calibrated standards were prepared using commercially available standards (AppliChem® Gmbh Ottoweg4, DE-64291 Darmstadt, Germany) to estimate mineral contents. Phosphorus content was estimated calorimetrically (UV/VIS spectrophotometer) at 350nm. Sodium and potassium were estimated using Flame photometer (Jenway PFP-7, UK). Analyzed minerals composition in MOSM based diets is presented in Table II.
Estimation of chromic oxide
Chromic oxide in test diets was added as an inert marker to determine minerals absorption. After experimental diets and feces ash samples oxidation with perchloric reagent, acid digestion method (Divakaran et al., 2002) through UV-VIS 2001 spectrophotometer at 350 nm was used to estimate chromic oxide content.
Digestibility calculation
Standard formula (NRC, 1993) was used to determine ADC% (apparent minerals digestibility coefficients) for test diets.

Carcass composition
Three fishes were randomly selected from each tank after the completion of experiment for proximate composition of whole fish body. Fish samples were thoroughly homogenized by mortar and pestle and analyzed by standard methods (AOAC, 1995). Samples were oven-dried at 105oC for 12 h to determine moisture contents. Crude protein (N × 6.25) was determined by micro kjeldahl apparatus whereas crude fat was determined by petroleum ether extraction method (Soxtec HT2 1045 system). Crude fiber was determined as loss on ignition of dried lipid-free residues after digestion with 1.25% H2SO4 and 1.25% NaOH, whereas ash was determined by sample ignition at 650oC for 12 h in electric furnace (Eyela-TMF 3100). Total carbohydrates were determined by using following formula: Total carbohydrate % = 100 - (crude protein % + crude fat % + crude fiber % + ash % + moisture %).
Hematological analysis
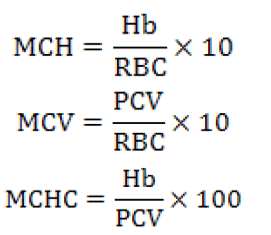
After the completion of 90 days experimental period fishes were tranquilized with 150 mg/1 tricane methanesulfonate solution (Wagner et al., 1997) for the collection of blood samples. The samples were sent to Molcare Lab, Biochemistry Department, University of Agriculture, Faisalabad for hematological analysis. Blood RBCs and WBCc were determined with a haemocytometer with improved Neubauer counting chamber (Blaxhall and Daisley, 1973) while Hb concentrations were estimated following Wedemeyer and Yastuke (1977). Haematocrit (PCV) was estimated by the Wintrobe and Westergreen method using micro haematocrit centrifuge (Blaxhall and Daisley, 1973) and heparinized capillary tubes of 25 mm. The MCH, MCV and MCHC were also calculated by following formulae:
Statistical analysis
At the end, one-way analysis of variance (Steel et al., 1996) was applied to analyze minerals absorption, carcass composition and hematological parameters. Differences among treatments were compared using Tukey’s Honesty Significant Difference Test and considered significant at p<0.05 (Snedecor and Cochran, 1991). Statistical analysis was completed by Co-Stat Computer Package (version 6.303, PMB 320, Monterey, CA, 93940 USA).
Results
The composition (%) of minerals in feces of C. mrigala fingerlings fed MOSM based diets is presented in Table III. The data shows that significantly (p˂0.05) higher concentrations of mineral were excreted through feces by fingerlings fed the control diet. Mineral absorption (%) by C. mrigala fingerlings fed MOSM based diets is presented in Table IV. Significant improvement in mineral absorption was observed by the addition of CA in MOSM based diets. The data shows that various minerals were absorbed
Table III.- Analyzed composition (%) of minerals in feces of C. mrigala fingerlings fed citric acid acidified MOSM based diets.
