Effect of Phytase Supplemented Diet on Whole Body Proximate Composition of Labeo rohita Fingerlings
Effect of Phytase Supplemented Diet on Whole Body Proximate Composition of Labeo rohita Fingerlings
Nuzhat Naseem, Sajid Abdullah and Sana Aziz*
Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad
ABSTRACT
Phytate, bound form of phosphorus and some other minerals, is present in plant products and it cannot be consumed by fish because there is no phytase activity in agestric fish. This study was performed to determine the impact on proximate composition of whole-body of Labeo rohita (fingerlings) fed distiller’s dried grains with solubles based diet, supplemented with phytase. Six different feeds were formulated by supplementing different concentrations of phytase. i.e. D1 without phytase supplementation, D2, D3, D4, D5 and D6 with 250, 500, 750, 1000 and 1250 FTU phytase per kilogram of diet, respectively. An experiment was conducted for 2 months. During this experiment, water quality parameters were also examined e.g pH, Dissolved oxygen and temprature. Results were examined by Tukey’s Honestly Significant Difference Test. Phytase supplementation decreased the moisture content and increased the crude ash, protein and fat significantly in proximate composition of whole-body of the fish and hence, improved the meat quality of Labeo rohita fingerlings.
Article Information
Received 28 September 2020
Revised 14 October 2020
Accepted 04 December 2020
Available online 06 July 2021
(early access)
Published 07 March 2022
Authors’ Contribution
NN executed the research. SA supervisor and planned the research. SA helped in compiling data and in writing the article.
Key words
Phytase, Fish, Proximate composition, Fingerling, Suplementation
DOI: https://dx.doi.org/10.17582/journal.pjz/20200928150918
* Corresponding author: sana.aziz1994@gmail.com
0030-9923/2022/0003-1479 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Aquaculture includes the rearing or culturing of aquatic organisms such as fish, crustaceans and molluscs. For most of the aquatic species, a major portion (60%) of the farm production cost usually goes to aquaculture diet (Cheng and Hardy, 2004). Considering the fish, aquaculture proves to be one of the most rapidly developing food industries in the world (Beveridge, 1984; Suguira et al., 1999). The main objective of aquaculture is to enhance the production of some required fish species to have maximum economic benefits (Goldburg and Triplett, 1997).
In aquafeeds, fishmeal is considered as one of the most important protein sources because of its balanced amino acid content and some important growth factors absent in plant based protein sources (Kousoulaki et al., 2009). Energy, minerals and essential fatty acids are also abundant in it (Webster et al., 1995). Anyhow, increasing requirement of fish meal leads to its reduced supply and high cost. So, to overcome these problems, other protein sources are explored for aquaculture (NRC, 1981). Thus, cost effective proteins are obtained from the plant source and considered to be the best alternate to fish meal (Gaylord et al., 2006).
Many sources of proteins are used for aquaculture but Distiller’s dried grain with solubles (DDGS) is considered as a cost effective source of protein for aquaculture diet comparative to fish meal (Bothast and Schlicher, 2005). It is collected as a by-product in the production of ethanol during the process of fermentation (Coyle et al., 2004; Wu et al., 1994).
DDGS contain lower phosphorus content as compared to animal based proteins i.e fish meal (Cheng and Hardy, 2004). Wheat distiller’s dried grain (WDG) is readily available, cost effective and poor in anti-nutritional factors, thus it can be used as a substitute in the aquaculture feed industry as a source of protein (Randall and Drew, 2010; Reveco, 2010). Supplementation of DDGS in the diet resulted in the maintenance of fish growth along with reduced feed cost.
Phytate, an anti-nutritional factor, is present in DDGS containing approximately 80% phosphorus bound with other minerals. Chemically, it is considered the free state of inositol hexa-kisphosphate and a polyanionic compound having six groups of phosphate that chelates with positive ions e.g., magnesium, iron, calcium, copper, potassium and zinc forming insoluble salts. Thus, digestion of the minerals in the fish are severely affected (NRC, 1993; Papatryphon et al., 1999).
