Effect of Varying Levels of Lipids and Proteins on the Growth Indices and Fatty Acid Profile of Labeo rohita (Rohu)
Effect of Varying Levels of Lipids and Proteins on the Growth Indices and Fatty Acid Profile of Labeo rohita (Rohu)
Jhan Zeb1*, Saiqa Tufail1, Nausheen Saboohi1, Zafar Samuel2, Asher Azeem3, Yaseen Amir4 and Sehrish Akram5
1Department of Zoology, Government Postgraduate College, Gojra, Pakistan.
2Department of Chemistry, Omea University, Omea, Sweden.
3Department of Fisheries, DCO Complex, Khanewal, Pakistan.
4Directorate of Colleges, Toba Tek Singh, Pakistan.
5Department of Zoology, University of the Punjab, Lahore, Pakistan.
ABSTRACT
This project was a 2×2 factorial design executed for the evaluation of optimum lipid/protein level in pelleted diets for Labeo rohita (Initial weight; 2.87±0.01g). Four pelleted diets varying in their lipid/protein levels i.e. 7.5/25, 9.5/25, 7.5/30 and 9.5/30% were formulated and hand fed for 90 days to four groups of 10 fish each. Results of the present study showed that L. rohita attained significantly higher average wet weights, fork and total lengths of 4.79±0.04g, 66.63±0.04mm and 74.67±0.04mm, respectively due to 9.5/30% lipid/protein diet (D4). None of the dietary lipid/protein levels in pelleted diet had a significant impact on either the condition or the survival of fish. Significantly higher (p<0.05) specific growth rate (SGR) and feed efficiency (FE) for L. rohita was also due to 9.5/30% lipid/protein diet (D4). The pelleted diets upon fatty acid analysis showed almost complete absence of linoleic acid, α-linolenic acid, ecosapentanoic acid (EPA) and docosahexanoic acid (DHA). However, these fatty acids were found to be present in the flesh of L. rohita at the end of the experiment. The sum of saturated fatty acids (SFA) was higher as compared to the sum of monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) in L. rohita flesh. Regardless of the feeding regimes there existed a higher proportion of n-3 fatty acids as compared to n-6 fatty acids in the flesh of L. rohita. Ratio of n-3/n-6 fatty acids in L. rohita flesh was significantly higher (p<0.05) due to 9.5/25% (D2) lipid/protein level in pelleted diet. Moisture contents of L. rohita varied significantly among the treatments, showing an inverse relationship with body fats. The feeding regime D4 (9.5/30% lipid/protein level) gave fish the highest flesh proteins i.e. 19.17±0.03%. The carcass lipid were generally higher in fish groups fed lower protein level whereas the fish groups fed higher protein level fetched comparatively lower body lipids. Body ash contents of L. rohita varied slightly among the treatments showing no obvious significant influence of varying lipid/protein levels.
Article Information
Received 09 December 2016
Revised 15 October 2020
Accepted 20 November 2020
Available online 08 March 2021
(early access)
Published 07 January 2022
Authors’ Contribution
JZ designed the experiments and wrote the manuscript. ST and NS conducted the experiments. ZS and SA helped with the fatty acid profiling. YA and AZ dealt with data analysis.
Key words
Saturated fatty acids (SFA), Monounsaturated fatty acids (MUFA), Polyunsaturated fatty acids (PUFA), Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA), Labeo rohita, Specific growth rate, GC analysis
DOI: https://dx.doi.org/10.17582/journal.pjz/20161209091247
* Corresponding author: [email protected]
0030-9923/2022/0002-0615 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
INTRODUCTION
Table fish is a food commodity that provides a balanced profile of nutrients to humans worldwide. Fish meat serves as a valuable human resource; contributing towards a nation’s economy and development (Teklu and Lema, 2015). Fish provides its consumers with an optimum mix up of essential fatty acids and all the crucial amino acids (Jabeen and Choudhary, 2011). Furthermore, is highly alimentative that provides energy required for daily human activities (Ljubojevic et al., 2013). Pakistan has been blessed with diverse fish fauna as well as fish habitats (Nazir et al., 2015). Unfortunately, least efforts have been exerted to channelize these abundant aquatic resources, towards commercial ventures.
Wild fishery is a natural resource that is renewable but at the same time it is highly vulnerable. Despite of their high rate of natural propagation these fisheries resources seems to be finite and limited (Sargent and Tacon, 1999). Fish catches in the natural environment are on the verge of decline. Only sustainable fish culture practices can bring this decline to compensation. Food production sectors such as poultry, livestock and fisheries need to be boosted through managerial practices for the provision of sustainable and secure animal proteins for human use (Nazir et al., 2015).
Labeo rohita is a commercial fish species, cultured throughout the sub-continent for its demand as food fish (Das et al., 2005). Flesh of L. rohita is also an important source of polyunsaturated fatty acids required for normal growth and development of humans (Memon et al., 2011). Taste acceptance, alimentative properties and commercial demand brings L. rohita substantial superiority over other locally reared fish species (Jabeen and Choudhary, 2011). Aquaculture is an expeditiously flourishing venture designed as a source of protein generation for human utilization (De Silva and Turchini, 2008). Intensive culture of fish causes a maximum yield per unit area at a minimum cost. In recent decades, intensive fish culture is preferred to be anticipated since it require fewer space and rapid fish tissue build up as compared to any other culture system. The main objective of this industrialized system is only to bring an increase in fish biomass within minimum possible time. Intensive culture of fish is a reliable means to promote food production in concomitant with human needs (Abid and Ahmed, 2009).
