Effect of Citrus Peels Mingled Diets on Carassius auratus Coloration
Effect of Citrus Peels Mingled Diets on Carassius auratus Coloration
Sumaira Abbas1, Muhammad Sultan Haider2,*, Fatima Kafayet1, Sana Ashraf2, Atifa Masood3 and Moazma Batool4
1Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Lahore
2Department of Zoology, The University of Lahore, Lahore
3Department of Botany, The University of Lahore, Lahore
4Department of Zoology, GC Women University, Faisalabad
ABSTRACT
Present project was an endeavor to use natural carotenoid sources to enhance skin color of gold fish Carassius auratus. Citrus peels were collected from local market dried and grinded. Organic solvent extraction was carried out by hexane and acetone mixture (1:1 v/v). Carotenoid concentration was determined by thin layer chromatography (TLC) and found satisfactory. By mixing fish meal, sunflower meal and rice polish four treatment diets, T1, T2, T3, were prepared and citrus peel powder was added @ 200g, 400g and 600g/ 1000g, respectively. While fourth one, control diet (To) was without citrus peels. These is nitrogenous diets (30% protein) were offered to Carassius auratus juvenile having body weight 20±7.54g in powder form for 92 days. Weight gain, length gain and FCR were calculated fortnightly. At completion of feeding trial, color intensity and pigment concentration was measured in Carassius auratus skin. Statistical analysis of results showed non-significant differences (P<0.05) among all treatments in weight gain and FCR. Maximum color intensity 1.52±0.08 and carotene concentration in lateral and tail region, 0.4±0.01 and 0.05 ±0.02 were recorded in T3 without any harmful effect. It is concluded that citrus peels are good, natural, low price Carotene source for color enhancement.
Article Information
Received 07 November 2016
Revised 22 July 2018
Accepted 23 October 2018
Available online 17 January 2020
Authors’ Contribution
MSH and S Abbas planned the research and wrote the manuscript. FK conducted research work. AM, MB and S Ashraf helped in data analysis and paper-writing.
Key words
Carotene, Goldfish, TLC, Growth, Color.
DOI: https://dx.doi.org/10.17582/journal.pjz/20161107041109
* Corresponding author: [email protected]
0030-9923/2020/0002-0519 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
Introduction
Now a days, ornamental fishes are rapidly gaining importance because of their aesthetic and commercial value in the export trade all over the world. Attractive colouration determines the commercial value of any ornamental fish. Pigmentation in the skin is responsible for colouration in the fish (James and Sampaths, 2003). Ornamental fish keeping is also a popular hobby, among young and old alike. It has been estimated that 1.5 to 2 million people worldwide keep marine ornamental fish aquaria (Mandal et al., 2010; Lim and Wong, 1997). Estimated value of marine ornamental trade is 200-330 million US$ per year (Degnai and Gur, 1992).
Carotenoids is the primary source of the pigmentation in fish skin. Fishes cannot synthesis carotenoid in natural environment, so they meet their carotenoid requirements by ingesting specific aquatic plants (Zhao et al., 2003). Dietary carotenoids play significant role in regulation of skin and muscle color in fish after absorption carotenoids are transformed into other carotenoids if necessary, and incorporated into tissues. Carotenoids are vital for healthy growth, metabolism, and reproduction, as well as color, in fish (Li et al., 2005). Astaxanthin is the main carotenoid pigment of red-pink coloured aquatic animals, being widely used in aqua-cultural processes due to its chemical stability (Madhupratap et al., 2001). The colour enhancing diets should contain additional natural pigments to enhance the colour of ornamental fish (Rickman et al., 2007).
Carotenoids in fish feed give yellow, red and pink color to fish skin, flesh and eggs. Some 600 species of plant and water organism contain these colorant substances (Irie and Seki, 2002; Furuita et al., 2003). Astaxanthin, which is the most effective coloring pigment exsists densely in water organisms such as gammarus, copepods etc and starfish (Ando et al., 1992).
Astaxanthin and canthaxanthin are widely prepared artificially and used as supplements in diets for goldfish and other ornamental fishes to induce the desired coloration (Kithara, 1983).