|
Diets |
Citric acid levels (%) |
PSE |
P |
|||||
|
Test diet-I (control) |
Test diet-II |
Test diet-III |
Test diet-IV |
Test diet-V |
Test diet-VI |
|||
|
Ca |
0.48a |
0.46a |
0.31b |
0.31b |
0.35b |
0.46a |
0.016 |
0 |
|
Na |
0.0048a |
0.0050a |
0.0034b |
0.0030b |
0.0034b |
0.0049a |
0.00016 |
0 |
|
K |
0.75a |
0.72a |
0.54b |
0.48b |
0.53b |
0.71a |
0.016 |
0 |
|
P |
1.11a |
0.91b |
0.70c |
0.68c |
0.70c |
0.93b |
0.017 |
0 |
|
Fe |
0.023ab |
0.025a |
0.018bc |
0.019bc |
0.018c |
0.024a |
0.001 |
0.0003 |
|
Cu |
0.0027a |
0.0025ab |
0.0022ab |
0.0020b |
0.0020b |
0.0023ab |
0.00012 |
0.0098 |
|
Zn |
0.022a |
0.023a |
0.020b |
0.020b |
0.019b |
0.023a |
0.001 |
0.0423 |
|
Mn |
0.013a |
0.012a |
0.009b |
0.009b |
0.010b |
0.013a |
0.0011 |
0.0624 |
|
Mg |
0.0044a |
0.0044a |
0.0036b |
0.0035b |
0.0041ab |
0.0046a |
0.00013 |
0.0002 |
|
Cr |
0.016a |
0.015a |
0.014a |
0.015a |
0.014a |
0.014a |
1.34E- |
0.001 |
|
Al |
0.00041a |
0.00034b |
0.00030c |
0.00029c |
0.00029c |
0.00033b |
0.00116 |
0.0005 |
For diet treatment, see Table I.
Table IV.- Minerals absorption (%) by C. mrigala fingerlings fed citric acid acidified MOSM based diets.
|
Diets |
Citric acid levels (%) |
PSE |
P |
|||||
|
Test diet-I (control) |
Test diet-II |
Test diet-III |
Test diet-IV |
Test diet-V |
Test diet-VI |
|||
|
Ca |
49.44d |
52.34c |
67.24a |
66.92a |
61.46b |
51.72cd |
0.4995 |
0 |
|
Na |
47.37c |
45.84c |
62.26b |
67.45a |
62.30b |
46.53c |
0.5257 |
0 |
|
K |
50.44d |
52.43cd |
64.33b |
67.62a |
63.73b |
53.42c |
0.533 |
0 |
|
P |
49.41d |
58.79c |
67.51b |
68.52a |
66.78b |
57.66c |
0.4706 |
0 |
|
Fe |
54.64b |
51.43c |
64.03a |
63.30a |
63.95a |
53.11bc |
0.5544 |
0 |
|
Cu |
53.54e |
58.47d |
62.52bc |
65.24a |
64.50ab |
61.78c |
0.456 |
0 |
|
Zn |
51.69c |
49.36d |
56.41b |
55.93b |
57.61a |
50.31cd |
0.4414 |
0 |
|
Mn |
50.34d |
53.43c |
67.57a |
66.85a |
60.68b |
54.12c |
0.5137 |
0 |
|
Mg |
55.71c |
56.38bc |
64.42a |
65.27a |
58.41b |
55.07c |
0.48067 |
0 |
|
Cr |
49.45cd |
48.74d |
52.23ab |
51.46abc |
53.14a |
50.43bcd |
0.4725 |
0 |
|
Al |
41.13c |
50.69b |
54.31a |
56.51a |
55.52a |
51.50b |
0.5447 |
0 |
For diet treatment, see Table I.
Table V.- Carcass proximate composition (%) of C. mrigala fingerlings fed citric acid acidified MOSM based diets.
|
Diets |
Citric acid levels (%) |
PSE |
P |
|||||
|
Test diet-I (control) |
Test diet-II |
Test diet-III |
Test diet-IV |
Test diet-V |
Test diet-VI |
|||
|
Crude protein |
54.75d |
56.00c |
60.54a |
58.70b |
58.34b |
56.62c |
0.15952 |
0 |
|
Crude fat |
9.23d |
11.47c |
13.01b |
13.52a |
12.82b |
11.73c |
0.17325 |
0 |
|
Ash |
9.34a |
9.63a |
8.26bc |
7.61c |
8.42b |
9.44a |
0.15539 |
0 |
|
Moisture |
7.10a |
6.42b |
6.14b |
5.17c |
5.46c |
6.40b |
0.12885 |
0 |
|
Crude fiber |
1.26a |
1.18b |
1.06c |
1.02c |
1.19b |
1.24a |
0.06726 |
0.0032 |
|
Carbohydrate |
18.32a |
15.31b |
10.98c |
13.99b |
13.77b |
14.57b |
0.3409 |
0 |
For diet treatment, see Table I.