The ongoing aim in fish culture is to promote the growth of fish by supplying the fish with least cost artificial diets. Fish is a cold blooded animal that requires a higher level of nutrients in their diet that can be made available to the fish through artificial diets (Kadhar et al., 2012). However, care should be taken into account in order to develop a sustainable feeding regime that is economically beneficial as well as efficient growth promoter of fish (Srivastava et al., 2013). Besides proteins, dietary lipids are also of utmost importance for fish because they serve as an energy source and offers protection against environmental stresses (Hsieh et al., 2007). Fish meat is a known source of omega-3 fatty acids that are required by cardiovascular patients to lower their serum cholesterol (Kandemir and Polat, 2007; Hosseini et al., 2010). Apart from these aforementioned alimentative properties, fish oil has been found to be an enriched source of docosahexaenoic acid (DHA) (22:6; n-3) as well as eicosapentaenoic acid (EPA) (20:5; n-3) that are meant to counter metabolic and other related syndromes (Breslow, 2006). Linoleic acid and α-linolenic acid are the major essential fatty acids that are of special interest since the humans lack an enzyme for the synthesis of these essential fatty acids (Nelson and Cox, 2008). Linoleic acid and α-linolenic acid act as a precursor of docosahexaenoic acid (DHA) as well as eicosapentaenoic acid (EPA), both of which play important role as components of cell membranes, vital for the cell support, developmental purposes and strengthening of the immune system (Memon et al., 2011). (EPA) and (DHA) both are valuable because these fatty acids bring prevention from cardiovascular diseases and other health disorders (Rasoarahona et al., 2004).
This investigation was pursued for the evaluation of fatty acid profile of L. rohita flesh fed various lipid/protein graded diets. The purpose was also to test the ability of this fish species to synthesize the essential fatty acid viz. linoleic acid and α-linolenic acid and the resulting benefits these fatty acids confer upon human health and wellbeing.
MATERIALS AND METHODS
Fish species and feeding regimes
Labeo rohita (Rohu) fingerlings with an initial average body weight of 2.87±0.01g (purchased from Government Fish Seed Hatchery, Peer Mahal) were reared in the Aquaculture Research Laboratory, Government postgraduate college, Gojra from April to June 2016 under approximately 12/12 light dark period. Fish were acclimatized to laboratory conditions for 15 days and then randomly distributed into 12 homogenous groups of 10 fish each. Each fish group was reared in triplicate in 100L capacity glass aquaria supplied with electric aerators. Triplicate groups of fish were fed four isoenergetic pelleted diets, differing in their lipid and protein levels (Table I). The four pelleted diets fed to fish contained 7.5/25, 9.5/25, 7.5/30 and 9.5/30% lipid/protein level and were designated as D1, D2, D3 and D4, respectively. Fish were fed to visual satiation manually, twice daily for a period of 15 days. Amount of feed consumed and the growth exhibited by the fish were monitored fortnightly.
Table I. Formulation and proximate composition (%) of pelleted diets for Labeo rohita.
|
Ingredients (lipid/protein %) |
D1 (7.5/25) |
D2 (9.5/25) |
D3 (7.5/30) |
D4 (9.5/30) |
|
Wheat |
15 |
17 |
15 |
13 |
|
Rice broken |
15 |
8 |
1 |
1 |
|
Rice polish |
8 |
12 |
8 |
8 |
|
Wheat bran |
8 |
7 |
8 |
7 |
|
Canola meal |
22 |
16 |
22 |
14 |
|
Sunflower meal |
7 |
15 |
7 |
18 |
|
Corn glutton |
5 |
5 |
5 |
5 |
|
Fish meal |
18 |
18 |
32 |
32 |
|
Corn oil |
1 |
1 |
1 |
1 |
|
Premix |
1 |
1 |
1 |
1 |
|
Proximate composition (%) |
||||
|
Moisturea |
7.77 |
6.57 |
7.63 |
6.71 |
|
Crude proteina |
25.09 |
25.34 |
30.20 |
30.33 |
|
Crude lipidsa |
7.65 |
9.45 |
7.59 |
9.43 |
|
Crude Asha |
4.57 |
5.5 |
5.94 |
6.70 |
|
Carbohydratesb |
54.92 |
53.14 |
48.64 |
46.83 |
|
Energy (Kcal/kg)c |
2534 |
2493 |
2520 |
2450 |
Note: Vitamin premix contains (gkg -1dry weight); Vit A, 3600000IU; Vit D3, 1200000IU; Vit E, 4200mg; Iron, 10000mg; β Carotene, 40mg; Zinc, 14000mg; Manganese, 16000mg; Casein, 1800mg; Cobalt, 160mg; Selenium, 60mg; Copper, 2400mg; Nicotinamide, 2400mg; Dicalcium phosphate, 80000mg. aEstimated in triplicate; bEstimated by difference (Moisture + Crude protein + Crude fat + Ash) – 100; cEstimated through Bomb Calorimeter (Parr Instrument Company Moline, USA).