Goldfish (Carassius auratus) is a widely cultured and commonly traded ornamental fish in the global aquarium business (Shahidi et al., 1998). Color is a major factor that determines the quality and market value of any ornamental fish (Kitahara, 1983). Skin pigmentation of goldfish has been accomplished by supplementing their diets with synthetic or extracted carotenoids, such as zeaxanthin, lutein or astaxanthin (Royes et al., 2006; Seyedi et al., 2013). From natural sources containing Carotenoids, the Red Yeast, Xanthophyllomyces dendrorhous (Wang et al., 2006), Spirulina (Wallat et al., 2002), Chlorella vulgaris, Haematococcus pluvialis and Arthrospira maxima (Gouveia et al., 2003) have already been used for pigmentation of Goldfish. Citrus (Citrus reticulate) peels have been tested as a protein source in some ornamental fish species (Reitain et al., 1997; Passos et al., 2007). However, nothing has been reported on its use as a pigment source for goldfish or other aquatic animals, except in freshwater crayfish, Cheraxquadri carinatus (Paulo and Antonio, 2005). So, recent effort has focused on use of this natural carotenoid source citrus (Citrus reticulate) peels as alternative to synthetic carotenoids in goldfish diet for color enhancement.
Materials and methods
Site and sample collection
Present experiment was conducted in Fish Hatchery and Laboratory, University of Veterinary and animal Sciences, Lahore, Pakistan. Goldfish of uniform size 20±7.54g were collected from ornamental fish market, Akbar Chowk, Lahore and acclimatized to laboratory conditions for one week before initiation of experiment. Complete randomized design (CRD) was used in this study. Citrus (Citrus reticulate) peels were collected from local market, dryed and grinded in powder form by electric grinder machine.
Pigment extraction from citrus peel
Veronica et al. (2010) was followed for pigment extraction from citrus peels. Citrus peel powder (200g) was weighed and immersed in 400 ml acetone solution and shaked well. The mixture was kept at room temperature, in darkness, for 12 h and continuously shaken by magnetic stirrer. Afterward mixture was filtered and evaporated on hot plate. Residues were extracted with petroleum ether and extract was left to saponify for 12 h at room temperature. Later, it was washed with 100 ml ether and diluted with 200 ml distilled water. Two phases were successively partitioned at this stage. The upper lipophilic phase contains the carotenoids. These carotenoids were concentrated on hot plate almost to dryness and stored till further use. The stored extract was dissolved in acetone and submitted to TLC on silica using petroleum ether and diethyl ether in same ratio.
The total carotenoids contents were calculated as μg per g weight of sample as follows:

Where, 10 is the dilution factor and 0.25 is the extinction coefficient.
Feeding trial
Fish feed was prepared by mixing traditional feed ingredients i.e. fish meal, sunflower meal and rice polish following Gupta et al. (2007) and were termed as T1, T2, T3 and T0. All diets were iso-nitrogenous with 30% protein level and were offered to fish in powdered form. Already ground citrus peels were mixed @200, 400, 600g/1000 g of basal diet in T1, T2 and T3, respectively, while fourth treatment diet T0 was control and prepared without citrus peels.
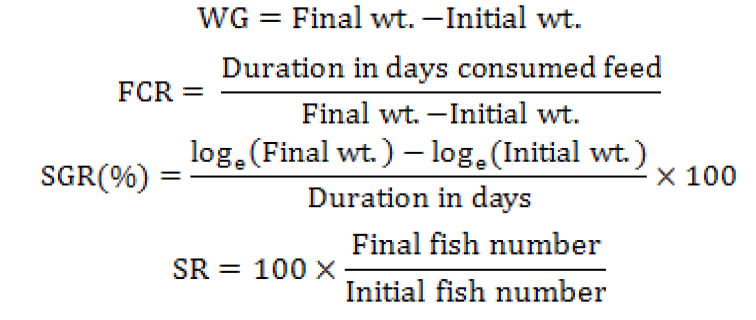
After preparation, proximate analysis of diets was performed for determination of total moisture, proteins, lipids and ash contents following AOAC (2000). When nutritional value of prepared diets was found satisfactory, prepared diets were offered to experimental fish in troughs @ 4% body weight for 90 days. The experiment was conducted in triplicate in 4 troughs having 70 liter volume capacity. In each experimental group for 90 days. Experimental groups were termed as G1, G2 and G3. In each group, fifteen fish approximately of equal size (20±7.54 g) were randomly selected and kept. Water quality parameters (dissolve oxygen level, temperature and pH) were recorded on daily basis while growth parameters like weight gain (WG), length gain (LG), feed conversion ratio (FCR), specific growth rate (SGR) and survival rate (SR) were calculated fortnightly. At the end of feeding trial, three fish were randomly selected from each treatment group and were subjected to pigment extraction procedure.