Table VI.- Hematological indices of C. mrigala fingerlings fed citric acid acidified MOSM based test diets.
|
Diets |
Citric acid levels (%) |
PSE |
P |
|||||
|
Test diet-I (control) |
Test diet-II |
Test diet-III |
Test diet-IV |
Test diet-V |
Test diet-VI |
|||
|
RBC (106mm-3) |
1.25d |
1.57c |
2.65ab |
2.83a |
2.54b |
1.63c |
0.04831 |
0 |
|
WBC (103mm-3) |
6.73d |
7.12c |
7.71a |
7.76a |
7.49ab |
7.55b |
0.06075 |
0 |
|
PLT |
54.36d |
60.76c |
63.76b |
65.96a |
63.52b |
60.34c |
0.09712 |
0 |
|
Hb (g/100ml) |
6.35d |
6.38d |
7.23c |
8.47a |
8.13b |
7.32c |
0.06269 |
0 |
|
PCV (%) |
21.44d |
22.24c |
23.60b |
24.51a |
23.45b |
22.66c |
0.16374 |
0 |
|
MCHC (%) |
25.99e |
27.70d |
32.28c |
33.81b |
34.93a |
32.12c |
0.17407 |
0 |
|
MCH (pg) |
38.64d |
38.86d |
42.01c |
49.97b |
56.84a |
50.02b |
0.10383 |
0 |
|
MCV (fl) |
92.26f |
103.99e |
184.87b |
187.11a |
183.01c |
173.69d |
0.16843 |
0 |
Data are means of three replicates. PSE =pooled SE = √MSE/n (where MSE=mean-squared error). WBC, white blood cell; RBC, red blood cell; PCV, packed cell volume; Hb, hemoglobin concentration; PLT, platelet; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration.
For diet treatment, see Table I.
significantly (p˂0.05) better at 2%, 3% or 4% CA levels. However, significantly lower mineral absorption was observed at 1% and 5% CA levels compared to other CA levels. Hence the results indicated that by increasing CA % in MOSM based diets, absorption of minerals by C. mrigala fingerlings also increased. Maximum minerals were absorbed at 2%, 3% or 4% CA levels while absorption of minerals decreased with further increase in CA %.
Carcass proximate composition (%) of C. mrigala fingerlings fed MOSM based diets is presented in Table V. Inclusion of CA in MOSM based diets caused significant variation in body proximate composition of C. mrigala among different treatments. Maximum body crude protein (60.54%) and crude fat (13.52%) contents were observed in C. mrigala fed 2% and 3% CA supplemented diets, respectively. Whereas, C. mrigala fed a 3% CA supplemented diet showed minimum ash, moisture and crude fiber in their body proximate composition. The results revealed maximum retention of crude protein and crude fat by C. mrigala at 2% and 3% CA levels, respectively in MOSM based diets.
Fingerlings of C. mrigala fed CA acidified MOSM based diets showed significant improvement (p<0.05) in hematological parameters compared to the control diet (Table VI). Comparison of means showed that maximum values of RBCs (2.83×106 mm-3), WBCs (7.76×103 mm-3), PLT (65.96), Hb (8.47 g/100ml), PCV (24.51%), and MCV (187.11 fl) were observed in fingerlings fed 3% CA acidified MOSM based diets. However maximum values of MCHC (34.93%), MCH (56.84 pg) were observed in fingerlings fed a 4% CA acidified MOSM based diet. Minimum values of above said hematological parameters were observed in fingerlings fed the control diet.