This problem is resolved by supplementing phytase in fish diet formulations. It belongs to a group of enzymes called myo-inositol hexa-phosphate phosphohydrolase. Phytase is added to fish diet to hydrolyze bound minerals in phytate, found in plant source diets, thus increasing the availability of nutrients to fish. Chelation of several cations with phytate is also impeded by this (Ravindran et al., 1995). Similarly, plants based protein digestibility is also enhanced with supplementation of phytase (Baruah et al., 2007a). Addition of phytase in aqua feeds also leads to reduction in water pollution by retaining the phosphorus in fish body and thus reducing its excretion in feces (Baruah et al., 2004).
Labeo rohita is considered an important Indian major carp that contributes almost eighty-seven percent of total freshwater aquaculture (ICLARM, 2001). It is heterosexual and usually cultured in a polyculture system. It has become very important in aquaculture because of its high commercial value (Jhingran, 1991). It can survive well in freshwaters with an altitude less than 549. Adult rohu is considered as both bottom and column feeders (Motwani and Bose, 1957). The main purpose of this study was to find out the impact of phytase on proximate composition of whole-body of Labeo rohita (fingerlings) fed phytase supplemented DDGS based diet.
Materials and methods
Labeo rohita (fingerlings) with average weight 3.25 g were brought from the Fish Seed Hatchery, Faisalabad. Acclimatization was done for 14 days in new trial conditions until trial has been started that lasted for 2 months. Trial fish were kept in tanks (UA system) of 70L capacity. 108 fish (18 fish/tank) were distributed in all treatments. Basal diet (Table I) was given to fingerlings once a day throughout the study period (Allan and Rowland, 1992). During this study period, different parameters of water quality, especially temperature, pH and dissolved oxygen were examined. pH was controlled between 7.4-8.6 and DO and temperature was controlled at 6.2 mg/L and 26.7oC. A water pipeline system was used to provide proper aeration to all treatments during the feeding trial.
For feed ingredients and experimental feeds dietary components were obtained from the local fish feed market and their chemical composition examined according to (AOAC, 2000), before the experimental diet was prepared. Before incorporating in the test diet (Table II) these feed materials were crushed and sieved until required particle size was attained.
In electric mixer, dried feed components were blended for 10-20 min, with gradual addition of fish oil during constant mixing. 0.5% chromic oxide, an inert marker, was also added. Six experimental diets were formulated namely D1, D2, D3, D4, D5 and D6 by supplementation of graded levels of phytase to these dried, mixed food materials at 0, 250, 500, 750, 1000 and 1250 FTU/Kg of diet, respectively. Dough of each test diet was prepared by adding 10-15% water and then pellets were formed by extruder.
Experimental diets were given to fingerlings by two percent of their live wet body weight. Feeding plates were adjusted in the tanks to avoid the removal of feed particles through sieve plates. Then 18 fish were stocked in each tank and all experimental diets with their replicates were allotted to all these treatments randomly. They were fed for three h then valves of each tank were opened to drain out the remaining diet. Diet particles were completely removed by washing tanks, then water was filled again in all the tanks and fish were restocked.
Table I. Composition (%) of experimental diet.
|
Ingredient |
Percentage |
|
Wheat flour |
7 |
|
Soybean meal |
15 |
|
Corn DDGS |
53 |
|
Fishmeal |
15 |
|
Vitamin C |
1 |
|
Fish oil |
6 |
|
Vitamin premix* |
1 |
|
Mineral mixture** |
1 |
|
Chromic oxide |
0.5 |
|
Choline chloride |
0.5 |
|
Total |
100 |
* Vitamin premix per Kg contains: Vitamin (A),15 M.I.U; Vitamin (B6), 4000 mg; Vitamin (B12), 9000 mg; Vitamin (D3), 3 M.I.U.; Vitamin (K3), 4000 mg; Nicotinic acid, 25000 mg; Vitamin (E), 6000 IU; Vitamin (B2), 6000 mg; Vitamin (B1), 5000 mg; Folic acid, 750 m; Vitamin (C), 15000mg; Calcium pantothenate, 10000mg. **Mineral granules per Kg contains: Copper, 600mg; Selenium, 3mg; Magnesium, 55gm; Calcium,155gm; Iodine, 40mg; Zinc, 3000 mg; Phosphorous,135gm; Cobalt, 40mg; Iron, 1000 mg; Sodium, 45gm.
