Effect of Selenium on Growth and Chemical Properties of Tomato Hybrid Salar F1
Research Article
Effect of Selenium on Growth and Chemical Properties of Tomato Hybrid Salar F1
Sadaqat Khan1, Saleem Ullah1* and Muhammad Sajid2
1Department of Agricultural Chemistry and Biochemistry, University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Horticulture, University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | An experiment was carried out in plots during 2016 and 2017 to evaluate the effect of different levels of selenium application in irrigation water and foliar spray on physicochemical parameters of tomato hybrid Salar F1 grown in simple plastic tunnels using randomized complete block design. The data showed that plant height in centimeter, buds, flower, leaves and leaflets in numbers per plants with a range of 131.33 to 176.67 cm, 60 to 105, 41.5 to 60.0, 50.66 to 77.167 and 7.607 to 15.33, 30.33 to 82.0, respectively were significantly affected (P<0.05) by the interaction of selenium application in irrigation and foliar spray in relation to season. The effect was also significant (P<0.05) on some minerals like Cu, Mn, Zinc, Mg and Se that ranged from 0.2080 to 0.3150, 0.1260 to 0.2520, 0.2012 to 0.2970 and 18.04 to 32.09, 0.2147 to 0.5257 mg/Kg, respectively. Most of the proximate parameters of leaves like moisture, ash, crude fiber, crude protein, crude fat ranged from 92.082 to 92.317, 3.097 to 3.85, 4.9133 to 5.2717, 5.0133 to 5.2867, 1.08 to 1.24, 0.0236 to 1.9567 g/100g and that of fruits with lesser values were also affected significantly (P<0.05). From the present study it was concluded that Se applied in the form of sodium selenite in irrigation and foliar spray considerably affected the physical parameter of tomato hybrid Salar F1, followed by proximate composition while minerals content was less affected. It is recommended that selenium may be added in moderate amount to plants for their physical well-being and for improvement of some chemical parameters.
Received | October 17, 2019; Accepted | January 21, 2021; Published | April 09, 2021
*Correspondence | Saleem Ullah, Department of Agricultural Chemistry, University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: saleemagrichemist@gmail.com
Citation | Khan, S., S. Ullah and M. Sajid. 2021. Effect of selenium on growth and chemical properties of tomato hybrid salar F1. Sarhad Journal of Agriculture, 37(2): 444-455.
DOI | https://dx.doi.org/10.17582/journal.sja/2021/37.2.444.455
Keywords | Tomato, Selenium, Hybrid
Introduction
Tomato is edible red berry fruit, belongs to South America region and it is used in daily foods in various forms. Tomato is rich in Lycopene and other essential nutrients. Tomato family is Solanaceae also called Nightshade that can reach a height of 1 to 5 meters i.e. 3–16 ft. The stem of tomato is tender and mostly creeps on ground or climb on other plants or supports. It is perennial in its native habitat, although often grown in temperate climates as annual crop.
Interest in the biological impacts of selenium (Se) is escalating because of its essentiality for humans and animals. Se in food mainly comes from plants sources and focus on effect of selenium uptake will prove its essentiality for fortification in plants. The deficiency of selenium causes keshan disease (fatal cardiomyopathy), kashin-beck disease. The selenium deficiency also badly affects the thyroid and immune system functions (Combs, 2001). In human, the nutritional function of Se is fulfilled by the selenoenzymes/ selenoproteins such as glutathione peroxidase, thioredoxin reductase and iodothyronine 5’-deiodinase that are involved in hormonal regulations. Humans need Se in their diet for at least 25 different proteins, mostly antioxidant enzymes and the recommended dietary allowance (RDA) is 55- 200 µg/day for an adult. It is for these reasons; there is a resurgence of interest in Se fortification in higher plants during the last decade researches. The importance of Se for plant growth has not yet been fully understood and a lot of work has to be done but may be helpful in bio-accumulation.
As studies revealed that Se is very important in human diet and its deficiency causes various diseases that compel the scientists to work for overcoming its deficiency in human diets. Deficiency of Se prevailed in various countries like UK, Australia, New Zealand, China and many others (Chen et al., 2002), where Se is deficient in their soils and crops grown in those countries contained negligible amount of Se (Combs, 2001).
Se in the plants mainly depends on soil concentration of selenium and soil properties e.g. Higher pH help in uptake of Se by plants (Chaney, 1994). Selenium concentration also related with plant sulphur content and with some of the soil components including CaCO3 and the ratio of sand and silt in the soil (Dhillon et al., 1992; Mayland et al., 1990). The present work is the continuation of such type of research where effect of selenium has been studied on tomato hybrid Salar F1 applied in the form of Na selenite in irrigation water and folair spray during two cropping season of tomato.
Materials and Methods
Study design and field layout
The experiment was carried out in Randomized Complete Block (RCB) factorial design with three 3 replications. The factors and their levels included foliar application of Se (F) with four levels (0, 5, 15, 20 mg/Kg) and Se application via irrigation water (I) with three (0, 50, 75 mg/Kg) levels replicated three times. The experiment was repeated over two seasons. Thus, the total numbers of treatment combinations in the experiment were 3×4×3= 36 per season. The plot size was 4x4 feet. All other agronomic practices for tomatoes cultivation were carried out as standard recommended in literature.
The following parameters were studied during the experiment:
Moisture content
The drying method was used for moisture determination (AOAC, 2016). A sample of two gram (W1) was weighted by electric balance in petri dishes with lids. Then the Petri plates with samples were completely dried in oven at a temperature of 105 °C. The samples were then covered with its lids and that was cooled in desiccator. When the plates with samples got cool then the sample was reweighted (W2). The moisture in g/100g was calculated as follows.
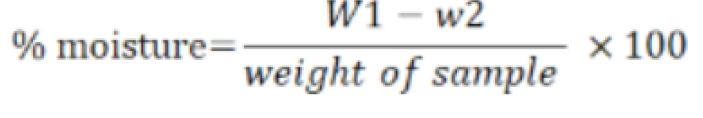
Where as:
W1 = Initial weight of Petri dish + sample; W2 = Final weight of Petri dish + sample.
Crude protein
For the determination of nitrogen percentage in tomato, Kjeldhal method was used (AOAC, 2016). For digestion of samples sulfuric acid in concentrated form was used in addition of digestion mixture (7g K2SO4:1g CuSO4). In the digestion tube, 2.0 g of samples was added with 15 mL of sulfuric acids and was heated up to 300 oC using digester. The greenish color digest was then cooled diluted to 100 mL with distilled water in volumetric flaks. For distillation 10 mL of the samples was taken and 40% Sodium hydroxide was added in the reaction tube of micro kjeldhal distillation apparatus. During heating with steam, ammonia was produced which was collected in the receiving flask containing boric acid (4%) with modified methyl red as indicator. Ammonia changed the colored yellow solution which then was titrated with 0.05 N HCl to calculate the percent nitrogen. The same procedure was repeated for blank and %N obtained was then multiplied with 6.25 as protein factor. The following formula was used.
