Effect of Different Diet’s Composition on Growth Performance and Survival Rate of Channa marulius
Research Article
Effect of Different Diet’s Composition on Growth Performance and Survival Rate of Channa marulius
Muhammad Khubaib1*, Muhammad Salman Khan1, Zia Ur Rahman2, Nafees Ahmad1, Muhammad Atif Haider1 and Mushtaq Ahmad Khan3
1Department of Zoology, Abdul Wali Khan University Mardan, Khyber Pakhtunkhwa, Pakistan; 2Department of Zoology, Shaheed Benazir Bhutto University, Sheringal Dir Upper, Khyber Pakhtunkhwa, Pakistan; 3Department of Agriculture, University of Swabi, Khyber Pakhtunkhwa, Pakistan.
Abstract | Channa is distributed throughout the subcontinent, highly resistive, spine less, adopted to low dissolved oxygen and is commercially important worldwide. The objective of this study is to investigate the effect of different diet on growth performance of Channa marulius to develop a rearing technique for promoting it aquaculture. Four different diets i.e. chicken liver, BSF-larvae, commercial feed and beef mince- having different protein levels were administered to compare their suitability as food for the rearing of C. marulius for 45 days. The fry fed with chicken liver and BSF-larvae showed significantly better result in terms of weight and length gain. The survival rate of C. marulius fed with chicken liver, BSF-larvae, commercial feed and beef mince were estimated as 90%, 85%, 80% and 60% respectively. The absolute weight of C. marulius fed with chicken liver, BSF-larvae, commercial feed and Beef mince were estimated as 2.24 g, 2.00 g, 1.9 g, and 1.86 g respectively while the value of the absolute length of C. marulius fed with chicken liver, BSF-larvae, commercial feed and Beef mince were estimated as 0.9 cm, 0.9 cm, 0.8cm, and 0.9 cm respectively. The value of SGR in term of weight is 1.803%, 1.727% 1.5% and 1.7% on chicken liver, BSF-larvae, commercial diet and beef mince respectively. While in term of length the value of SGR is 0.32%, 0.35%, 0.36% and 0.37% on chicken liver, BSF-larvae, commercial diet and beef mince, respectively. The difference in growth performance observed were highly significant (P ˂ 0.05). The relative growth rate was also higher on chicken liver and BSF-larvae and SGR was high on the Chicken liver. It is, therefore determined that C. marulius can be culture on animal-based protein diet for profitable farming. By creating more enticing and delectable artificial feed, changing feeding strategy, and making more attractive artificial feed, additional study is required to reduce the generation of shooters at juvenile stages.
Received | January 04, 2024; Accepted | August 12, 2024; Published | October 04, 2024
*Correspondence | Muhammad Khubaib, Department of Zoology, Faculty of Biological Science, Abdul Wali Khan University, Mardan, Pakistan; Email: [email protected]
Citation | Khubaib, M., M.S. Khan, Z.U. Rahman, N. Ahmad, M.A. Haider and M.A. Khan. 2024. Effect of different diet’s composition on growth performance and survival rate of Channa marulius. Sarhad Journal of Agriculture, 40(4): 1135-1143.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.4.1135.1143
Keywords | Channa marulius, BSF-larvae, Commercial feed, Protein, Growth indices, Survival rate
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Aquaculture is one of the crucial food-supply sectors that ensure the provision of nutritional needs to consumers around the world (Mirghaed et al., 2018). Aquaculture is predicted to play a key role in food security, income, and economic community, supplying nutritious food to a growing human population (FAO, 2020). Protein one of the most significant component needed to improve fish growth rate. Dietary protein offers both type of amino acid’s needed to sustain body energy and synthesis of body protein (Hossain et al., 2010). The requirement of protein for animals varies depending on their age, species, size, reproductive status, and environment (NRC, 2011). Smaller fish have greater protein demands as compared to large size fish. Efficient growth is influenced by the amount of high-quality protein in diet, especially for carnivorous species (Lee et al., 2002).