Table II. Chemical composition (%) of feed (Dry basis).
|
Diets |
Phytase (FTU/kg) |
Dry matter (%) |
Crude protein (%) |
Crude fat (%) |
|
D1 |
0 |
91.835 |
33.335 |
9.575 |
|
D2 |
250 |
92.15 |
32.93 |
10.355 |
|
D3 |
500 |
92.275 |
32.8 |
10.15 |
|
D4 |
750 |
92.08 |
32.76 |
10.31 |
|
D5 |
1000 |
92.065 |
33.105 |
10 |
|
D6 |
1250 |
92.025 |
32.945 |
9.65 |
Test diets and whole body samples were blended (by mortar and pestle) and then examined through standard Protocols (AOAC, 2000). To determine the dry matter, 1g sample was oven dried for 12h at 105°C and transferred to desiccator for 5 min, then final weight of sample is recorded. This process is repeated till the required weight is obtained. Weight loss was considered as the moisture content. Crude ash was determined by igniting sample at 65°C keeping it in electric furnace for 12 h to attain constant weight. Crude fat analysis was performed through ether extraction (Bligh and Dyer, 1995) using soxhlet system and crude protein analysis through micro kjeldahl method.
Dry matter, crude ash, fat and protein are then determined through following formulas:
Dry matter = 100 – Moisture contents %
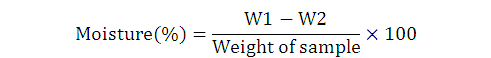
Where, W1 is weight of sample before drying+ Petri dish, and W2 is weight of sample after drying+ Petri dish.
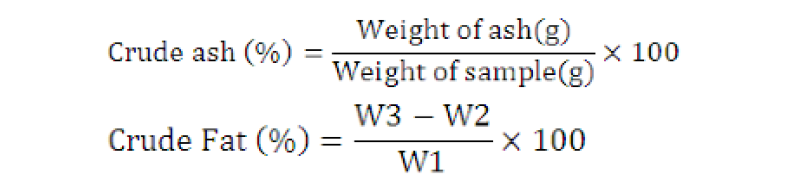
Where, W1 is weight of sample used (g), W2 is weight of blank extraction cup (g), and W3 is weight of residue + extraction cup (g).
% of Crude protein contents = N2 x 6.25
Where, 0.014 is standard volume of 0.1 N H2SO4 which is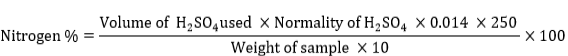 used to neutralize 1 ml of NH3, 250 is digestion mixture dilution, 100 is N2 percentage, and 10 is volume of diluted and digested sample.
used to neutralize 1 ml of NH3, 250 is digestion mixture dilution, 100 is N2 percentage, and 10 is volume of diluted and digested sample.
One-way analysis of variance was applied on resulted data of whole body proximate (Steel et al., 1996). For comparison of means, Tukey’s Honestly Significant Difference Test was performed and P<0.05 was considered significant (Snedecor and Conhran, 1991).
Results and discussion
In current study, we used DDGS as fish diet because DDGS has more protein and fat content and less anti-nutritional substances (phytate) found in most of the other plant protein sources. Thus phytase is supplemented to DDGS for the digestibility of phytate. Phytases are actually a group of enzymes, which are considered very important to hydrolyze phytate in plants (Vohra and Satyanarayana, 2003).
Addition of different levels of phytase affects the % of moisture contents, in whole-body proximate of Labeo rohita (Table III). Results (P<0.05) showed that phytase supplementation reduces the moisture in fingerling body of the L. rohita which implies that dry matter has increased in fish as compared to control diet and this increase in dry matter remained concentration dependent. Maximum decrease in moisture content was observed in D3 containing 500 FTU/kg phytase. Minimum decrease in moisture in the whole-body of the Labeo rohita was in the diet D6 with 1250 FTU phytase. The results of our study are similar to those of Cheng and Hardy (2004) and Debnath et al. (2005) in which dry matter of fish increased significantly by increasing its digestibility co-efficient and meat quality of fish is also improved.