Discussion
Phytate present in plant feed ingredient chelates with minerals and makes them unavailable to fish by reducing their availability and absorption (Hussain et al., 2011a). Dietary CA inclusion in plant based diets enhances the activities of intestinal digestive enzymes (Shah et al., 2015b) which helps in releasing minerals from the phytic acid complex (Baruah et al., 2007) and thus enhances dietary minerals absorption by fish (Sarker et al., 2005). The results of present study also revealed that C. mrigala fingerlings fed MOSM based diets supplemented with CA excreted significantly lower minerals through feces compared to control diets. Supplementation of CA in MOSM based diets significantly enhanced Ca, Na, K, P, Fe, Cu, Zn, Mn, Mg, Al and Cr absorption by C. mrigala fingerlings compared to control diets. These results coincide with the findings of Rabia et al. (2017) who reported improved absorption of P, Na, K, Ca, Mg, Cu, Zn, Mn and Fe in the body of fish fed CA supplemented diets as compared to control diet. Highest absorption of Ca (67.24 %), Fe (64.03 %) and Mn (67.57 %) was observed at 2% CA level. Whereas significantly higher (p<0.05) absorption of Na, K, P, Cu, Mg and Al was observed at 3% CA level. In agreement to our results Baruah et al. (2005) also reported significantly better mineral absorption by L. rohita fingerlings fed 3% CA acidified diet. Baruah et al. (2007) reported that dietary CA supplementation at 3 % significantly enhanced the absorption of Na, K, P, Fe, Mg, Mn, Ca, N and Cu. Khajepour and Hosseini (2010, 2011 and 2012) reported significant increase in Ca and P content of muscle and serum when fed 2% or 3% CA supplemented diets. Hisano et al. (2017) also reported relative improvement in Ca and P in Pacu juveniles fed 3% CA acidified diet compared to control diet. In contrary to our results Sarker et al. (2007) reported that supplementation of 1% CA was adequate in retention of nutrient and keeping aquatic loading levels low. Moreover, Zhu et al. (2015) reported no significant impact of CA on minerals absorption by juvenile yellow cat fish. However, absorption of particular nutrient may be species specific and also depend on the feed ingredients used. This area needs further research (Baruah et al., 2007).
Fish flesh is considered a better protein source than eggs, milk, cereals and other animal proteins because of balanced fatty acid and amino acid profiles along with essential minerals (Hussain et al., 2011b). Through proximate analysis scientist monitor the health and physiological condition of fish (Saliu et al., 2007; Aberoumad and Pourshafi, 2010). Results of present study revealed significant improvement in body proximate composition of C. mrigala fingerlings fed CA supplemented MOSM based diets compared to control diet. By the addition of CA, crude protein and crude fat contents increased while moisture, ash, crude fiber and carbohydrate contents decreased. In agreement to present study Reda et al. (2016) also reported significant improvement in carcass composition of Nile tilapia fed acidified diet. Nuez-Ortin (2011) reported that Nile tilapia retained significantly higher protein and fat when fed acidified diets. In contrary to our results, Sarker et al. (2007) and Zhu et al. (2015) reported no significant effect of CA on body proximate composition of red sea bream Pagrus major and yellow catfish Pelteobagrus fulvidraco, respectively. This contradiction in results of various scientists may be due to the different feed composition, method of feed formulation, ecological variables and species difference and therefore needs further exploration.
Hematological studies are least studied in fish, necessary to access fish health and to check the quality of formulated diets (Schutt et al., 1997; Shahzad et al., 2016). The results of present study revealed no adverse effect of CA acidified MOSM based diets on hematological indices of C. mrigala fingerlings. Acidification of MOSM based diets with CA improved growth and nutrients availability to fish from diet which in turn also improved hematological indices. The results showed the safe use of CA in the diet of C. mrigala fingerlings to improve body status. The improvement in hematological indices may be attributed to the liberation of, Fe P, Ca and Cu from MOSM based diets by acid supplementation (Khajepour and Hosseini, 2010, 2012). The results are in agreement with Kubena (1996) and Baruah et al. (2009) who also reported positive impact of nutrients availability on fish hematology and immune system although no effect of CA on RBCs count was observed (Baruah et al., 2009). Whereas, positive effect of dietary acidification on fish blood WBCs, RBCs, platelets, Hb, MCV and MCH counts is also reported by Reda et al. (2016). Most of the hematological indices of C. mrigala fingerlings showed significant improvement at 3% CA level. In agreement to our study Baruah et al. (2009) and Khajepour et al. (2011) also reported significantly (P <0.001) improved blood Hct and Hb in fish fed 3% CA added diet.