Fish growth performance
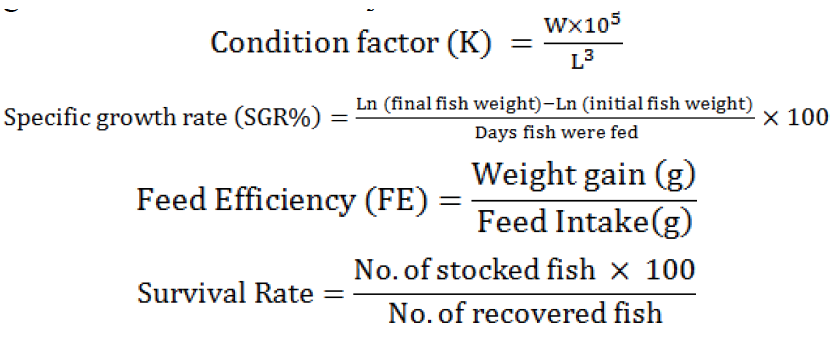
The following formulae were applied to calculate fish growth and feed efficiency.
Aftermath of the trial
For the determination of fish flesh proximate composition and fatty acid profile each of the four experimental groups of fish were starved for 24 h and then sampled randomly to begin the analysis procedure. Each group of fish were filleted, freeze dried, ground into fine powder and stored at -20ºC, packed in screw-top glass bottles until used for proximate composition or fatty acid profile.
Proximate composition of fish flesh and pelleted diets
Proximate composition of fish flesh and pelleted diets were analyzed through AOAC (2006). All the methods were run in triplicate and a very brief description of the methods is as follows. For the determination of moisture, the flesh and diet samples were oven dried at 102ºC for 24 h. Crude protein was determined by Kjeldhal method (Crude protein= Nitrogen×6.25). Crude lipids were estimated through extraction with chloroform: methanol (2:1); crude ash was determined in a muffle furnace at 550ºC for 6 h; carbohydrates were determined by subtracting the sum of moisture, crude protein, crude lipids and crude ash by 100.
Fatty acid methyl esters (FAME) preparation
Fatty acid methyl esters (FAME) synthesis method (Indrati et al., 2005) was employed for the fatty acid analysis of fish flesh and pelleted diets. 100mg powdered fish flesh and or diet in triplicate was taken into 10ml screw top glass bottles. 4ml mixture (1.7ml methanol: 0.3ml H2SO4: 2ml Chloroform) was added to it. Each bottle was vortexed for 20 seconds and tightly closed by a teflon cap to prevent any leakage. For the purpose of carrying out trans-esterification each bottle was placed in heating block at 90ºC for 90 minutes. After the completion of trans-esterification process each bottle was cooled to room temperature and leaking bottles were discarded. Now 1ml of distilled water was added to each bottle and allowed to stand for a minute. Two phases were developed the upper phase was discarded whereas the lower phase containing the FAME was dried with anhydrous Na2SO4. Each sample was transferred into polypropylene eppendorf tube that was tightly closed with a lid, labeled and refrigerated until GC (Gas Chromatography) analysis.
Gas chromatography (GC) analysis
Gas chromatography was performed at Central High-Tech Laboratory, University of Agriculture, Faisalabad, Pakistan. All tests were done on a gas chromatogram GC-17A (SHIMADZU) equipped with flame ionization detector and a fused silica capillary SGE column. Each sample was allowed to run in a gas chromatograph using nitrogen as a carrier gas on the mobile phase. The length of capillary column was 624 to 30 m and the diameter was 3.0µ to 0.32 id. The flow rate of the gas was 30cm per min. The peaks were identified by using external FAME standards (BP 1250mV, 10 samples, per second, peak width 0.020 min and the threshold level, 0.050 mV). The detection was done by FID at 2500C and at a pressure of 5.57 psi. The temperature of column was set and held at 100ºC for 1 min. The temperature was then raised at 15ºC/minute up to 240ºC. The temperature of the injector port was set at 260ºC. Fatty acids were determined by comparing the retention times of FAME with a standard component FAME mixture. GC analyses were performed in triplicate and the results were expressed as mean value ± standard deviation.
Water quality attributes
For the purpose of maintaining a healthy environment for fish rearing the water quality attributes i.e. water temperature, pH and dissolved oxygen were measured through meters i.e. HANNA HI-8053, HI-8520 and HI-9146, respectively on daily basis.
Statistical analysis
The data were subjected to one factor and two factor analysis of variance (ANOVA) at a significance level of 0.05. Significance of means was further tested through Tukey’s Honestly Significant Difference posthoc test. All the statistical analyses were performed through the statistical software, Statistix® (version 8.1; Analytical Statistix Software, Tallahassee, USA).
RESULTS
Fish growth and feed efficiency
Results pertaining to variations in fish average weight, fork length, total length, condition factor, specific growth rate, feed efficiency and fish survival are presented in Table II. The growth performance of the fish i.e. final average weight, fork and total length increased significantly
Table II. Growth and feed efficiency of Labeo rohita fed varying lipid/protein levels (%).
|
Parameters |
D1(7.5/25) |
D2(9.5/25) |
D3(7.5/30) |
D4(9.5/30) |
|
Initial weights (g) |
2.87±0.01 |
2.87±0.01 |
2.85±0.01 |
2.87±0.01 |
|
Final weights (g) |
4.22±0.03c |
4.23±0.03c |
4.69±0.03b |
4.79±0.04a |
|
Initial fork lengths (mm) |
56.3±0.03 |
58.4±0.03 |
56.3±0.03 |
57.90±0.04 |
|
Final fork lengths (mm) |
64.22±0.03d |
65.02±0.03c |
65.37±0.04b |
66.63±0.04a |
|
Initial total lengths (mm) |
65.1±0.07 |
63.8±0.02 |
62.2±0.03 |
63.6±0.04 |
|
Final total lengths (mm) |
72.42±0.04d |
72.50±0.02c |
73.90±0.03b |
74.67±0.04a |
|
Condition factor |
1.10±0.00a |
1.10±0.00a |
1.13±0.00a |
1.12±0.01a |
|
Specific growth rate |
0.80±0.05c |
0.78±0.02c |
0.97±0.06b |
1.10±0.04a |
|
Feed efficiency |
0.14±0.01c |
0.14±0.00c |
0.17±0.01b |
0.19±0.01a |
|
Fish Survival |
100±0.00a |
100±0.00a |
100±0.00a |
100±0.00a |
Similar alphabets in the same row are not significantly different (p>0.05).