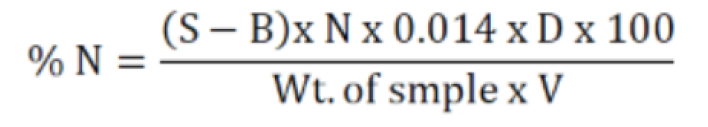
Crude fat
Crude fat was determined by Soxhlet apparatus method (AOAC, 2016). The extraction solvent was petroleum ether (40-60°C). Each sample (1.0 g) was weighed and wrapped in filter paper, kept in the thimble and transferred into the extraction tube. The round bottom flask was weighed and one third of it was filled with petroleum ether and connected to the extraction tube. After various siphonings, the flasks were dried and cooled and weighed. Crude fats were calculated as under.
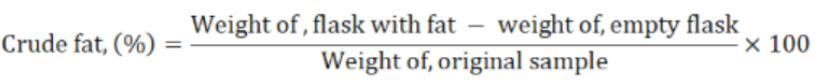
Crude fiber
Crude fiber was determined by acid base digestion method (AOAC, 2016). For acid digestion, 200 ml of 2% H2SO4 was taken in a 500 mL beaker and 2.0 g sample was added into it. The samples were placed on water bath for 30 min. The digested was then filtered through muslin cloth. The acid digest was transferred into 500 mL beaker containing 200 mL of 2% NaOH. After alkali digestion the sample was filtered again with muslin cloth and weighted and dried in oven completely at 100 oC. After drying the digest was transferred into pre-weighted crucible and kept at 550 oC in muffle furnace. The crucible was weighted again and kept in desiccator for cooling. The crude fiber percentage was determined as under:
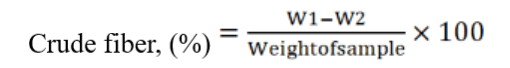
Total ash
Ash content was determined by combustion method (AOAC, 2016). Two grams of ground tomato sample was taken in a crucible and weight was taken (W1). The sample in crucible was charred with blowing flame and then ignited in muffle furnace at 550 oC into grayish white residues. The samples were cooled in desiccator. It was weighed again (W2) accurately and ash contents was calculated as.
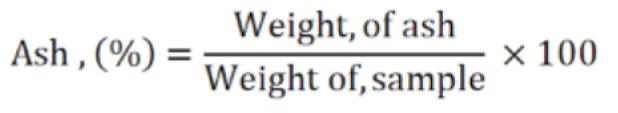
Nitrogen free extracts (NFE)
The nitrogen free extract (NFE) is the total digestible carbohydrate of tomato plant and fruit samples. It was calculated through subtraction as follows:
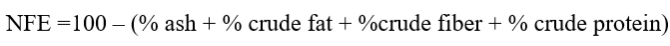
Minerals analysis
Tomato samples were analyzed for its minerals contents by following (AOAC, 2016) procedures. Sodium and potassium were determined by flame photometer. Flame photometer was used for Sodium and Potassium determination. Other minerals i.e. Cu, Ca, Fe, Mg, Mn, P, Se and Zn were analyzed through atomic absroption.
Preparation of acid digests
The tomato samples (1.0 g) were digested to colorless liquid with conc. HNO3 and perchloric acid (HClO4) at the ratio of 7:3 in medium sized digestion tubes using hot plates. These digests were diluted to 100 ml with distilled water.
Na and K
Flame photo meter (PEP7) was used for determination of Na and K in the tomato samples.
Preparation of standard curve: Technical grade NaCl and KCl (Murk) was taken and 100 ppm solution was made for Na and K in double distilled water. These solutions were then diluted to 4 different concentrations of 20, 40, 80 and 100 ppm itself. The emission reading for these dilutions were noted and standard curves were developed using MS excel for further quantitative determination of Na and K in the samples.
Sample assay: The acid digest of tomato samples was taken in beakers and emission reading was noted by Flame photometer using Na and K filters. The amount of Na and K was calculated in mg/Kg which was then mathematically change into mg/100 g.
Phosphorus determination
Phosphorus was determined by molybdate method. For molybdate solution 7.5 g of ammonium paramolybdate (NH4) Mo7O24.4H2O was dissolved in 250 mL of deionized water. Similarly, for H2SO4 solution 70 mL of concentrated sulfuric acid was added to 450 mL of DI water. The ascorbic acid solution was prepared by dissolving 13.5 g of ascorbic acid in 250 mL of DI water. Potassium antimonyl-tartrate solution was prepared by adding 0.34 g in 250 mL of DI water. Then mixed reagent was obtained by adding together 100 mL ammonium molybdate, 250 mL sulfuric acid, 100 mL ascorbic acid, and 50 mL of potassium antimonyl-tartrate solutions. A stock solution of phosphorus 10 mM was prepared from 0.6805 g of KH2PO4 (fw = 136.09) in 100 mL DI water using a volumetric flask. For secondary stock solution of 50 mM, 100 µL of the primary stock solution was diluted up to 100 mL with deionized water using a volumetric flask. Then 1.0 mL from diluted solution was taken and 4.0 mL coloring reagent and 15 mL of water were added. It was kept for 15 min. to develop color. Absorption reading was taken at 880 nm wave length using spectrophotometer. Similarly, absorption readings of all the samples were taken and the data were compared with standard curve as under.

Micro mineral analysis
Micro mineral was determined by flame Atomic absorption spectrophotometer (Perkin Elmer) using the respective cathode lamp of the minerals. The sample was sucked through the flame and the reading was noted in ppm. For calibration of the instrument the standard solution of the required minerals was used provided with machine.
Agronomic characteristics
Plant height: Measuring tape was used to measure plant height from the base to the tip of the plant.
Number of flowering buds per plant: Buds as they are the initial form of flower were counted when it started growing on plant.
Number of flowers per plant: Buds then changed in the flower and when this phenomenon was started the flower count was started.
Number of fruits per plant: Flowers changed into fruits and as this phenomena was started the fruits were counted.
Chlorophyll analysis
For chlorophyll measurement the instrument used was AT leaf chlorophyll meter. The unit used was µg/g.
Lycopene analysis
For Lycopene measurement spectrophotometer sp 3000 was used. The reagents of HPLC grade was used where they were taken in the 2:1:1 including hexane, acetone and ethanol for lycopene extraction from tomato. After washing the tomato was turned into juice in blender. A portion of juice 100 µL was taken through pipette into 20 mL screw cap tube. In this juice, 8.0 mL of the mix solvents were mixed and vortexed. Then the sample was kept in the dark for incubation at room temperature. I.0 mL of distilled water was added and vertexed again. The sample was kept undisturbed for about 20 min. where two phases were formed. In this procedure distilled water was used as blank. The upper layer was taken into a prewashed cuvette. The maximum absorption was 503 nm. First the spectrophotometer was made zero with blank.