Table III shows that supplementation of different phytase levels increases the crude protein level in the whole body of the Labeo rohita (fingerlings). Greatest increase was observed in D3 containing 500 FTU/kg phytase and the minimum increase was found in D2 with 250 FTU phytase/Kg of the diet. In the same line of our study, the same observations were made in rainbow trout which is fed with the feed supplemented with 500 FTU microbial phytase/Kg of diet and Rohu fed with 1000 FTU phytase studied by Cheng and Hardy (2002), Rabia et al. (2017), and Debnath et al. (2005).
Table III. Effect of different levels of phytase supplementation on moisture (%) in whole-body proximate of Labeo rohita (fingerlings).
|
Diet |
Phytase level (FTU/kg) |
Moisture (%) |
Crude protein (%) |
Crude fat (%) |
Crude ash |
|
D1 |
0 |
69.91a |
19.4e |
5.7b |
2.63c |
|
D2 |
250 |
68.82c |
19.69d |
5.2d |
3.12a |
|
D3 |
500 |
68.38d |
20.45a |
5.19d |
2.99ab |
|
D4 |
750 |
68.48d |
20.19b |
5.59c |
2.92b |
|
D5 |
1000 |
69.26b |
20.17b |
6.08a |
3.02ab |
|
D6 |
1250 |
69.27b |
19.9c |
6.07a |
3.12a |
|
Pooles SE |
0.03434 |
0.03379 |
0.02821 |
0.02769 |
|
|
ANOVA |
|
P-value |
P-value |
P-value |
P-value |
|
Phytase |
|
.0000*** |
.0000*** |
.0000*** |
.0001*** |
Values showing different superscripts in each row were statistically significant at p<0.05.
Pooled SE= √MSE/n (MSE, mean squared error; n, replications). Values values, means of two replications
Supplementation of different levels of phytase results in an increase in the crude fat and crude ash in proximate composition of Labeo rohita (fingerlings) (Table III). Highest increase in crude fat contents was found in D5 containing 1000 FTU phytase per kg of diet. Minimum increase in crude fat was detected in diet D3 with 500 FTU/kg phytase.
Highest increase of crude ash was in D2 and D6 containing 250 and 1250 FTU/kg phytase respectively. Minimum increase in crude ash was detected in diet D4 with 750 FTU phytase/Kg of diet. Cheng and Hardy (2004) fed the rainbow trout with DDGS based phytase supplemented diet and showed an increased digestibility co-efficient value of ash content which is actually inorganic matter in fish represented as minerals that comes to be 7.3-99.7%.
Conclusions
Supplementation of phytase significantly (p<0.05) decreased the moisture contents (%) and increased the crude protein (%), crude fat (%) and crude ash (%) in the whole body of L. rohita fingerlings. Phytase supplementation improved the meat quality of Labeo rohita fingerlings.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Allan, G.L. and Rowland, S.J., 1992. Austasia. Aquacult., 6: 39-40.
AOAC. 2000. Association of Official Analytical Chemists, Arlington, VA.
Baruah, K., Pal, A.K., Sahu N.P. and Debnath, D., 2004. World Fish Center Quarter, 27: 15-19.
Baruah, K., Pal, A.K., Sahu N.P. and Debnath, D., 2007a. J. World Aquacult. Soc., 38: 129-137. https://doi.org/10.1111/j.1749-7345.2006.00081.x
Beveridge, M.C.M., 1984. Cage and pen fish farming. Carrying capacity models and environmental impact. FAO Fisheries Technical paper no. 255. FAO, Rome, Italy.