Conclusion
In conclusion CA supplementation in MOSM based diet caused significant improvement in overall minerals absorption, carcass proximate composition and hematological indices of C. mrigala fingerlings. These parameters showed significantly better improvement at 3% CA level. Hence, 3% CA acidified MOSM based diet is recommended for better minerals absorption and carcass proximate composition of C. mrigala fingerlings without any negative impact on fish hematological indices.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Aberoumad, A. and Pourshafi, A., 2010. Chemical and proximate composition properties of different fish species obtained from Iran. World J. Fish. Mar. Sci., 2: 237-239.
Abo-state, H., Hammouda, Y., El-nadi, A. and Abozaid, H., 2014. Evaluation of feeding raw moringa (Moringa oleifera Lam.) leaves meal in Nile tilapia fingerlings (Oreochromis niloticus) diets. Glob. Vet., 13: 105-111.
Allan, G.L. and Rowland, S.J., 1992. Development of an experimental diet for silver perch (Bidyanusbidyanus). Austasia Aquacult., 6: 39-40.
AOAC, 1995. Official methods of analysis, 15th ed. Association of Official Analytical Chemists, Washington, DC, pp. 1094.
Baruah, K., Pal, A.K., Sahu, N.P., Debnath, D. and Yengkokpam, S., 2009. Dietary Crude protein, citric acid and microbial phytase interacts to influence the hemato-immunological parameters of rohu, Labeo rohita, Juveniles. World Aquacul. Soc., 40: 824-831. https://doi.org/10.1111/j.1749-7345.2009.00304.x
Baruah, K., Pal, A.K., Sahu, N.P., Jain, K.K., Mukherjee, S.C. and Debnath, D., 2005. Dietary protein level, microbial phytase, citric acid and their interactions on bone mineralization of Labeo rohita (Hamilton) juveniles. Aquacul. Res., 36: 803-812. https://doi.org/10.1111/j.1365-2109.2005.01290.x
Baruah, K., Sahu, N.P., Pal, A.K., Debnath, D. and Yengkokpam, S.A., 2007. Interactions of dietary microbial phytase, citric acid and crude protein level on mineral utilization by rohu, Labeo rohita (Hamilton) juveniles. J. World Aquacul. Soc., 38: 238-249. https://doi.org/10.1111/j.1749-7345.2007.00092.x
Blaxhall, P.C. and Daisley, K.W., 1973. Routine haematological methods for use with fish blood. J. Fish Biol., 6: 771-781. https://doi.org/10.1111/j.1095-8649.1973.tb04510.x
Boling-Frankenbach, S.D., Snow, J.L., Parsons, C.M. and Baker, D.H., 2001. The effect of citric acid on the calcium and phosphorus requirements of chicks fed corn-soybean meal diets. Poult. Sci., 80: 783-788. https://doi.org/10.1093/ps/80.6.783
Chuks, P.E., Mgbenka, B.O. and Ezeonyejiaku, C.D., 2013. Moringa plant and its use as feed in aquaculture development: A review. Anim. Res. Int., 10: 1672-1680.
Davis, D.A. and Arnold, C.R., 2000. Replacement of fishmeal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 185: 291-298. https://doi.org/10.1016/S0044-8486(99)00354-3
Divakaran, S., Leonard, G.O. and Lan, P.F., 2002. Note on the methods for determination of chromic oxide in shrimp feeds. J. Agric. Fd. Chem., 50: 464-467. https://doi.org/10.1021/jf011112s
FAO, 2012. The state of world fisheries and aquaculture 2012. Rome, Italy, pp. 209.
FAO, 2016. The state of world fisheries and aquaculture 2016. Contributing to food security and nutrition for all. Rome, Italy, pp. 18.
FAO, 2007. FAO yearbook of fishery statistics. Aquacul. Prod., 100: 55-56.
Francis, G., Makkar, H.P.S. and Becker, K., 2001. Anti-nutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture, 199: 197-227. https://doi.org/10.1016/S0044-8486(01)00526-9
Greiner, R. and Konietzny, U., 2006. Phytase for food application. Fd. Technol. Biotechnol., 44: 125-140.
Hisano, H., Sanchez, M.S.S. and Nascimento, M.S., 2017. Citric acid as a feed additive in pacu Piaractus mesopotamicus (Holmberg, 1887) diets. J. appl. Ichthyol., 33: 478-484. https://doi.org/10.1111/jai.13289
Hossain, M.A., Pandey, A. and Satoh, S., 2007. Effects of organic acids on growth and phosphorus utilization in red sea bream Pagrus major. Fish Sci., 73: 1309-1317.