Table III. Fatty acid profile (g 100g-1) of pelleted diet offered to Labeo rohita.
|
Fatty acids |
Formulae |
D1 (7.5/25) |
D2 (9.5/25) |
D3 (7.5/30) |
D4 (9.5/30) |
|
Heptanoic acid |
C 7:0 |
0.17 |
0.18 |
0.16 |
0.19 |
|
Decanoic acid |
C 10:0 |
0.03 |
0.05 |
0.02 |
0.06 |
|
Dodecanoic acid |
C 12:0 |
0.10 |
0.02 |
0.01 |
0.04 |
|
Myristic acid |
C 14:0 |
0.43 |
0.52 |
0.44 |
0.59 |
|
Pentadecanoeic acid |
C 15:0 |
0.07 |
0.06 |
0.09 |
0.10 |
|
Palmitic acid |
C 16:0 |
8.40 |
7.33 |
7.45 |
8.56 |
|
Stearic acid |
C 18:0 |
4.31 |
4.28 |
4.32 |
4.63 |
|
Arachidic acid |
C 20:0 |
1.91 |
1.78 |
1.85 |
1.99 |
|
Docosanoic acid |
C 22:0 |
2.00 |
1.97 |
2.12 |
2.35 |
|
Palmitoleic acid |
C 16:1 n-7 |
0.04 |
0.01 |
0.05 |
0.06 |
|
Oleic acid |
C 18:1 n-9 |
14.78 |
13.66 |
13.89 |
14.98 |
|
Erucic acid |
C 22:1 n-9 |
28.45 |
27.97 |
27.58 |
28.65 |
|
Nervonic acid |
C 22:1 n-9 |
2.30 |
2.00 |
2.36 |
2.45 |
|
Linoleic acid |
C 18:2 n-6 |
0.01 |
0.01 |
0.01 |
0.01 |
|
α-linolenic acid |
C 18:3 n-3 |
0.01 |
0.01 |
0.01 |
0.01 |
|
EPA |
C 20:5 n-3 |
0.01 |
0.01 |
0.01 |
0.01 |
|
DHA |
C 22:6 n-3 |
0.01 |
0.01 |
0.01 |
0.01 |
|
Σ SFA* |
17.42 |
16.01 |
16.46 |
18.51 |
|
|
Σ MUFA¶ |
45.57 |
43.64 |
43.88 |
46.14 |
|
|
Σ PUFAǀ |
0.04 |
0.04 |
0.04 |
0.04 |
|
|
Σ n-3ǂ |
0.03 |
0.03 |
0.03 |
0.03 |
|
|
Σ n-6ǁ |
0.01 |
0.01 |
0.01 |
0.01 |
|
|
n-3/n-6† |
3.00 |
3.00 |
3.00 |
3.00 |
EPA, Ecosapentanoic acid; DHA, Docosahexanoic acid; *Sum of saturated fatty acids; ¶Sum of monounsaturated fatty acids; ǀSum of polyunsaturated fatty acids; ǁSum of omega 6 fatty acids; ǂSum of omega 3 fatty acids; †Ratio of omega 3/omega 6 fatty acids.
(p<0.05) as the level of lipid/ protein ratio increased in the pelleted diets. None of the lipid/ protein level in the pelleted diet was able to cause any significant variation in the condition or survival of the fish. Both specific growth rate and feed efficiency of the fish were significantly (p<0.05) higher as 1.10±0.04 and 0.19±0.01, respectively due to 9.5/30% lipid/protein level in pelleted diets.
Fatty acid profile of pelleted diets and fish flesh
The fatty acid profile of the pelleted diets is presented in Table III. The pelleted diets upon fatty acid analysis showed almost complete absence of linoleic acid, α-linolenic acid, EPA and DHA. Fatty acid profile of Labeo rohita flesh is presented in Table IV. The sum of saturated fatty acids (SFA), monosaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) in the flesh of L. rohita plateaued at the highest lipid/protein level i.e. 9.5/30% (D4). However, a gradual decrease in lipid/protein level of the pelleted diets caused a general decline in SFA, MUFA and PUFA of fish flesh. The principal omega-6 fatty acid recorded in the fish flesh was linoleic acid whereas principal omega-3 fatty acids recorded were α-linolenic acid, EPA and DHA. The concentrations of linoleic acid, α-linolenic acid, EPA and DHA in fish flesh increased significantly (p<0.05) with an increase in lipid/protein level of the pelleted diet. Ratio of n-3/n-6 fatty acids in L. rohita flesh was significantly higher (p<0.05) due to 9.5/25% (D2) lipid/protein level in pelleted diet.