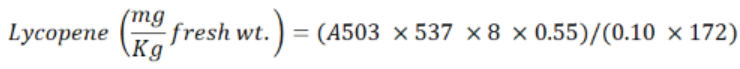
The formula showed that 537 g/mole is the molecular weight of Lycopene, 8 mL is the volume of mixed solvent, 0.55 is the volume ratio of the upper layer to the mixed solvents, 0.10 g is the weight of tomato added, and 172 mM-1 is the extinction coefficient for Lycopene in hexane
Statistical design
Data was analyzed using the statistical package statistix 8.1 (USA) and the significant differences between treatments was determined using least significant difference (LSD) test for main as well as interaction effects.
Results and Discussion
A field experiment was conducted in two consecutive seasons to study the effect of selenium on plant growth parameters, proximate composition, mineral and other chemical constituents of tomato cultivar Salar F1, in randomized complete block design with three factors factorial experiment.
The data of growth parameters (Table 1) showed that average height (cm), no of leaves, leaflets, buds, flowers and fruits per plant ranged from 131.33 to 176. 67, 6.67 to 15.33, 30.33 to 82, 61.33 to 116.33, 50.67 to 77.17, 12 to 58.67, respectively. Different factors and their interaction (Table 5) showed that height was significantly affected (P<0.05) by all factors and their interaction except season and season into irrigation interaction. The number of leaves/plant was significantly affected by season and foliar versus irrigation interaction while number of leaflets/plant was affected by all the three factors and their interactions. Number of buds/plant were also affected by irrigation, season and by the interaction of irrigation with foliar and season. Number of flower/plant was affected by foliar, season and by the interaction of foliar with irrigation and season while
Table 1: Physical parameters of tomato hybrid Salar F1 as affected by Selenium application (mg/Kg) in irrigation and as foliar spray in two seasons.
|
IA |
FA |
SS |
hieght/p |
Leaves/Plant |
Leaflets/Plant |
Buds/Plant |
Flowers/Plant |
Fruits/plant |
|
1 |
1 |
a |
155 defg |
7.667 cd |
30.333 k |
61.33f |
50.667h |
26.333 cde |
|
b |
141 hij |
8 cd |
50 fdg |
82.67cd |
66.333bcd |
50.667 ab |
||
|
2 |
a |
166.33 abcd |
8.000cd |
36.667 jk |
65.67ef |
53.167gh |
38.667 abcd |
|
|
b |
171.33 abc |
10.333 abcd |
52.333 fd |
94.00bc |
62.500cdefg |
45 abc |
||
|
3 |
a |
148 ghi |
9.667 bcd |
34 k |
61.67f |
51.000h |
24 cde |
|
|
b |
176.67 a |
9.667 bcd |
49 ghi |
76.33de |
64.167bcde |
21 de |
||
|
4 |
a |
140.67 hij |
8.667 cd |
38 jk |
70.00def |
60.667cdefgh |
38.667 abcd |
|
|
b |
137.33 ij |
15.333 a |
82 a |
84.00cd |
77.167a |
49.667 ab |
||
|
2 |
1 |
a |
153.33 efgh |
8.667 cd |
39.667 hijk |
82.00cd |
66.333bcd |
50.333 ab |
|
b |
131.33 j |
12 abc |
60 def |
116.33a |
71.000ab |
39.667 abcd |
||
|
2 |
a |
160.67 bcdefg |
9.000cd |
39.333 hijk |
71.33def |
59.167cdefgh |
12 e |
|
|
|
b |
153.33 efgh |
9.667 bcd |
46 ghij |
114.33a |
67.833bcd |
58.667 a |
|
|
3 |
a |
158.67 cdefg |
6.667 d |
34 k |
65.00ef |
58.667defgh |
20 de |
|
|
b |
161.33 bcdef |
10 bcd |
53.333 efg |
102.67ab |
61.000cdefg |
31 bcde |
||
|
4 |
a |
171 abc |
7.667 cd |
36.667 jk |
67.00ef |
58.833defgh |
32 bcde |
|
|
b |
173 ab |
11.333 abcd |
67.667 bcd |
102.33ab |
63.500bcdef |
39.333 abcd |
||
|
3 |
1 |
a |
157.33 defg |
8.667 cd |
39 ijk |
66.67ef |
56.000efgh |
30 bcde |
|
b |
154 defg |
11.667 abcd |
78.667 a |
92.00bc |
67.500bcd |
30 bcde |
||
|
2 |
a |
165.67 abcde |
7.667 cd |
33 k |
64.00ef |
54.833fgh |
33.667 bcd |
|
|
b |
164.67 abcde |
14.667 ab |
71.333 abc |
99.67bc |
56.000efgh |
41 abcd |
||
|
3 |
a |
163.67 bcde |
8.333 cd |
37.667 jk |
66.00ef |
58.833defgh |
33.667 bcd |
|
|
b |
164.67 abcde |
12.333 abc |
77.333 ab |
104.00ab |
59.667defgh |
43.667 abc |
||
|
4 |
a |
164.33 abcde |
7.667 cd |
37.333 jk |
60.67f |
58.833defgh |
38 abcd |
|
|
b |
149 fghi |
9.667bcd |
64 cde |
105.00ab |
64.500bcde |
34.667 bcd |
number of fruits/plant was highly affected by season and by the interaction of the three factors.
The present study was in agreement with the work done by Nancy et al. (2014) who reported that the application of Se can increase fruit yield by increasing shoot length. Akbulut and Cakir (2010), and Djanaguirman et al. (2005) also concluded that selenium application increases leave numbers by decreasing leaf abscission which assisted the present study. Han-Wens (2010) reported that Se increase meiosis of meristematic cells that may result in higher leaves number. Increase in leaf numbers may be due to stimulation of cell division, increase photosynthesis, chlorophyll and carbon fixation (Malik et al., 2011). Fu et al. (2011) reported that application of Se at moderate level can promote plant growth parameters like leaves, buds etc. Prins et al. (2011) reckoned to the increase of buds with application of the selenium. Xue et al. (2001) and Djanaguiraman et al. (2005) work on rice, lettuce and soybean and reported same results of increasing yield with application of selenium. Chi et al. (2017) also founded that Se application can increase number of fruits.
The mineral content of tomato fruits (Table 2) showed considerable variation under the effect of different factors. Among different minerals in mg/kg Cu ranged from 0.186 to 0.324, Mn from 0.119 to 0.257, Zn from 0.125 to 0.301, Fe from 1.244 to 3.09, Ni from 0.211 to 0.92, Pb from 0.119 to 0.614, Cr from 0.011 to 0.331, Se from 0.19 to 0.564, Ca from 72.279 to 128.35 and Mg was ranged from 18.029 to 33.02, respectively. The effect of factors and their interaction (Table 6) on different mineral showed that Cu was significantly affected by foliar application of Se and its interaction with irrigation. Zn, Fe, Ni, Cr, Pb and Mg were significantly affected by Foliar, Irrigation application and also by season. The interaction of foliar and irrigation applications was also significant in case of these minerals. Mg was also affected by the interaction of Foliar and Season. Ca, Mn and Se were affected by foliar and irrigation applications and also by their interaction.