Fish growth parameters were calculated by following formulas:
Pigment extraction from fish tissue
At the end of feeding trial, color pigment was extracted from fish tissue following Sant’Anna et al. (2005). One gram of fish body tissue was taken and mixed with 2.5 g of anhydrous sodium sulphate and gently meshed. Then 5ml of chloroform was added in it and left overnight at 0°C. Chloroform formed a clear 1-2 cm layer of carotenoids on upper surface of this mixture. The layer was removed and its optical density was recorded at ƛ470nm using spectrophotometer, taking 0.3 ml of chloroform diluted aliquots mixed with 3ml absolute ethanol. Blank tube was used as standard for comparison. Wavelength at which maximum absorption was recorded was used for calculation of carotenoid concentration. Calculations were made by formula given below:
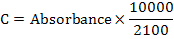
Where, C is concentration (µg/g for tissue), 2100 is E (1%, 1 cm) or extinction coefficient of carotenoids in hexane at 472 nm and 10000 is the scale factor.
Skin colour analysis
For color analysis skin samples were taken from from lateral and tail regions. Reflectance spectroscopy and Portable Minolta Chroma Meter CR-300 (Konica USA) was used for color analysis following (Storebakken, 1987).
Statistical analysis
For statistical analysis SAS Version 9.4 was used. One way analysis of variance (ANOVA) was applied on data to find out the significant differences among means. Results were considered significant at (p< 0.05).
Results and discussions
Proximate analysis
Four treatment diets were prepared by mixing fish meal, sun flower meal and rice bran. Citrus peel (Citrus reticulate) powder was incorporated in diets at different ratios. According to Govind (2013) nutrients required for commercial and ornamental fish are same as per other animals.
Table I.- Nutritive evaluation of different treatment diets based on different ratio of citrus peel powder.
|
Tr. |
Moisture |
Protein |
Fat |
Ash |
Orange peel g/kg |
|
T1 |
4.89±0.57b |
31.88±0.52a |
7.53±1.32b |
6.58±0.60b |
200 |
|
T2 |
6.69±0.43a |
32.03±0.15a |
9.40±0.63a |
8.41±0.76a |
400 |
|
T3 |
5.08±0.83b |
32.10±0.29a |
6.69±0.52b |
8.79±0.58a |
600 |
|
To |
6.05±0.66ab |
31.81±0.21a |
4.75±0.84c |
5.70±0.50b |
- |
Means with similar letters in a column are statistically non-significant (p<0.05).
According to Wang et al. (2006) various nutrients vary in proportion according to fish type. Protein concentrations vary from 30-32% in diets. Proximate analysis of four treatment diets was performed following AOAC (2000). All treatment diets were isonitrogenous and protein level was maintained at 30%. While moisture, fat and ash ratio vary in all diets, the values are presented in Table I.
According to Ho et al. (2014) protein is most crucial nutrient in fish feed and its requirements vary according to ornamental fish species, size, feeding rate and water quality parameters. According to Sim et al. (2005) protein requirement of guppy (C. reticulate) is 30-35%%, goldfish (C. auratus) is 30%. By observing these arguments it can be said that our prepared feed is suitable for ornamental fish. According to Wilson (1994) and Halver and Hardy (2002) fish growth rate mostly solely depends upon protein utilization rate. Remaining parameters like moisture, protein, fat and ash contents were also within range as described by different authors (Sargent et al., 1995; Li and Galtin, 2008). However, the concentrations of orange peel vary in all treatment diets.
Rf value is ratio between distance travelled by a pigment, solute and the solvent. In other words Rf = (distance moved by solute) / (distance moved by solvent) (Hartely and Kennedy, 2006).