Proximate composition of pelleted diets and fish flesh
The formulation and proximate composition of the pelleted diets were in conformation to the each other with respect to lipid/protein levels (Table I). The proximate composition (moisture, crude protein, crude lipids, crude ash and carbohydrates) of L. rohita flesh are summarized in Table V. The moisture contents of L. rohita flesh plateaued when fed 7.5/30% lipid/protein diet (D3). Crude protein content value of L. rohita flesh were highest as 19.17±0.03% as a result of feeding the fish 9.5/30% lipid/protein diet (D4), followed by D2, D1 and D3. On the other hand, lipid contents of fish flesh were generally higher in fish groups fed lower protein level i.e. D1 and D2 as compared to fish groups fed higher protein level i.e. D3 and D4. Ash contents varied slightly among the treatments showing no significant influence of varying lipid/protein levels in pelleted diets. Fish group fed lowest lipid/protein level i.e. 7.5/25% (D1) in pelleted diet fetched significantly higher (p<0.05) carbohydrates in their flesh.
Water quality management
The temperature, pH and dissolved oxygen of the aquarium water did not varied significantly (p>0.05) across the treatments (Table VI).
Table IV. Fatty acid profile (g 100g-1) of Labeo rohita flesh at final harvest.
|
Fatty acids |
Formulae |
D1 (7.5/25) |
D2 (9.5/25) |
D3 (7.5/30) |
D4 (9.5/30) |
p value |
|
Dodecanoic acid |
C 12:0 |
0.39±0.07c |
0.42±0.07c |
0.56±0.09b |
0.91±0.05a |
p<0.01 |
|
Myristic acid |
C 14:0 |
7.14±0.04d |
8.11±0.07c |
8.62±0.06b |
8.83±0.04a |
p<0.01 |
|
Pentadecanoeic acid |
C 15:0 |
1.51±0.13b |
1.41±0.15b |
2.00±3.60a |
2.45±0.07b |
p<0.01 |
|
Palmitic acid |
C 16:0 |
29.68±0.10b |
27.54±0.06d |
28.16±0.09c |
30.14±0.06a |
p<0.01 |
|
Stearic acid |
C 18:0 |
11.45±0.11d |
12.11±0.03c |
13.07±0.06b |
13.79±0.10a |
p<0.01 |
|
Palmitoleic acid |
C 16:1 n-7 |
0.55±0.13b |
0.63±0.05b |
0.77±0.15b |
1.10±0.04a |
p<0.01 |
|
Oleic acid |
C 18:1 n-9 |
10.42±0.11d |
11.31±0.09b |
10.57±0.11c |
12.63±0.10a |
p<0.01 |
|
Erucic acid |
C 22:1 n-9 |
0.41±0.05c |
0.52±0.12b |
0.61±0.04b |
1.16±0.07a |
p<0.01 |
|
Linoleic acid |
C 18:2 n-6 |
6.43±0.13c |
5.31±0.04d |
6.59±0.07b |
7.48±0.09a |
p<0.01 |
|
α-linolenic acid |
C 18:3 n-3 |
2.15±0.06d |
3.10±0.04c |
3.42±0.13b |
4.12±0.06a |
p<0.01 |
|
EPA |
C 20:5 n-3 |
3.11±0.03c |
3.09±0.04c |
3.77±0.09b |
4.32±0.07a |
p<0.01 |
|
DHA |
C 22:6 n-3 |
4.99±0.01d |
5.11±0.04c |
5.81±0.08b |
6.18±0.06a |
p<0.01 |
|
Σ SFA* |
50.17c |
49.59c |
52.41b |
56.12a |
||
|
Σ MUFA¶ |
11.38c |
12.46b |
11.95c |
14.89a |
||
|
Σ PUFAǀ |
28.06d |
29.07c |
31.54b |
36.99a |
||
|
Σ n-3ǂ |
10.25d |
11.30c |
13.00b |
14.62a |
||
|
Σ n-6ǁ |
6.43b |
5.31c |
6.59b |
7.48a |
||
|
n-3/n-6† |
1.59c |
2.13a |
1.97b |
1.95b |
Similar alphabets in the same row are not significantly different (p>0.05). for abbreviations and other statistical details, see Table II.
Table V. Proximate composition of Labeo rohita flesh fed varying lipid/protein levels (%).
|
Flesh parameters |
D1(7.5/25) |
D2(9.5/25) |
D3(7.5/30) |
D4(9.5/30) |
|
Moisture (%) |
76.00±2.00c |
78.00±2.00b |
82.00±2.64a |
78.00±2.64b |
|
Crude Protein (%) |
18.37±0.02ab |
19.00±1.73a |
15.24±0.02b |
19.17±0.03a |
|
Crude Lipids (%) |
1.35±0.01a |
1.32±0.03a |
0.75±0.02c |
1.02±0.01b |
|
Crude Ash (%) |
1.01±0.04b |
1.03±0.04ab |
1.05±0.03a |
1.04±0.04ab |
|
Carbohydrates (%) |
3.27±0.01a |
0.65±0.02d |
0.99±0.01b |
0.77±0.03c |
Similar alphabets in the same row are not significantly different (p>0.05).
Table VI. Water quality attributes during 90 days of the experiment.
|
Quality parameters |
D1(7.5/25) |
D2(9.5/25) |
D3(7.5/30) |
D4(9.5/30) |
|
Water Temperature (°C) |
29.01±0.30a |
29.02±0.43a |
29.16±0.09a |
28.91±0.06a |
|
DO (mgL-1) |
6.170±0.02a |
6.170±0.03a |
6.162±0.03a |
6.177±0.03a |
|
pH |
8.025±0.03a |
8.005±0.03a |
8.025±0.04a |
8.072±0.04a |
Similar alphabets in the same row are not significantly different (p>0.05).