Table 2: Mineral content (mg/Kg*) of tomato hybrid Salar F1 as affected by Selenium application (mg/Kg) in irrigation and foliar spray in two seasons.
|
IA |
FA |
SS |
Cu |
Mn |
Zn |
Fe |
Ni |
Pb |
Cr |
Se |
Ca |
Mg |
|
0 |
0 |
a |
0.2190 efgh |
0.1253k |
0.2367fghi |
1.2476i |
0.6633def |
0.1987ij |
0.1146e |
0.2147h |
72.285l |
18.04n |
|
b |
0.2110gh |
0.1610ghij |
0.2370fghi |
1.3053hi |
0.8000ab |
0.2287hi |
0.1206e |
0.2317fgh |
72.653l |
18.28n |
||
|
5 |
a |
0.2500cdef |
0.1507ghijk |
0.2717bc |
2.0866cde |
0.8200a |
0.5710a |
0.0163l |
0.2507fg |
128.027a |
32.42b |
|
|
b |
0.2350defgh |
0.1593ghij |
0.2843ab |
2.1876bcd |
0.8413a |
0.5850a |
0.0530hij |
0.2600f |
128.18a |
32.90b |
||
|
10 |
a |
0.2280defgh |
0.1700efghi |
0.2830ab |
2.0506de |
0.4800ij |
0.3480cd |
0.0270kl |
0.5200a |
112.036d |
28.24e |
|
|
b |
0.2730bc |
0.1737efgh |
0.2970a |
2.3250ab |
0.6247efg |
0.3723cd |
0.0340jkl |
0.5257a |
112.386d |
28.68d |
||
|
15 |
a |
0.2316defgh |
0.1260k |
0.2407fghi |
1.4130ghi |
0.5467ghi |
0.2768e |
0.1723d |
0.2233gh |
86.817j |
21.66lm |
|
|
b |
0.2543cdef |
0.1510ghijk |
0.2253ij |
1.4616gh |
0.5767fgh |
0.2757ef |
0.1750d |
0.2293fgh |
87.453j |
21.97l |
||
|
50 |
0 |
a |
0.2290defgh |
0.2073bcd |
0.2012k |
2.1896bcd |
0.5088hi |
0.3482cd |
0.0813fg |
0.2413fgh |
106.228f |
26.97g |
|
b |
0.2336defgh |
0.2357ab |
0.2107jk |
2.2890abc |
0.5527ghi |
0.3690cd |
0.0823fg |
0.2388fgh |
106.446ef |
27.73f |
||
|
5 |
a |
0.2173 fgh |
0.1610ghij |
0.2703bcd |
2.0473de |
0.5333ghi |
0.1877j |
0.0418ijk |
0.4410b |
107.205ef |
26.99g |
|
|
b |
0.2223 efgh |
0.1487ghijk |
0.2813ab |
2.0650de |
0.6163efg |
0.2017ij |
0.0495hij |
0.4420b |
107.133ef |
27.15g |
||
|
10 |
a |
0.2570 cde |
0.1383ijk |
0.2477efgh |
1.5710g |
0.5767fgh |
0.3377d |
0.2333c |
0.2300fgh |
101.348h |
25.28ij |
|
|
b |
0.2660cd |
0.1683fghi |
0.2720bc |
1.5090gh |
0.6633def |
0.3777c |
0.2290c |
0.2380fgh |
101.816h |
25.51hi |
||
|
15 |
a |
0.2080h |
0.2520a |
0.1290m |
2.3020ab |
0.2170m |
0.1243k |
0.1566d |
0.2397fgh |
84.425k |
21.52m |
|
|
b |
0.2326defgh |
0.2357ab |
0.1480l |
2.3590ab |
0.2357lm |
0.1303j |
0.1663d |
0.2483fgh |
84.777k |
21.66lm |
||
|
75 |
0 |
a |
0.2113gh |
0.1740efgh |
0.2537def |
2.0296de |
0.6667def |
0.2330ghi |
0.0570hi |
0.3527de |
103.046g |
25.75h |
|
b |
0.2200efgh |
0.1677fghij |
0.2607cde |
2.0800cde |
0.7013cde |
0.2400fgh |
0.0632gh |
0.3480e |
103.15g |
25.92h |
||
|
5 |
a |
0.2483cdefg |
0.1450hijk |
0.2595cde |
1.8290f |
0.6900cde |
0.4572b |
0.1238e |
0.2404fgh |
114.686c |
28.86d |
|
|
b |
0.2266defgh |
0.1350jk |
0.2513efg |
1.9000ef |
0.7247bcd |
0.4703b |
0.1313e |
0.2427fgh |
114.306c |
28.79d |
||
|
10 |
a |
0.2230 efgh |
0.2017cde |
0.2085jk |
2.1725bcd |
0.3158kl |
0.2678efg |
0.0889f |
0.3837cd |
98.232i |
24.88k |
|
|
b |
0.2393cdefgh |
0.2213abc |
0.2303hi |
2.4860a |
0.3940jk |
0.2660efg |
0.0898f |
0.3900c |
98.343i |
24.93jk |
||
|
15 |
a |
0.3073ab |
0.1957cdef |
0.2323hi |
2.3260ab |
0.7833abc |
0.2173hij |
0.2856b |
0.3513de |
119.207b |
29.56c |
|
|
b |
0.3150a |
0.1787defg |
0.2350ghi |
2.4280a |
0.8250a |
0.2293hi |
0.3070a |
0.3610cde |
119.766b |
29.43c |
IA: Irrigation Application; FA: Foliar Application; SS: Seasons; *: ppm; The means followed by same letters are not statistically significant at P< 0.05.
Copper is essential for cellular metabolism (Ivanova et al., 2010). Mn is essential mineral and intervening in several metabolic processes, mainly in photosynthesis and as an enzyme antioxidant-cofactor (Millaleo et al., 2010). Hu et al. (2015) also reported that Se application can improve Mn and Zn, Ni, Ca and other minerals in the plants. Arvy (1992) reported that Se when applied on moderate level affects Mn, Co, Zn, P and Mo. Zinc is present in many proteins so important for cellular metabolism (Ivanova et al., 2010). Selenium can increase uptake of Zn when applied at moderate level (Fu et al., 2011). Iron plays an important role in redox reaction and electron transport chain. It is also important for photosynthesis and respiration (Kim and Guerinot, 2007). Li et al. (2018) reported that when Se is applied to the plants, it increases the concentration of iron. He et al. (2007) also reported that application of Se in lettuce had increased mineral content. Soil pH, acidity, salinity and related factors affect the mineral availability to the plants.
He et al. (2007) worked on application of Se in lettuce and Ying et al. (2014) worked on rice who reported that application of Se can decrease Pb concentration. Qing et al. (2015) reported that Se application in foliar form decrease chromium concentration. They also reported that Se can detoxify Cr by minimizing super oxide free radicals that are produced by Cr in leaves. Selenium helps in plant growth. It delays leaf senescence. It increases oxidative stress in plant cause due to UV light Germ et al. (2007). Application of Se can increase Se concentration in tomato fruit (Nancy and Indra, 2014). Lee et al. (2007) also found the same results. Smith and Watkinson (1984) also found that application of Se can increase Se in tomato. Se is essential for plants because it helps in holding together cell walls and have structural role in cell wall and membrane (White et al., 2003).