Hussain, S.M., Afzal, M., Rana, S.A., Javid, A. and Iqbal, M., 2011a. Effect of phytase supplementation on growth performance and nutrient digestibility of Labeo rohita fingerlings fed on corn gluten meal based diets. Int. J. Agric. Biol., 13: 916-922.
Hussain, B., Mahboob, S., Hassan, M., Liaqat, F., Sultana, T. and Tariq, H., 2011b. Comparative analysis of proximate composition of head from wild and farmed Catla catla. J. Anim. Pl. Sci., 21: 207-210.
Hussain, M., Hussain, S.M., Iqbal, R., Javid, A., Shahzad, M.M., Arsalan, M.Z. and Riaz, D., 2017. Effect of citric acid acidified Moringa oleifera seed meal based diet on nutrients digestibility and growth performance of Cirrhinus mrigala fingerlings. Int. J. Agric. Biol., 19: 719-725. https://doi.org/10.17957/IJAB/15.0346
Hussain, S.M., Ahmad, N., Javid, A., Shahzad, M.M., Hussain, M. and Arsalan, M.Z.H., 2018. Effects of phytase and citric acid supplemented corn gluten (30%) meal based diets on the mineral digestibility of Cirrhinus mrigala fingerlings. Turk. J. Fish. aquat. Sci., 18: 501-507.
Jorquera, M., Martinez, O., Maruyama, F., Marschiner, P. and Mora, D.L., 2008. Current and future biotechnology applications of bacterial phytases and phytase-producing bacteria. Microbes Environ., 23: 182-191. https://doi.org/10.1264/jsme2.23.182
Khajepour, F. and Hosseini, S.A., 2010. Mineral status of juvenile beluga (Huso huso) fed citric acid supplemented diets. World appl. Sci. J., 11: 682-686.
Khajepour, F. and Hosseini, S.A., 2011. Effect of dietary citric acid supplementation and partial replacement of dietary fish meal with soybean meal on calcium and phosphorus of muscle, scute and serum of Beluga, Huso huso. Afr. J. Biotechnol., 10: 14652-14655. https://doi.org/10.5897/AJB11.1312
Khajepour, F. and Hosseini, S.A., 2012. Calcium and phosphorus status in juvenile Beluga (Huso huso) fed citric acid-supplemented diets. Aquacul. Res., 43: 407-411. https://doi.org/10.1111/j.1365-2109.2011.02843.x
Khajepour, F., Hosseini, S.A. and Hoseini, S.M., 2011. Study on some hematological and biochemical parameters of juvenile beluga (Huso huso) fed citric acid supplemented diet. Glob. Vet., 7: 361-364.
Kubena, K.S. and Mcmurray, D.N., 1996. Nutrition and the immune system: a review of nutrient-nutrient interactions. J. Am. Diet. Assoc., 96: 1156-1164. https://doi.org/10.1016/S0002-8223(96)00297-0
Lovell, R.T., 1989. Fish nutrition and feeding. Van Nostrand Reinhold Co., New York. https://doi.org/10.1007/978-1-4757-1174-5
Majhi, S., 2013. Nutritional value of Moringa oleifera as a dietary supplement. Thesis submitted in partial fulfillment of the requirements for the degree of Master of Pharmacy, Department of Pharmaceutical Technology Jadavpur University, Kolkata, 700032 India.
NRC, 1993. Nutrient requirements of fish. National Research Council, National Academies Press, Washington, pp. 114.
Nuez-Ortin, W., 2011. Gustor-Aqua: An effective solution to optimize health status and nutrient. Int. Aquafeed, 2011: 18-20.
Rabia, S., Afzal, M. and Shah, S.Z.H., 2017. Nutrient digestibility performance by rohu (Labeo rohita) juveniles fed acidified and phytase pre-treated sunflower meal-based diet. J. appl. Anim. Res., 45: 331-335. https://doi.org/10.1080/09712119.2016.1190731
Rana, K.J., Siriwardena, S. and Hasan, M.R., 2009. Impact of rising feed ingredient prices on aquafeeds and aquaculture production. In: FAO fisheries and aquaculture technical paper, Vol. 541. FAO, Rome, Italy.