DISCUSSION
Fish growth and feed efficiency
In this investigation the growth performance i.e. final average weight, fork and total length of L. rohita treated with 9.5/30% lipid/protein diet (D4) were significantly (p<0.05) higher than the fish fed with any other lipid/protein level. Satpathy et al. (2003) also obtained optimal growth of L. rohita when fed diet at 30% protein and 10% lipids therefore, this 9.5/30% level in pelleted diets may be deemed optimal for maximum yield of L. rohita under intensive culture condition. Condition factor is the growth parameter that represents the degree of well-being of cultured fish species, with respect to their wet body weight and total length (Javed, 2015). None of the dietary lipid/protein levels in pelleted diet had any significant impact on either the condition or the survival of fish in the present study, indicating the potential of all these lipid/protein levels to keep fish supple under each treatment. Kim et al. (2012) also demonstrated a lack of impact of varying lipid levels in olive flounder diet on its condition. Zeb (2016) also recorded 100% fish survival when pond raised cyprinids were fed pelleted diets varying in their protein contents. Specific growth rate of fish is the estimation of fish growth under specific period of time whereas feed efficiency is a growth parameter that explains relationship of feed with the growth of fish. Specific growth rate and feed efficiency of L. rohita were significantly (p<0.05) effected due to varying lipid/protein levels. Increase in dietary protein level along with an increase in dietary lipids caused a significant (p˂0.05) increase in L. rohita specific growth rate and feed efficiency. Aminikhoei et al. (2015) also observed a significant improvement in the specific growth rate and feed efficiency of Cyprinus carpio with a unit increase in the lipid/protein level in pelleted diets.
Fatty acid profile of pelleted diets and fish flesh
The pelleted diets upon fatty acid analysis showed almost complete absence of linoleic acid, α-linolenic acid, EPA and DHA. However, these fatty acids were found to be present in the flesh of L. rohita at the end of the experiment. Paiko et al. (2010) also observed the occurrence of DHA in the flesh of Channa striatus when fed pelleted diets either deficient or contained very low levels of DHA. The occurrence of these fatty acids in finally harvested L. rohita flesh demonstrated the ability of this fish species to synthesize these fatty acids even when not provided in their diet. This might have occurred due to the possession of certain enzymes called desaturases and elongases by the fish that caused the desaturation and ultimate synthesis of these fatty acids (Zheng et al., 2009). Dietary lipid source is one of the major factors that influenced the fatty acids profile of fish flesh (Choi and Lee, 2015). In this investigation corn oil was used as a lipid source in the pelleted fish diets. The sum of SFA, MUFA and PUFA in the flesh of L. rohita plateaued at the highest lipid/protein level i.e. 9.5/30% (D4). Sharma et al. (2010) also observed similar higher values of the sum of SFA, MUFA and PUFA in L. rohita fed with pelleted diet against wild L. rohita. The sum of SFA was higher as compared to the sum of MUFA and PUFA in L. rohita flesh in the present study. Similar findings were also reported by Ozparlak (2013) where he also observed a higher percentage of SFA in Cyprinus carpio flesh against the percentage of PUFA. These findings suggest that these carp species possibly contain a higher SFA percentage in their flesh against MUFA or PUFA percentage. The n-3/n-6 fatty acids balance is one of the most important attribute of edible human food (Ghomi et al., 2012). Human diet all over the world is susceptible to n-3/n-6 fatty acid imbalance; through containing a higher proportion of n-6 PUFA as compared to n-3 PUFA (Strobel et al., 2012). Any diet containing a higher proportion of n-3 PUFA and a lower proportion of n-6 PUFA are considered suitable for human health (Nelson and Cox, 2008). The results of the present study showed the occurrence of a higher proportion of n-3 fatty acids as compared to n-6 fatty acids in L. rohita flesh regardless of the treatments. This shows the significance of L. rohita flesh as an edible product containing most favorable fatty acid profile.
Proximate composition of pelleted diets and fish flesh
Proximate analysis of the pelleted diets generally conformed to the formulated lipid/protein levels. Moisture contents of L. rohita varied significantly among the treatments, showing an inverse relationship with body fats. Increase in moisture causes decrease in fat contents and vice versa. Similar inverse moisture/fat relation has also been reported by Ashraf et al. (2011) but for other carp species. The feeding regime D4 (9.5/30% lipid/protein level) gave fish the highest flesh proteins i.e. 19.17±0.03%. Significantly higher (p<0.05) average body weight, fork and total length of L. rohita at this lipid/protein level i.e. D4 may be a direct consequence of accumulated flesh proteins. Kim et al. (2016) also observed similar results while studying the growth performance of juvenile parrot fish, Oplegnathus fasciatus in relation to carcass proteins. The carcass lipid were generally higher in fish groups fed lower protein level whereas the fish groups fed higher protein level fetched comparatively lower body lipids. Fingerlings of Channa straitus also accumulated higher body lipids when fed lower dietary proteins and vice versa (Paiko et al., 2010). Body ash contents of L. rohita varied slightly among the treatments showing no obvious significant influence of varying lipid/protein levels. Ash contents also did not show significant differences due to dietary protein for juvenile parrot fish (Kim et al., 2016) or showed slight differences in response to dietary lipids for Juvenile black fin sea bream (Rahim et al., 2015). The water quality parameters were within the permissible range for healthy fish culture.