Table 3: Proximate composition (%) of tomato hybrid Salar F1 fruits as affected by Selenium application (mg/Kg) in irrigation and foliar spray in two seasons.
|
IA |
FA |
SS |
C. Fat |
C. Prtn |
Ash |
C Fibr |
Moisture |
Lycopene |
|
1 |
1 |
a |
0.19 ghi |
0.65 ghi |
1.13 abcde |
1.64 i |
93.083 k |
2.82 b |
|
b |
0.22 efg |
0.67 fghi |
1.16 ab |
1.8333 bcd |
93.127 jk |
2.3567 mn |
||
|
2 |
a |
0.14 j |
0.68 efghi |
1.01 ghijkl |
1.56 j |
93.15 ijk |
2.6833 c |
|
|
b |
0.1667 ij |
0.7133 bcdef |
1.0667 cdefgh |
1.6333 i |
93.167 ghij |
2.4233 ijkl |
||
|
3 |
a |
0.2167 efgh |
0.72 abcdef |
1.09 bcdefg |
1.76 efg |
93.247 cdef |
2.5733 d |
|
|
b |
0.25 cde |
0.7533 abc |
1.14 abc |
1.8233 bcde |
93.283 abcd |
2.4867 fghi |
||
|
4 |
a |
0.24 cdef |
0.6267 i |
0.9767 ijklm |
1.75 fg |
93.157 hij |
2.84 b |
|
|
b |
0.2767 bc |
0.6633 fghi |
1.0633 cdefgh |
1.8033 cdef |
93.193 fghij |
2.4167 jklm |
||
|
2 |
1 |
a |
0.18 hi |
0.6833 defghi |
0.86 p |
1.6833 hi |
93.223 defgh |
2.7333 c |
|
b |
0.2167 efgh |
0.7167 bcdef |
0.95 lmn |
1.72 gh |
93.273 abcd |
2.35 n |
||
|
2 |
a |
0.2767 bc |
0.65 ghi |
1.05 efghij |
1.8367 bcd |
93.17 ghij |
2.4967 efg |
|
|
b |
0.3 ab |
0.6633 fghi |
1.0933 bcdef |
1.88 ab |
93.217 defghi |
2.3267 n |
||
|
3 |
a |
0.21 fgh |
0.74 abcd |
1.1967 a |
1.7733 defg |
93.257 cdef |
2.8 b |
|
|
b |
0.2367 def |
0.7567 ab |
1.1333 abcd |
1.7967 cdef |
93.303 abc |
2.43 hijk |
||
|
4 |
a |
0.2967 ab |
0.6967 cdefgh |
1.0433 fghijk |
1.65 i |
93.123 jk |
2.92 a |
|
|
b |
0.3267 a |
0.72 abcdef |
1.1067 bcdef |
1.7233 gh |
93.167 ghij |
2.49 efgh |
||
|
3 |
1 |
a |
0.1867 ghi |
0.7133 bcdef |
0.92 mnop |
1.8733 abc |
93.2 efghi |
2.9467 a |
|
b |
0.21 fgh |
0.7333 abc |
0.9967 hijklm |
1.91 a |
93.227 defgh |
2.3633 lmn |
||
|
2 |
a |
0.2733 bcd |
0.7433 abc |
0.8633 op |
1.7967 cdef |
93.167 ghij |
2.5467 def |
|
|
b |
0.31 ab |
0.7767 a |
0.9433 lmno |
1.8533 abc |
93.23 defg |
2.4567 ghij |
||
|
3 |
a |
0.22 efg |
0.6433 hi |
0.9633 klm |
1.6333 i |
93.267 bcde |
2.7233 c |
|
|
b |
0.2533 cde |
0.6667 fghi |
1.0567 defghi |
1.68 hi |
93.337 ab |
2.46 ghij |
||
|
4 |
a |
0.21 fgh |
0.6767 efghi |
0.88 nop |
1.8533 abc |
93.283 abcd |
2.5533 de |
|
|
b |
0.2433 cdef |
0.7033 bcdefg |
0.97 jklm |
1.88 ab |
93.343 a |
2.3667 klmn |
IA: Irrigation Application; FA: Foliar Application; SS: Seasons; The means followed by same letters are not statistically significant at P< 0.05.
It helps to decrease leaf senescence also plays an important role in photosynthesis and nucleic acid synthesis. Increasing Se application can increase Mg concentration (Kopsell et al., 2000).
Table 3 showed the effect of selenium application on proximate composition of tomato fruits. It was examined that crude fat ranged from 0.14 to 0.3267%, crude protein from 0.6267 to 0.7767%, ash from 0.86 to 1.1967%, crude fiber from 1.56 to 1.91%, moisture 93.083 to 93.343 % and lycopene 2.3567 to 2.9467 µg/g. Factors and interaction showed their effect on these proximate composition of fruits. Crude fat, ash and moisture were significantly affected by all the three factors and by the interaction of foliar and irrigation applications. Crude protein was affected by season and by the interaction of foliar and irrigation. Crude fiber was affected by irrigation, season, interaction of foliar and irrigation and by the interaction of irrigation and season. Lycopene was affected by foliar and season applications and by interaction all the three factors.
Table 4 showed the effect of selenium application on proximate composition of tomato leaves which showed that ash 3.1933 to 3.85%, crude fiber 5.03 to 5.2733%, crude fat 0.0296 to 0.0309%, crude protein 1.0967 to 1.24%, moisture from 92.083 to 92.317%, chlorophyll before flowering 0.024 to 0.0309 µg/g, chlorophyll after flowering 0.0352 to 0.043 µg/g. The factors effect on these parameters of tomato leaves showed that crude fat, ash, chlorophyll before and after flowing was significantly affected by all the three factors i.e. foliar and irrigation application and season and by the interaction of Foliar and irrigation. However, the chlorophyll of leaves after flowing was additionally affected by irrigation and season interaction and by overall interaction of the three factors. Crude proteins were affected by season and moisture by irrigation. These both parameters were also affected by the interaction of foliar and irrigation factors.