Rauf, A., 2015. Acute toxicity and effects of malathion exposure on behavior and hematological indices in Indian carp, Cirrhinus mrigala (Hamilton). Int. J. aquat. Biol., 3: 199-207.
Reda, R.M., Mahmoud, R., Selim, K.M. and El-Araby, I.E., 2016. Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellf. Immunol., 50: 255-262. https://doi.org/10.1016/j.fsi.2016.01.040
Richter, N., Siddhuraju, P. and Becker, K., 2003. Evaluation of nutritional quality of moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.). Aquaculture, 217: 599-611. https://doi.org/10.1016/S0044-8486(02)00497-0
Saliu, J.K., Joy, O. and Catherine, O., 2007. Condition factor, fat and protein content of five fish species in Lekki Lagoon, Nigeria. Life Sci., 4: 54-57.
Sarker, S.A., Satoh, S. and Kiron, V., 2005. Supplementation of citric acid and amino acid-chelated trace element to develop environment-friendly feed for red sea bream, Pagrus major. Aquaculture, 248: 3-11. https://doi.org/10.1016/j.aquaculture.2005.04.012
Sarker, S.A., Satoh, S. and Kiron, V., 2007. Inclusion of citric acid and/or amino acid-chelated trace elements in alternate plant protein source diets affects growth and excretion of nitrogen and phosphorus in red sea bream Pagrus major. Aquaculture, 262: 436-443. https://doi.org/10.1016/j.aquaculture.2006.10.007
Schutt, D.A., Lehmann, J., Goerlich, R. and Hamers, R., 1997. Haematology of swordtail, Xiphophorus helleri. I: Blood parameters and light microscopy of blood cells. J. appl. Ichthyol., 13: 83-89. https://doi.org/10.1111/j.1439-0426.1997.tb00106.x
Shah, S.Z.H., Afzal, M., Khan, S.Y., Hussain, S.M. and Habib, R.Z., 2015a. Prospects of using citric acid as fish feed supplement. Int. J. Agric. Biol., 17: 1-8.
Shah, Z.H., Afzal, M., Hussain, S.M., Fatima, M., Bilal, M., Ahmed, T. and Habib, R.Z., 2015b. Supplementation of citric acid and phytase improves the digestive enzymes activities in Labeo rohita fingerlings. Biologia, 61: 63-68.
Shahzad, M.M., Hussain, S.M., Jabeen, F., Hussain, A.I., Arsalan, M.Z., Ahmad, N., Rehan, M.M. and Riaz, D., 2016. Carcass composition and hematological study of Catla catla fingerlings fed on phytase supplemented Moringa oleifera leaf meal (MOLM) based diet. J. appl. environ. Biol. Sci., 9: 57-68.
Snedecor, G.W. and Cochran, W.G., 1991. Statistical methods, 8th Ed. Iowa State University Press, Americans, USA, pp. 503.
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1996. Principles and procedures of statistics, 3rd Ed. McGraw Hill International Book Co. Inc., New York, USA, pp. 336-352.
Tacon, A.G.J., Hasan, M.R. and Subasinghe, R.P., 2006. Use of fishery resources as feed inputs to aquaculture development: Trends and policy implications. In: FAO fisheries circular, Vol. 1018. FAO, Rome, Italy.
Wagner, E.J., Jensen, T., Arndt, R., Routledge, M.D. and Brodwich, Q., 1997. Effects of rearing density upon cutthroat hematology, hatchery performance, fin erosion and general health and condition. Prog. Fish-Cult., 59: 73-187. https://doi.org/10.1577/1548-8640(1997)059<0173:EORDUC>2.3.CO;2
Wedemeyer, G.A. and Yastuke, W.T., 1977. Clinical methods for the assessment of the effects of environmental stress on fish health. U.S. Fish and Wildlife Service, pp. 89.
Zhu, Y., Ding, Q., Chan, J., Chena, P. and Wang, C., 2015. The effects of concurrent supplementation of dietary phytase, citric acid and vitamin D3 on growth and mineral utilization in juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture, 436: 143-150. https://doi.org/10.1016/j.aquaculture.2014.11.006
To share on other social networks, click on any share button. What are these?