CONCLUSIONS
The lipid/protein level i.e. 9.5/30% in pelleted diets may be deemed optimal for maximum yield, specific growth rate, feed efficiency and survival of Labeo rohita under intensive culture condition. This study also demonstrated the potential existence of desaturases and elongases in L. rohita, as this fish was able to synthesize the essential fatty acids without being provided in diet. There existed the occurrence of a higher proportion of n-3 fatty acids as compared to n-6 fatty acids in L. rohita flesh, indicating the significance of L. rohita flesh as an edible product conferring health benefits to humans. However, further research need to be sought in order to study the mechanism by which these desaturases and elongases operate in the fish.
ACKNOWLEDGEMENT
The authors are extremely grateful to the Principal, Government Postgraduate College, Gojra, Prof. Munir Ahmed for the provision of funds and facilities that enabled us to execute this project.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abid, M. And Ahmed, M.S., 2009. Growth responses of Labeo rohita fingerlings fed with different feeding regimes under intensive rearing. J. Anim. Pl. Sci., 19: 45-49.
Aminikhoei, Z., Choi, J. and Lee, S.M., 2015. Optimal dietary protein and lipid levels for growth of juvenile Israeli carp Cyprinus carpio. Fish. aquat. Sci., 18: 265-271. https://doi.org/10.5657/FAS.2015.0265
AOAC, 2006. Official methods of analysis. 18th Edition, Association of Official Analytical Chemists, Gaithersburgs, MD.
Ashraf, M., Zafar, A., Rauf, A., Mehboob, S. and Qureshi, N.A., 2011. Nutritional values of wild and cultivated silver carp (Hypophthalmichthys molitrix) and grass carp (Ctenopharyngodon idella). Int. J. Agric. Biol., 13: 210-214.
Breslow, J.L., 2006. n-3 fatty acids and cardiovascular disease. Am. J. clin. Nutr., 83: 1477-1482S. https://doi.org/10.1093/ajcn/83.6.1477S
Choi, J. and Lee, S.M., 2015. Effects of dietary lipid sources on body fatty acid composition of Chinese longsnout catfish Leiocassis longirostris Gunther. Fish. aquat. Sci., 18: 359-365. https://doi.org/10.5657/FAS.2015.0359
Das, T., Pal, A.K., Chakraborty, S.K., Manush, S.M., Sahu, N.P. and Mukherjee, S.C., 2005. Thermal tolerance, growth and oxygen consumption of Labeo rohita fry (Hamilton, 1822) acclimated to four temperatures. J. therm. Biol., 30: 378-383. https://doi.org/10.1016/j.jtherbio.2005.03.001
De Silva, S.S. and Turchini G.M., 2008. Towards understanding the impacts of the pet food industry on world fish and seafood supplies. J. Agric. environ. Ethics, 21: 459-467. https://doi.org/10.1007/s10806-008-9109-6
Ghomi, M.R., Nikoo, M. and Pourshamsian, K., 2012. Omega-6 and omega-3 essential fatty acid ratio in cultured beluga sturgeon. Comp. clin. Paththol., 21: 479-483. https://doi.org/10.1007/s00580-012-1495-5
Hosseini, S.V., Kenari, A.A., Rezaei, M. and Nazari, R.M., 2010. Influence of the in vivo addition of alpha-tocopheryl acetate with three lipid sources on the lipid oxidation and fatty acid composition of Beluga surgeon, Huso huso, during frozen storage. Fd. Chem., 118: 341-348. https://doi.org/10.1016/j.foodchem.2009.04.131
Hsieh, S.L., Hu, C.Y., Hsu, Y.T. and Hsieh, T.J., 2007. Influence of dietary lipids on the fatty acid composition and stearoyl-CoA desaturase expression in hybrid tilapia (Oreochromis niloticus × O. aureus) under cold shock. Comp. Biochem. Physiol., 147: 438-444. https://doi.org/10.1016/j.cbpb.2007.02.010
Indrati, E., Majid, A.M.I., Hashim, R. and Chong, A., 2005. Direct FAME synthesis for rapid total lipid analysis from fish oil and cod liver oil. J. Fd. Comp. Anal., 18: 161-170. https://doi.org/10.1016/j.jfca.2003.12.007
Jabeen, F. and Choudhry, A.S., 2011. Chemical compositions and fatty acid profiles of three freshwater fish species. Fd. Chem., 125: 991-996. https://doi.org/10.1016/j.foodchem.2010.09.103
Javed, M., 2015. Chronic dual exposure (water borne+dietary) effects of cadmium, zinc and copper on growth and their bioaccumulation in Cirrhina mrigala. Pak. Vet. J., 35: 143-146.
Kadhar, M.A., Mustafa, M.S., Christrarasu, P., Arunkumar, M.S. and Ali, A.J., 2012. Growth responses of two indian major carps Catla catla (Hamilton) and Cirrhinus mrigala (Hamilton) fingerlings fed diets containing different nutritional supplements. Int. J. appl. Biol. Pharm. Tech., 3: 280-285.
Kandemir, S. and Polat, N., 2007. Seasonal variation of total lipid and total fatty acid in muscle and liver of Rainbow trout (Oncorhynchus mykiss) reared in Derbent Dam Lake. Turk. J. Fish. aquat. Sci., 7: 27-31.