Table 4: Proximate composition (%) of tomato hybrid Salar F1 leaves as affected by Selenium application (mg/Kg) in irrigation and foliar spray in two seasons.
|
IA |
FA |
SS |
C. Fat |
C. Prtn |
Ash |
C. Fibr |
Moisture |
Chlor before flowers |
Chlor after flowers |
|
1 |
1 |
a |
1.7333 cde |
1.0967 hi |
3.24 hijk |
5.1067 ab |
92.25 ab |
0.0251 kl |
0.0424 a |
|
b |
1.78 bcd |
1.14 efgh |
3.4667cdefg |
5.1433 a |
92.093 cd |
0.024 lm |
0.0382 ef |
||
|
2 |
a |
1.77 cd |
1.1533 defg |
3.2933 fghij |
5.2567 a |
92.083 d |
0.0322 bc |
0.0391 de |
|
|
b |
1.8067 bc |
1.19 abcde |
3.3867defgh |
5.2867 a |
92.153 bcd |
0.0314 cd |
0.0362 hij |
||
|
3 |
a |
1.9267 a |
1.1133 ghi |
3.7733 a |
5.03ab |
92.18 abcd |
0.0347 a |
0.0382 ef |
|
|
b |
1.9567 a |
1.16 defg |
3.81 a |
5.0733 ab |
92.213 abcd |
0.0336 ab |
0.0352 jk |
||
|
4 |
a |
1.47 i |
1.1733 cde |
3.5867 bc |
5.1333 ab |
92.227 abc |
0.0318 cd |
0.0411 bc |
|
|
b |
1.4967 hi |
1.2233 ab |
3.6933 ab |
5.1767 a |
92.267 ab |
0.0309 cde |
0.0377 fg |
||
|
2 |
1 |
a |
0.0296 efg |
1.1533 defg |
3.1933 ijk |
5.2767 a |
92.2 abcd |
0.0296 efg |
0.0385 def |
|
b |
0.0288 gh |
1.2033 abcd |
3.2733 hij |
5.02 ab |
92.24 ab |
0.0288 gh |
0.0366 gh |
||
|
2 |
a |
0.0253 kl |
1.08i |
3.1367 jk |
5.11 ab |
92.193 abcd |
0.0253 kl |
0.0413 bc |
|
|
b |
0.0236 m |
1.1533 defg |
3.2067 ijk |
5.1633 a |
92.243 ab |
0.0236 m |
0.0348 k |
||
|
3 |
a |
0.0249 klm |
1.1167 fghi |
3.79 a |
5.19 a |
92.26 ab |
0.0249 klm |
0.0421 ab |
|
|
b |
0.0239 lm |
1.1733 cde |
3.85 a |
5.2333 a |
92.293 a |
0.0239 lm |
0.0363 hi |
||
|
4 |
a |
0.0286 ghi |
1.17 cdef |
3.2467 hijk |
5.26 a |
92.183 abcd |
0.0286 ghi |
0.043 a |
|
|
b |
0.0280 hi |
1.2333 ab |
3.3133efghi |
5.0133 ab |
92.237 ab |
0.0280 hi |
0.0391 de |
||
|
3 |
1 |
a |
0.0272 ij |
1.1833 bcde |
3.46 cdef |
5.1633 a |
92.277 ab |
0.0272 ij |
0.0410 c |
|
b |
0.0257 jk |
1.24 a |
3.53 bcd |
5.2467 a |
92.317 a |
0.0257 jk |
0.037 gh |
||
|
2 |
a |
0.0309 cde |
1.1433 efgh |
3.0967k |
4.7067 b |
92.267 ab |
0.0309 cde |
0.0394 d |
|
|
b |
0.0299 efg |
1.19 abcde |
3.1767 ijk |
5.12 ab |
92.303 a |
0.0299 efg |
0.0384 def |
||
|
3 |
a |
0.0288 gh |
1.1467 efgh |
3.2433 hijk |
5.14 a |
92.18 abcd |
0.0288 gh |
0.0375 fg |
|
|
b |
0.0279 hi |
1.19 abcde |
3.29 ghij |
5.1567 a |
92.203 abcd |
0.0279 hi |
0.036 hij |
||
|
4 |
a |
0.0304 def |
1.0933 hi |
3.4467cdefg |
5.2233 a |
92.23 abc |
0.0304 def |
0.0385 def |
|
|
b |
0.0292 fgh |
1.1433 efgh |
3.5333 bcd |
5.2733 a |
92.237 ab |
0.0292 fgh |
0.0355 ijk |
IA: Irrigation Application; FA: Foliar Application; SS: Seasons; The means followed by same letters are not statistically significant at P< 0.05.
Table 5: Effect of foliar, irrigation applications and Season and their interaction on chemical parameters of tomato hybrid Salar F1 Leaves.
|
Factors and Interactions |
Hieght/ p |
Leaves/P |
Leaflets/P |
Buds/P |
Flowers/P |
Fruits/P |
|
Foliar |
Sig. |
Sig. |
Sig. |
|||
|
Irrigation |
Sig. |
Sig. |
Sig. |
|||
|
Season |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
|
|
Foliar x Irrigation |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
|
|
Foliar x Season |
Sig. |
Sig. |
||||
|
Irrigation x Season |
Sig. |
Sig. |
Sig. |
|||
|
Foliar x Irrigation x Season |
Sig. |
Sig. |
Sig. |
* Significant at 5% probability (P<0.05).
Table 6: Effect of foliar, irrigation applications and Season and their interaction on mineral content of tomato hybrid Salar F1.
|
Factors and Interactions |
Ca |
Cr |
Cu |
Fe |
Mg |
Mn |
Ni |
Pb |
Se |
Zn |
|
Foliar |
Sig.* |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
|
Irrigation |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
|
|
Season |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
||||
|
Foliar x Irrigation |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
|
Foliar x Season |
Sig. |
|||||||||
|
Irrigation x Season |
||||||||||
|
Foliar x Irrigation x Season |
* Significant at 5% probability (P<0.05).
Table 7: Effect of foliar, irrigation applications and Season and their interaction on chemical parameters of tomato hybrid Salar F1 fruits.
|
Factors and Interactions |
Fruit C.Fat |
FC. Prtn |
Fruit Ash |
Fruit Fibr |
Moisture |
Lycopene |
|
Foliar |
Sig. |
Sig. |
Sig. |
Sig. |
||
|
Irrigation |
Sig. |
Sig. |
Sig. |
Sig. |
||
|
Season |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
|
Foliar x Irrigation |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
|
Foliar x Season |
Sig. |
|||||
|
Irrigation x Season |
Sig. |
Sig. |
||||
|
Foliar x Irrigation x Season |
Sig. |
* Significant at 5% probability (P<0.05).
Table 8: Effect of foliar, irrigation applications and Season and their interaction on chemical parameters of tomato hybrid Salar F1 leaves.
|
Factors and Interactions |
Leaves C. Fat |
Leaves C. Prtn |
Leaves ash |
LeavesC.Fibr |
Leaves Moisture |
Chlor before flowers |
Chlor after flowers |
|
Foliar |
Sig. |
Sig. |
Sig. |
Sig. |
|||
|
Irrigation |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
||
|
Season |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
||
|
Foliar x Irrigation |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
Sig. |
|
|
Foliar x Season |
|||||||
|
Irrigation x Season |
Sig. |
||||||
|
Foliar x Irrigation x Season |
Sig. |
* Significant at 5% probability (P<0.05).