Bligh, E.G. and Dyer, W.J., 1995. Can. J. Biochem. Physiol., 37: 911-917. https://doi.org/10.1139/o59-099
Bothast, R.J. and Schlicher, M.A., 2005. Appl. Microbiol. Biotechnol., 67: 19-25. https://doi.org/10.1007/s00253-004-1819-8
Cheng, Z.J. and Hardy, R.W., 2002. Aquacult. Nutr., 8: 271-277. https://doi.org/10.1046/j.1365-2095.2002.00219.x
Cheng, Z.J. and Hardy, R.W., 2004. J. appl. Aquacult., 15: 101-113. https://doi.org/10.1300/J028v15n03_08
Coyle, S.D., Mengel, G.J., Tidwell, J. and Webster, C.D., 2004. Aquacult. Res., 35: 365-370. https://doi.org/10.1111/j.1365-2109.2004.01023.x
Debnath, D., Pal, A.K., Sahu, N.P., Jain, K.K., Yengkokpam, S. and Mukherjee, S.C., 2005. Aquacult. Res., 36: 180-187. https://doi.org/10.1111/j.1365-2109.2004.01203.x
Gaylord, T.G., Teague, A.M. and Barrows, F.T., 2006. J. World Aquacult. Soc., 37: 509-517. https://doi.org/10.1111/j.1749-7345.2006.00064.x
Goldburg, R. and Triplett, T., 1997. Environmental effects of aquaculture in the United States. EDF, Washington (DC).
ICLARM (International Center for Living Aquatic Resources Management). 2001. Genetic improvement of carp species in Asia: Final report. Asian development bank regional technical assistance No. 5711. International Center for Living Aquatic Resources Management, Penang, Malaysia.
Jhingran, V.G., 1991. Fish and fisheries of India. Hindustan Publication Company, New Delhi, India.
Kousoulaki, K., Albrektsen, S., Langmyhr, E., Olsen, H.J., Campbell, P. and Aksnes, A., 2009. Aquaculture, 289: 74-83. https://doi.org/10.1016/j.aquaculture.2008.12.034
Motwani, M.P. and Bose, B.B., 1957. Proc. natl. Inst. Sci. India, 23: 1-16.
NRC (National Research Council), 1981. Nutrient requirement of domestic animals no. 16. Nutrient requirement of coldwater fishes. National Academy Press, Washington, DC.
NRC (National Research Council), 1993. Nutrient requirements of fish. National Academy Press, Washington, DC. pp. 114.
Papatryphon, E., Howell, R.A. and Soares, J.H., 1999. J. World Aquacult. Soc., 30: 161-173. https://doi.org/10.1111/j.1749-7345.1999.tb00863.x
Rabia, S., Afzal, M. and Shah, S.Z.H., 2017. J. appl. Anim. Res., 45: 331-335. https://doi.org/10.1080/09712119.2016.1190731
Randall, K.M. and Drew, M.D., 2010. Anim. Feed Sci. Technol., 159: 138-142. https://doi.org/10.1016/j.anifeedsci.2010.05.011
Ravindran. V., Bryden, W.L. and Kornegay, E.T., 1995. Avian Poult. Biol. Rev., 6: 125-143.
Reveco, F.E., 2010. Effect of fractionation on nutritional value of wheat distillers grains for rainbow trout, Oncorhynchu smykiss. In: Animal and poultry science. University of Saskatchewan, Canada, p. 108.
Snedecor, G.W. And Conhran, W.G., 1991. Statistical methods. Iowa State Univ. Press, Ames, USA.
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1996. Principles and procedures of statistics, 3rd Ed. McGraw Hill International Book Co. Inc., New York. USA.
Sugiura, S.H., Raboy, V., Young, K.A., Dong, F.M. and Hardy, R.W., 1999. Aquaculture, 170: 285-296. https://doi.org/10.1016/S0044-8486(98)00414-1
Vohra, A. and Satyanarayana, T., 2003. Crit. Rev. Biotechnol., 23: 29–60. https://doi.org/10.1080/713609297
Webster, C., Tidwell, J., Goodgame-Tiu, L. and Yancey, D., 1995. In: Nutrient utility and techniques in aquaculture (eds. C. Lim and D.J. Sessa). AOCS, Illinois, USA. pp. 189-198.
Wu, V.Y., Rostagi, R.R., Sessa, D.J. and Brown, P.B., 1994. J. Am. Oil Chem. Soc., 71: 1041-1043. https://doi.org/10.1007/BF02542277
To share on other social networks, click on any share button. What are these?