Calculated Rf values of extracted pigments are presented in Table II. According to our results T1 showed lycopene and xanthophyl with RF value 0.88 and 0.86, respectively. In T2 leutine and pheophytin were found with Rf value 0.68 and 0.71. While in T3 xanthophyl, α and ß-carotene were found having Rf value of 0.89 and 0.90. These values are in line with values as described by Gouveia et al. (2003) and Donato et al. (2003). They described Rf values for xanthophyl, chlorophyl b and a and carotene, as 0.16, 0.32, 0.44, and 0.91, respectively. So our selected orange peel powder has enough concentration of carotenoids and can be used as color enhancer in ornamental fish diet.
Table II.- Rf value and colors pattern of different plates showed under UV lamp.
|
Tr. No |
Replicate No |
Rf Value |
Colors |
Pigment name |
|
1 |
1 |
0.88 |
Red-Orange |
ß-carotene Zeaxanthin Astaxanthin |
|
2 |
0.86 |
Yellow |
||
|
3 |
0.87 |
Yellow |
||
|
2 |
1 |
0.88 |
Yellow – brown |
ß-carotene α−carotene Zeaxanthin |
|
2 |
0.89 |
Yellow – brown |
||
|
3 |
0.81 |
Olive Green |
||
|
3 |
1 |
0.89 |
Yellow – Orange |
−carotene Xanthophyll ß-carotene |
|
2 |
0.90 |
Yellow – orange |
||
|
3 |
0.88 |
Yellow – orange |
Growth parameters
Fish growth parameters like weight gain, length gain, FCR, SGR and survival rate were recorded fortnightly (Table III). All treatments showed non-significant variation for weight gain among all treatment diets, however in case of FCR T1 and T3 showed significant difference (p<0.05) from other two treatments T2 and T4. Total weight gain was recorded as 39.76±1.12, 40.63±1.43, 34.85±1.61and 42.34±1.21 among T1, T2, T3 and T0. While FCR was recorded as 3.31±1.74, 2.93±0.85, 3.87±1.07 and 2.43±1.37 among T1, T2, T3 and T0. This variation in weight gain can be justified by Macartney (1996) and Sales and Janssens (2003), who described that in captivity ornamental fish may show variation in protein digestibility. The difference in FCR value among four treatments can be justified by Pannevis (1993) and Lim et al. (2001). They stated that along with protein other components like lipids, carbohydrates and minerals also effect growth rate of fish. As there is variation in feed proximate analysis results so there is also variation in FCR values in our treatment diet results.
Table III.- Weight gain, length gain, FCR, SGR, and survival rate of Carassius auratus fed on different treatment diets.
|
Tr. |
Total weight gain |
Total length gain |
FCR |
SGR |
Survival % |
|
T1 |
39.76±1.12a |
12.51±2.56c |
3.31±1.74a |
0.95a |
100% |
|
T2 |
40.63±1.43a |
13.37±3.18b |
2.93±0.85b |
1.27b |
100% |
|
T3 |
34.85±1.61ab |
13.81±1.12b |
3.87±1.07a |
0.91a |
100% |
|
T0 |
42.34±1.21a |
15.50±1.25a |
2.43±1.37b |
1.29b |
100% |
Means with similar letters in a column are statistically non-significant (p<0.05).
Table IV.- Carotenoid concentration in lateral, tail region (μg/g) and color intensity in muscle region of Carassius auratus in different treatment groups of fish tissue.
|
Sample |
T0 |
T1 |
T2 |
T3 |
|
Lateral region |
0.03±0.03a |
0.3±0.04b |
0.4±0.01b |
0.8±0.01c |
|
Tail region |
0.02±0.01a |
0.03±0.02a |
0.05±0.02b |
0.06±0.01b |
|
Muscle color |
1.23±0.15a |
1.32±0.17ab |
1.52±0.08b |
1.72±0.11c |
Muscle color was observed by comparing muscle sample to Salmonids Roche TM Color Card. Means with similar letters in a column are statistically non-significant (p<0.05).
Color assessment
Recoded color assessment and total number of carotenoid calculated are presented in Table IV. During experiment all groups were refractive to color enhancer. Skin color was estimated by comparing muscle sample with Salmonids Roche TM Color Card (Lim et al. 2001). In lateral region, carotenoid contents were measured as 0.3±0.04, 0.4±0.01, 0.8±0.01 and 0.03±0.03 in T1, T2, T3 and T0, while in tail region the carotenoid concentration was recoded as 0.03±0.02, 0.05±0.02, 0.06±0.01 and 0.02±0.01 for T1, T2, T3 and T0, respectively. Carotenoids are categorized as micronutrient and attractant for fish. For ornamental fish addition of carotenoids in fish diet enhance color pigment concentration in fish muscle (Hardy and Barrows, 2002). As the concentration of carotenoids increase in fish diet its color also enhanced.