Results of Abdullahi et al. (2016) were similar with the present study. Crude fat provides energy for metabolic processes structural component in membrane and important in intracellular signals. Song et al. (2015) reported and suggested that selenium application at optimum level can increase crude fat by increasing linoleic acid and sterol. Fernando et al. (2018) and Zhu et al. (2017) also reported the same result. Hu et al. (2003) suggested that crude protein content might be increased due to increase in different amino acid. Ježek et al. (2011) studied that application of Se can increase total amino acid when applied at flowering stage of Chrysanthemum. They further reported that when Se is sprayed on potato it would increase phenylalanine. The work done by Nancy and Arulselvi (2014) also agreed with the present study who reported that application of Se increases minerals content so in turn increased ash content. Hu et al. (2015) also agreed with Chen and Arvy study. So in variation or increase in minerals may result in the increase or decrease of ash content. Fu et al. (2011) also reported similar result. Turakainen et al. (2004) reported that selenium application can increase starch content in potato edible part. So it may be possible that Se application in tomato also increases fruit fiber which is a part of carbohydrate. Godina et al. (2016) reported that Selenium application has positive effect on moisture of leaves. All these study support the present data. Godina et al. (2016) also reported that application of Se on tomato plant improves the content of % total dry matter (TDM) in fruits. Zhu et al. (2017) reported that application of Se can increase total polysaccharide. Turakainen et al. (2004) reported that selenium application can improve total starches in potato leaves. So Selenium might cause increase in crude fiber content of tomato leaves. Xue et al. (2001) also reported that Se increase chlorophyll content at low concentration. Pennanen et al. (2002) also agreed with the present study and reported that Se protect Chloroplast enzymes and hence increase biosynthesis of photosynthetic pigments.
Lycopene affect the antioxidant activity of tomato (Chang and Liu, 2008). Lycopene act as antioxidant and promote decrease in DNA damage (Yildiz and Baysal, 2007). Results of the present study were contradicted with those reported by Pezzarossa et al. (2013). The reason may be the concentration and way of application of Se. Lee et al. (2007) also reported that lycopene was increased in ripe fruit of tomato after selenium application. Secondary metabolism and composition may be affected by selenium accumulation in fruit at ripening. Lycopene content increases because carotenoid biosynthetic path way is affected by Se accumulation. Sams et al. (2011) reported that when Arabidopsis was treated with Se, it regulated a key step in the carotenoid biosynthesis i.e. phytoene synthetase. However, in tomato fruit, Se may affect other carotenoid genes and enzymes (Sams et al., 2011).
Conclusions and Recommendations
Selenium affected most of the growth parameters of tomato plant including height, leaves, flowers and buds with its moderate level application through irrigation. Proximate composition was also affected by selenium application with its moderate level; However here the foliar application was more effective than irrigation, in relation to the effect of season. Selenium increased most of the minerals, including Cu, Zn and Se etc. By comparing irrigation versus foliar application of the selenium, the irrigation application proved to be more efficient. Selenium should be applied in moderate amount. Application should be conducted mainly through irrigation because it is more efficient than foliar application. Selenium also have adverse effect on chlorophyll concentration that may cause unhealthy plant growth, so should be avoided in higher doses. Se effect should be checked on other cultivars or hybrids for further investigation
Novelty Statement
Novelty of this research is the addition of selenium in moderate amount to tomato hybrid Salar F1 for their physical well-being and improvement of some chemical constituents.
Author’s Contribution
Sadaqat Khan: Conducted the research.
Saleem Ullah: Supervised the whole study.
Muhammad Sajid: Monitored the experiments.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdullahi, I., Ismail, Abdullahi, Nasiru, A.A. Muhammad, Ibrahim and A. Salisu. 2016. Proximate, mineral and vitamin analysis of fresh and canned tomato. Biosci. Biotechnol. Res. Asia, 13(2): 1163-1169. https://doi.org/10.13005/bbra/2147
Akbulut, M. and S. Cakir. 2010. The effects of Se phytotoxicity on the antioxidant systems of leaf tissues in barley (Hordeum vulgare L.) seedling. Plant Physiol. Biochem., 48: 160–166. https://doi.org/10.1016/j.plaphy.2009.11.001
AOAC, 2016. Official method of analysis. USA.
Arvy, M.P., 1992. Some aspects of selenium relationships in soils and plants. Commun. Soil Sci. Plant Anal., 23: 1397–1407. https://doi.org/10.1080/00103629209368675
Chaney, R.L., 1994. Trace metal movement: Soil plant systems and bioavailability of biosolids applied metals. In: Sewage sludge: Land utilization and the environment.
Chang, C.H. and Y.C. Liu. 2008. Evaluation of the antioxidative performance of tomato extracts obtained by different methods. J. Sci. Food Agric., 88: 612-618.
Chen, L., F.J. Yang, H. Xu, Q. Yun, Y. Hu, G. Zhang and Pan. 2002. Determination of selenium concentration of rice in China and effect of fertilization of selenite and selenate on Se content of rice. J. Agric. Food Chem., 50: 5128−5130. https://doi.org/10.1021/jf0201374
Chi, S., X. Weihong, L. Jun, W. Weizhong and X. Zhiting. 2017. Effect of exogenous selenium on activities of antioxidant enzymes, cadmium accumulation and chemical forms of cadmium in tomatoes. Int. J. Agric. Biol., ISSN Print: 1560–8530.
Combs, G.F., 2001. Selenium in global food systems. Br. J. Nutr., 85: 517−547. https://doi.org/10.1079/BJN2000280
Dhillon, K.S., S.S. Bawa and S.K. Dhillon. 1992. Selenium toxicity in some plants and soils of Punjab. J. Indian Soc. Soil Sci., 40(1): 132-136.
Djanaguiraman, M., D. Durga Devi, A.K. Shanker, J.A. Sheeba and U. Bangarusamy. 2005. Selenium an antioxidative protectant in soybean during senescence. Plant Soil, 272(1–2): 77–86. https://doi.org/10.1007/s11104-004-4039-1
Fernando, C.L., O. Karliana, M.R.Maria, P. Joao, P. Ines, C.R. Jose, E.L. Antonio, S.A. Ana, S.C. Paula, R.B. Ana, P.P. Isabel, M.S. Maria, F.P. Maria, H.R. Fernando. 2018. Selenium bio fortification of rice grains and implications on macronutrients quality. J. Cereal Sci., (81): 22-29. https://doi.org/10.1016/j.jcs.2018.03.010
Fu, D.D., M.L. Duan, D.L. Liang, S. S. Wang and X.P. Wu. 2011. Effects of selenite and selenate on growth and nutrient absorption of pakchoi. Plant Nutr. Fertiliz. Sci., 17, 358–365.
Germ, M., S. Vekoslav and K. Ivan. 2007. Metabolic importance of selenium for plants. Eur. J. Plant Sci. Biotechnol., 1(1): 91-97.
Godina, R.G., C., Pournavab, R. Foroughbakhch and M.A. Benavides. 2016. Effect of selenium on elemental concentration and antioxidant enzymatic activity of tomato plants. J. Agric. Sci. Tech., 18: 233-244.