Kim, D.K., Kim, K.D., Seo, J.Y. and Lee, S.M., 2012. Effects of dietary lipid source and level on growth performance, blood parameters and flesh quality of sub-adult olive flounder (Paralichthys olivaceus). Asian-Aust. J. Anim. Sci., 25: 869-879. https://doi.org/10.5713/ajas.2011.11470
Kim, K.W., Moniruzzaman, M., Kim, K.D., Han, H.S., Yun, H., Lee, S. and Bai, S.C., 2016. Effects of dietary protein levels on growth performance and body composition of juvenile parrot fish, Oplegnathus fasciatus. Int. aquat. Res., 8: 239-245. https://doi.org/10.1007/s40071-016-0139-9
Ljubojevic, D., Trbovic, D., Lujic, J., Cabrilo, O.B., Kostic, D., Novakov, N. and Cirkovic, M., 2013. Fatty acid composition of fishes from inland waters. Bulg. J. agric. Sci., 19: 62-71.
Memon, N.N., Talpur, F.N., Bhanger, M.I. and Balouch A., 2011. Changes in fatty acid composition in muscle of three farmed carp fish species (Labeo rohita,Cirrhinus mrigala,Catla catla) raised under the same conditions. Fd. Chem., 126: 405-410. https://doi.org/10.1016/j.foodchem.2010.10.107
Nazir, K., Yongtong, M., Kalhoro, M.A., Memon, K.H., Mohsin, M. and Kartika, S., 2015. A preliminary study on fisheries economy of Pakistan: Plan of actions for fisheries management in Pakistan. Can. J. Basic. appl. Sci., 3: 7-17.
Nelson, D.L. and Cox, M.M., 2008. Lehninger principles of biochemistry 5th Edition, W.H. Freeman, USA.
Ozparlak, H., 2013. Effect of seasons on Fatty acid composition and n-3/n-6 ratios of muscle lipids of some fish species in Apa Dam Lake, Turkey. Pakistan J. Zool., 45: 1027-1033.
Paiko, M.A., Hashim, R., Chong, A.S.C., Yogarajah, L. and Sayed, A.F.M.E., 2010. Influence of different sources and levels of dietary protein and lipid level on the growth, feed efficiency, muscle composition and fatty acid profile of snakehead Channa striatus fingerlings. Aquacult. Res., 41: 1365-1376. https://doi.org/10.1111/j.1365-2109.2009.02425.x
Rahim, A., Abbas, G., Waryani, B., Ghaffar, A., Monwar, M., Rehman, M. and Dastagir, G., 2015. Influence of varying dietary lipid levels on growth, feed conversion and chemical composition of meat and liver of the juvenile blackfin sea bream, Acanthopagrus berda (Forsskal 1775). Pakistan J. Zool., 47: 1467-1473.
Rasoarahona, J.R.E., Barnathan, G., Biachini, J.P. and Gaydou, E.M., 2004. Annual evolution of fatty acid profile from muscle lipids of the common carp (Cyprinus carpio) in Madagascar inland waters. J. Agric. Fd. Chem., 52: 7339-7344. https://doi.org/10.1021/jf048993y
Sargent. J.R. and Tacon, A.G.J. 1999. Important information development of farmed fish: A nutritionally necessary alternative to meat. Nutr. Soc., 58: 377-383. https://doi.org/10.1017/S0029665199001366
Satpathy, B.B., Mukherjee, D. and Ray, A.K., 2003. Effects of dietary protein and lipid levels on growth, feed conversion and body composition in rohu, Labeo rohita (Hamilton), fingerlings. Aquaculture, 9: 17-24. https://doi.org/10.1046/j.1365-2095.2003.00223.x
Sharma, P., Kumar, V., Sinha, A.K., Ranjan, J., Kithsiri, H.M.P. and Venkateshwarlu, G., 2010. Comparative fatty acid profilesof wild and farmed tropical freshwater fish rohu (Labeo rohita). Fish Physiol. Biochem., 36: 411–417. https://doi.org/10.1007/s10695-009-9309-7
Srivastava, P.P., Jena, J.K., Chowdhary, S., Sharma, P., Raizada, S. and Dayal, R., 2013. Performance of catla (Catla catla) fingerlings reared on locally available feed ingredients. J. Anim. Feed. Res., 3: 153-158.
Strobel, C., Jahries, G. and Kuhnt, K., 2012. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipid. Hlth. Dis., 11: 144. https://doi.org/10.1186/1476-511X-11-144
Teklu, D. and Lema, A., 2015. Optimization of time and temperature for smoking of Nile tilapia for a better preservation of protein and gross energy value. Nutr. Fd. Sci., 5: 1-9.
Zeb, J., 2016. Optimization of protein level in supplementary feeds for fish rearing under semi-intensive composite pond culture systems. PhD thesis, University of Agriculture, Faisalabad, Pakistan.
Zheng, X.Z., Ding, Z.K., Xu, Y., Monroig, O., Morais, S. and Tocher, D. R., 2009. Physiological role of fatty acyl desaturase and elongases in marine fish: Characterization of cDNAs offatty acyl Δ6 desaturase and and elovl5 elongase of cobia (Rachycentron canadum). Aquaculture, 290: 1-2. https://doi.org/10.1016/j.aquaculture.2009.02.010
To share on other social networks, click on any share button. What are these?