Kruger et al. (2001) reported that when swordtail fish was fed with 40-80 mg kg-1 astaxanthin equivalent of orange peel carotenoids for eight weeks, it scored the highest for customer preferences and its muscle color had no difference with those treated with astaxanthin (Carophyll Pink TM) at same concentration. All groups showed enhancement in muscle color. Muscle color concentration was recorded as 1.32±0.17, 1.52±0.08, 1.72±0.11 and 1.23±0.15 for T1, T2, T3 and T0, respectively. These results are in agreement with results of Woods (2003), who reported similar findings when rainbow trout fed with from H. roretzis extract.
Conclusions
It can be concluded from above experiment that citrus (Citrus reticulate) peel is a good source of carotenoids and when given in feed positively effect color pattern in goldfish (Carrassius auratus).
Acknowledgements
The authors would like to express their gratitude to Higher Education Commission of Pakistan (HEC) and University of Veterinary and Animal Sciences, Lahore for facilitating the current project.
Statement of conflict of interest
The authors declare no conflict of interest.
References
Ando, S., Yamauchi, H., Hatano, M. and Heard, W.R., 1992. Comparison of muscle compositions between red- and white-fleshed Chinook salmon (Oncorhynchus tshawytscha). Aquaculture., 103: 359-365. https://doi.org/10.1016/0044-8486(92)90178-N
AOAC, 2000. Official method of analysis. 17th Edition,, MD, USA. Method no. 925.10, 65.17, 974.24, 992.16. The Association of Official Analytical Chemists, Gaithersburg.
Degani, G. and Gur, N., 1992. Growth of juvenile Tricogasterleeri (Bleeker, 1952) on diets with various protein levels. Aquacult. Fish. Manage., 23: 161-166.
Donato, M., Vilela, M.H. and Bandarra, N.M., 2003. Fatty acids, sterols, α-tocopherol and growth of goldfish (Carassius auratus) larvae and juvenile. J. Anim. Vet. Adv., 4: 654-658.
Furuita, H., Tanaka, H., Yamamoto, T., Suzuki, N. and Takeuchi, T., 2003. Supplemental effect of vitamin A in diet on the reproductive performance and egg quality of the Japanese flounder Paralichthyso livaceus (T and S). Aquacul. Res., 34: 461-467. https://doi.org/10.1046/j.1365-2109.2003.00831.x
Rymes, G.A., 2005. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Fd. Sci. Tech., 16: 389-406. https://doi.org/10.1016/j.tifs.2005.02.006
Gouveia, L., Rema, P., Pereira, O. and Empis, J., 2003. Colouring ornamental fish (Cyprinus carpio and Carassius auratus) with microalgal biomass. Aquacul. Nutr., 9: 123-129. https://doi.org/10.1046/j.1365-2095.2003.00233.x
Govind, P. 2013. Feed formulation and feeding technology for fishes. J. Appl. Anim. Res., 44: 24-26
Govindpandey, T., 2013. Feed formulation and feeding technology in fishes. Int. Res. J. Pharm., 4: 23-30. https://doi.org/10.7897/2230-8407.04306
Gupta, S.K., Jha, A.K., Pal, A.K. and Venkateshwarlu, G., 2007. Use of natural carotenoids for pigmentation in fishes. Nat. Prod. Rad., 6: 46-49.
Halver, J.E. and Hardy, R.W., 2002. Fish nutrition. In: The lipids (Eds. J.R. Sargent, D.R. Tocher and G. Bell). 3rd Edition, Academic Press, California, 182-246.
Hartley, R.C. and Kennedy, M.W., 2004. Are carotenoids a red herring in sexual display? Trends Ecol. Evol., 19: 353–354.
Hardy, R.W. and Barrows, F.T., 2002. Diet formulation and manufacture. In: Fish nutrition (eds. J.E. Halver and R.W. Hardy), 3rd edition. Academic Press, London, pp. 505-600.
Ho, A.L., Zong, S. and Lin, J., 2014. Skin color retention after dietary carotenoid deprivation and dominance mediated skin coloration in clown anemone fish, Amphipriono cellaris. AACL Bioflux, 7: 103-115.