Han-Wens, S., H. Jing, L. Shu-Xuan and K. Wei-Jun. 2010. Protective role of selenium on garlic growth under cadmium stress. Commun. Soil Sci. Plant Anal., 41: 1195–1204. https://doi.org/10.1080/00103621003721395
He, P.P., X.Z. Lv and G.Y. Wang. 2007. Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ. Int., 30(2): 167-172. https://doi.org/10.1016/S0160-4120(03)00167-3
Hu, Q., J. Xu and G. Pang. 2003. Effect of selenium on the yield and quality of green tea leaves harvested in early spring. J. Agric. Food Chem., 51: 3379–3381. https://doi.org/10.1021/jf0341417
Hu, X.R., W.B. Dong and R. Liu. 2015. Effects of the addition of selenium on trace element concentrations in Danshen (Salvia miltiorrhiza). Anal. Lett., 48: 513–525. https://doi.org/10.1080/00032719.2014.947536
Ivanova, E.M., V.P. Kholodova and V.V. Kuznetsov 2010. Biological effects of high copper and zinc concentrations and their interaction in rapeseed plants. Fiziologiya Rastenii, 57(6): 864–873.
Ježek, P., J. Hlušek, T. Lošák, M. Jůzl, P. Elzner, S. Kráčmar, F. Buňka and A. Martensson. 2011. Effect of foliar application of selenium on the content of selected amino acids in potato tubers (Solanum tuberosum L.). Plant Soil Environ., 57: 315-320. https://doi.org/10.17221/57/2011-PSE
Kim, S.A. and M.L. Guerinot. 2007. Mining iron: Iron uptake and transport in plants. FEBS Lett., 581: 2273–2280. https://doi.org/10.1016/j.febslet.2007.04.043
Kopsell, D.A., W.M. Randle and H.A. Mills. 2000. Quantitative, chemically specific imaging of selenium nutrient accumulation in leaf tissue of rapid-cycling Brassica oleracea response to increasing sodium selenate concentrations. J. Plant Nutr. Soil Sci., 23: 927-935. https://doi.org/10.1080/01904160009382071
Lee, G.J., B.K. Kang, T.I. Kim, T.J. Kim and J.H. Kim. 2007. Effects of different selenium concentrations of the nutrient solution on the growth and quality of tomato fruit in hydroponics. Acta Hortic., 761: 443-448. https://doi.org/10.17660/ActaHortic.2007.761.61
Li, X., B. Li and Y. Yang. 2018. Effects of foliar selenite on the nutrient components of turnip (Brassica rapa var. rapa Linn.). Front. Chem., 6: (42): 1-7. https://doi.org/10.3389/fchem.2018.00042
Malik, J.A., S. Kumar, P. Thakur, S. Sharma, R. Kaur, R. Kaur, D. Pathania, K. Bhandhari, N. Kaushal, K. Singh, A. Srivastav and H. Nayyar. 2011. Promotion of growth in mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol. Trace Elem. Res., 143: 530–539. https://doi.org/10.1007/s12011-010-8872-1
Many, J., Nirmala, B. Radhika and T. Ganesan. 2014. Nutrient analysis of tomato wine. Int. J. Innov. Sci. Eng. Technol., 1(8): 97-100. https://doi.org/10.9790/2402-081497100
Mayland, H.F., L.P. Gough and K.C. Stewart. 1990. Selenium mobility in soils and its absorption, translocation, and metabolism in plants. Proc. 1990 Billings Land Reclam. Symp. Selenium in Arid and Semiarid Environments, Western United States, Billings, Montana. 55-64.
Millaleo, R., M. Reyes-Díaz, A.G. Ivanov and M.L. Mora. 2010. Alberdi Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr., 10(4): 476 – 494. https://doi.org/10.4067/S0718-95162010000200008
Nancy, D. and P.I. Arulselvi. 2014. Effect of Selenium Fortification on Biochemical Activities of Tomato (Solanum lycopersicum) Plants. Indo Am. J. Pharm. Res., 4: 3997-4005.
Nancy, D.P. and I. Arulselvi. 2015. Effect of selenium fortification on vegetative and reproductive growth in tomato (Solanum lycopersicum). Biotechnology, 5(3): 22-49.
Pennanen, A., T. Xue and H. Hartikainen. 2002. Protective role of selenium in plant subjected to severe UV irradiation stress. J. Appl. Bot. Food Qual., 76: 66–76.
Pezzarossa, B., I. Rosellini, F. Malorgio, E. Borghesi and P. Tonutti. 2013. Effects of selenium enrichment of tomato plants on ripe fruit metabolism and composition. Proc. 7th Int. Postharvest Symp. Eds.
Prins, C.N., J.H. Laura, F.Q. Colin, A.H. Elizabeth and Pilon-Smits. 2011. Effects of selenium accumulation on reproductive functions in Brassica juncea and Stanleya pinnata. J. Exp. Bot., 62 (15): 5633–5640. https://doi.org/10.1093/jxb/err247
Qing, X., Z. Xiaohu, H. Chengxiao, W. Peng, Z. Ying, Z. Xuan, W. Pengcheng, S.H. Shi, J. Fen and Q. Chanjuan. 2015. Selenium alleviates chromium toxicity by preventing oxidative stress in cabbage (Brassica campestris L. ssp. Pekinensis) leaves. Ecotoxicol. Environ. Saf., 114: 179-189. https://doi.org/10.1016/j.ecoenv.2015.01.026
Sams, C.E., D.R. Panthee, C.S. Charron, D.A. Kospell and J.S. Yuan. 2011. Selenium regulates gene expression for glucosinolate and carotenoid biosynthesis in Arabidopsis. J. Am. Soc. Hortic. Sci., 136: 23-34. https://doi.org/10.21273/JASHS.136.1.23
Smith, G.S. and J.H. Watkinson. 1984. Selenium toxicity in perennial Rye grass and white clover. New Phytol., 97: 557-564. https://doi.org/10.1111/j.1469-8137.1984.tb03619.x
Song, Y.R., X.G. Jiang, S.F. Peng, F.C.J. Li, Liu and D. Chen. 2015. Effect of selenium content on the quality and functional components of selenium-riched Camellia oleifera oil. J. Chin. Inst. Food Sci. Technol., 15: 142–149.
Turakainen, M., Hartikainen, Helinä and M.S. Mervi. 2004. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem., 52(17): 5378–5382. https://doi.org/10.1021/jf040077x
White, J., Phillip and R.B. Martin. 2003. Calcium in plants. Ann. Bot., 92: 487-511. https://doi.org/10.1093/aob/mcg164
Xue, T., H. Hartikainen and V. Piironen. 2001. Antioxidative and growth- promoting effect of selenium on senescing lettuce. Plant Soil, 237: 55–61. https://doi.org/10.1023/A:1013369804867
Yildiz, H. and B. Taner. 2007. Color and lycopene content of tomato puree affected by electroplasmolysis. Int. J., 10: 3. https://doi.org/10.1080/10942910600909063
Ying, H, G.J. Norton, G. Duan, Y. Huang and Y. Liu. 2014. Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant Soil, 384(1–2): 131–140. https://doi.org/10.1007/s11104-014-2189-3
Zhu, L., W. Peng, Z. Wenjing, H. Feng and C. Xiangxiang. 2017. Effects of selenium application on nutrient uptake and nutritional quality of Codonopsis lanceolata. Sci. Hortic., pp. 574-580. https://doi.org/10.1016/j.scienta.2017.06.064
To share on other social networks, click on any share button. What are these?