Irie, T. and Seki, T., 2002. Retinoid composition and retinal localization in the eggs of teleost fishes. Comp. Biochem. Physiol., 131: 209-219. https://doi.org/10.1016/S1096-4959(01)00496-1
James, R. and Sampath, K., 2003. Effect of meal frequency on growth and reproduction in the ornamental red swordtail (Xiphophorus helleri). Isr. J. Aquacult-Bamid., 55: 197-207.
Kitahara, T., 1983. Behavior of carotenoids in the chum salmon (Oncorhynchus keta) during anadromous migration. Comp. Biochem. Physiol., 76: 97-101.
Kruger, D.P., Britz, P.J. and Sales, J., 2001. The influence of live feed supplementation on growth and reproductive performance of swordtail (Xiphophorus helleri, Heckel, 1848) broodstock. Aquarium Sci. Conserv., 3: 265-273. https://doi.org/10.1023/A:1013150314719
Li, H.X., Tyndale, S.T., Heath, D.D. and Letcher, R.J., 2005. Determination of carotenoids and all-trans-retinol in fish eggs by liquid chromatography-electrospray ionizationtandem mass spectrometry. J. Chromat., 816: 49-56.
Li, P. and Gatlin D.M., 2006. Nucleotide nutrition in fish: current knowledge and future applications. Aquaculture, 251: 141–152.
Lim, L.C. and Wong, C.C., 1997. Use of the rotifer, Brachionuscalyciflorus Pallas, in freshwater ornamental fish larvae culture. Hydrobiologia, 358: 269-273. https://doi.org/10.1023/A:1003101423349
Lim, L.C., Sho, A., Dhert, P. and Sorgeloos, P., 2001. Production and application of on-grown Artemia in freshwater ornamental fish farm. Aquacul. econ. Manage., 5: 211-228.
Macartney, A., 1996. Ornamental fish nutrition and feeding. In: Manual of companion: Animal nutrition and feeding (Eds. N.C. Kelly and J.M. Wills). British Small Animal Veterinary Association, Gloucester shire, UK, pp. 244–251.
Mandal, B., Mukherjee, A. and Banerjee, S., 2010. Growth and pigmentation development efficiencies in fantail guppy, Poecilia reticulate fed with commercially available feeds. Agric. Biol. J. N. Am., 1: 1264-1267. https://doi.org/10.5251/abjna.2010.1.6.1264.1267
Madhupratap, M., Nair, K.N.V., Gopalakrishnan, T.C., Haridas, P., Nair, K.K.C., Venugopal, P. and Mangesh, G., 2001. Arabian Sea oceanography and fisheries of the west coast of India. Curr. Sci., 81: 355-361.
Pannevis, M.C. and Earle, K.E., 1994. Nutrition of ornamental fish: Water soluble vitamin leaching and growth of Paracheirodon innesi. J. Nutr., 124: 2633-2635. https://doi.org/10.1093/jn/124.suppl_12.2633S
Passos, J., Saretzki, G., Ahmed, S., Nelson, G., Richter, T., Peters, H., Wappler, I., Birkett, M., Harold, G. and Schaeuble K., 2007. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol., 5: 110-112.
Paulo, R. and Antonio G., 2005. Effect of microalgal biomass concentration and temperature on ornamental goldfish (Carassius auratus) skin pigmentation. Aquacult. Nutri., 11: 19–23.
Reitain, K., Rainuzzo, J.R., Oie, G. and Olsen, Y., 1997. A review of the nutritional effects of algae in marine fish larvae. Aquaculture, 155: 207-221. https://doi.org/10.1016/S0044-8486(97)00118-X
Gouveia, L., Rema, P. and Gouveia, L., 2003. Colouring ornamental fish (Cyprinus carpio and Carassius auratus) with microalgal biomass. Aquacult. Nutr., 9: 123-129. https://doi.org/10.1046/j.1365-2095.2003.00233.x
Rickman, J.C., Bruhn, C.M. and Barrett, D.M., 2007. Nutritional composition of fresh, frozen, and canned fruits and vegetables II. Vitamin A and carotenoids, vitamin E, minerals and fiber. J. Sci. Fd. Agric., 87: 1185-1196. https://doi.org/10.1002/jsfa.2824
Royes, J., Murie, D.J. and Francis-Floyd, R., 2006. Effects of varying dietary protein and lipid levels on growth performance and hepatocyte changes in juvenile African cichlids (Pseudotropheus socolofi and Haplochromi sahli). J. World Aquacul. Soc., 37: 48-59. https://doi.org/10.1111/j.1749-7345.2006.00006.x
Sales, J. and Janssens, P.J.G., 2003. Nutrient requirements of ornamental fish. Rev. aquat. Living Resour., 16: 533-540. https://doi.org/10.1016/j.aquliv.2003.06.001
Sant’Anna, B.S., Zangrande, C.M. and Reigada, A.L., 2005. Utilization of shells of the snail Achatina fulica Bowdich, 1822 (Mollusca, Gastropoda) by the hermit crab Clibanarius vittatus (Bosc, 1802) (Decapoda, Anomura) in the São Vicente Estuary, São Paulo, Brazil. Invest. Marinas, 33: 217-219. https://doi.org/10.4067/S0717-71782005000200010
Sargent, J.R., Bell, M.V., Bell-Henderson, R.J. and Tocher, D.R., 1995. Requirement criteria for essential fatty acids. J. appl. Ichthyol., 11: 183-198. https://doi.org/10.1111/j.1439-0426.1995.tb00018.x
Seyedi, S.M., Sharifpour, I., Ramin, M. and Jamili, S., 2013. Effect of dietary astaxanthin on survival, growth, pigmentation clownfish, Amphipriono cellaris, cuvier. Ind. J. Fund. appl. Life. Sci., 3: 391-395.
Shahidi, F., Metusalach, J. and Brown, J.A., 1998. Carotenoid pigments in seafoods and aquaculture. Crit. Rev. Fd. Sci. Nutr., 38: 1-67. https://doi.org/10.1080/10408699891274165
Sim, S.Y., Rimmer, M.A., Toledo, J.D., Sugama, K., Rumengan, I., Williams, K.C. and Phillips, M.J., 2005. A practical guide to feeds and feed management for cultured groupers. NACA, Bangkok, Thailand, pp. 18.
Storebakken, T., Foss, P., Schiedt, K., Austreng, E., Liaaen-Jensen, S. and Manz, U., 1987. Carotenoids in diets for salmonids. IV. Pigmentation of Atlantic salmon with astaxanthin, astaxanthin dipalmitate and canthaxanthin. Aquaculture, 65: 279-292. https://doi.org/10.1016/0044-8486(87)90241-9
Veronica, S., Kefalas, P. and Socaciu, C., 2010. Patterns of carotenoid pigments extracted from two orange peel wastes (valencia and navel var.). J. Fd. Biochem., 34: 101-110. https://doi.org/10.1111/j.1745-4514.2009.00267.x
Wallat, G.K., Luzuriaga, D.A., Balaban, M.O. and Chapman, F.A., 2002. Analysis of skin color development in live goldfish using a color machine vision system. N. Am. J. Aquacult., 64: 79-84. https://doi.org/10.1577/1548-8454(2002)064<0079:AOSCDI>2.0.CO;2
Wang, Y.J., Chien, Y.H. and Pan, C.H., 2006. Effects of dietary supplementation of carotenoids on survival, growth, pigmentation, and antioxidant capacity of characins, Hyphessobrycon callistus. Aquaculture, 261: 641-648. https://doi.org/10.1016/j.aquaculture.2006.08.040
Wilson, R.P., 1994. Utilization of dietary carbohydrate by fish. Aquaculture, 124: 67-80. https://doi.org/10.1016/0044-8486(94)90363-8
Woods, C., 2003. Growth and survival of juvenile seahorse Hippocampus abdominalis reared on live, frozen and artificial foods. Aquaculture, 220: 287-298. https://doi.org/10.1016/S0044-8486(02)00639-7
Zhao, D.Y., Aldini, G., Johnson, E.J., Rasmussen, H., Kraemer, Woolf, H., Musaeus, N., Krinsky, N.I., Russell, R.M. and Yeum, K.J., 2003. Modification of lymphocyte DNA damage by carotenoid 371 supplementation in postmenopausal women. Am. J. Clin. Nutri., 83: 163–169.
To share on other social networks, click on any share button. What are these?