Snakehead is a carnivorous, air-breathing, freshwater fish species that belongs to the Channidae family and is distributed in freshwater areas of Southeast Asia. (Wallady et al., 2022). The Channidae family consists of just two genera, with 26 currently recognized species is extensively dispersed across the Iran, South Asia, Far East (Li, 2006) India, among these three species C. marulia, C. striata, and C. punctate can be find in Pakistan in different environmental habitats. (Rahman et al., 2012; Chowdhury et al., 2021) C. marulius (Hamilton, 1822) is commonly known as the bull’s eye snake head or great snake head. C. marulius is considered as the species complex (Britz et al., 2017). Snakeheads are opportunistic carnivores and can breathe air, they are frequently exported and sold alive, which increases the risk of purposeful or unintentional introduction to habitats where they do not belong (Courtenay and Williams, 2004). C. marulius a large snakehead fish, is a fast-growing species with a high market value and customer preference (Khan et al., 2012). C. marulius is raised as a food fish for humans and is commercially taken from the wild (Dua and Kumar, 2006). Snakehead is regarded as a commercially significant species in its native area, and as a result, certain wild populations in India have been declining due to its use in local human diets (Habib et al., 2011; Ali et al., 2013). C. marulius is a carnivorous fish which consume a variety of animals as food such as fish, frog, snakes, insect, earthworms, tadpoles, water birds and mice (Qin and Fast, 1996).
According to Raizada et al. (2012) that C. marulius fry require about 540–600 g kg-1 of protein, and they may be raised to fingerling size on specially designed diets. Insect larvae and pupae are typically rich in protein 40-70% (Rumpold and Schlüter, 2013). The FAO strongly recommends the use of insects as human food and animal feed as a tool for poverty alleviation (FAO, 2010). Many insects are potentially suitable for animal feed (van Huis et al., 2013). One such dipteran, the black soldier fly (BSF) (Hermetia illucens) has had a great deal of success in several tests replacing fishmeal with its larval meals, the black soldier fly larvae BSFL have the crude protein value stays at an average of 24-28 percent from day-old larvae through prepupal stage (Gangadhar et al., 2018). A previous investigation revealed a useful correlation between food protein content and growth efficiency (Raizada et al., 2012). Sagada et al. (2017) demonstrated the protein requirement of C. argus is about 48%. The purpose of this study is to give adequate and optimal protein for excellent development of the C. marulius through balanced diet.
Materials and Methods
Fish sampling
The specimen were collected from river Indus district Swabi Khyber Pakhtunkhwa, with the help of scope nets, cast net and hand net and transported to the Department of Zoology, Abdul Wali Khan University Mardan on the same day in oxygen filled polythene bags. The species were acclimatized for a week to laboratory condition.
Experimental design and diets feedings
The feeding trial was conducted in four replicates with four variable diets. The experimental feed was prepared according to the formula (Table 1), consists of Chicken liver, BSF-larvae, commercial feed and Beef mince and stored in the refrigerator at 4 oC. On the basis of feeding the replicates were divided as he controlled group fed with chicken liver, group second was fed with the BSF-larvae, group third was fed with commercial feed and the group fourth was fed with the beef mince. The study was continued for 45 days, and the feed was given twice a day. Each aquarium was stocked with 20 species of the target fish and provide 8% of the target feed according to the body weight twice a day. The feed ratio was adjusted in the whole experimental trials. Before stocking, the average initial biomass of the fry was measured and
Table 1: Feed formulation for C. marulius.
|
Ingredient |
Weight |
Percentage |
Chicken liver (g) |
BSF-larvae (g) |
commercial feed (g) |
Beef mince (g) |
|
Protein source |
450 |
45% |
450 |
450 |
450 |
450 |
|
Soyabean meal |
160 |
16% |
160 |
160 |
160 |
160 |
|
Wheat bran |
170 |
17% |
170 |
170 |
170 |
170 |
|
Corn meal |
110 |
11% |
110 |
110 |
110 |
110 |
|
Rice polish |
40 |
4% |
40 |
40 |
40 |
40 |
|
Premixes (Vitamin + Mineral) |
15 |
1.5% |
15 |
15 |
15 |
15 |
|
Vitamin C |
6 |
0.5% |
6 |
6 |
6 |
6 |
|
Soyabean oil/ fish oil |
50 |
5% |
50 |
50 |
50 |
50 |
was not significantly differ across treatments. Physio-chemical parameters of water were checked regularly and 50 % aquarium water was replaced with fresh water every day.
Water quality
Physico-chemical parameters of water were examined regularly during the experimental trails. Digital thermometer was used to measure the temperature, dissolved oxygen (DO) (YSI 55 Incorporated, Yellow Springs, Ohio, 4387, USA) and an APA Kit was used to measure the pH, total alkalinity, ammonia, nitrate, and nitrite levels. The data were recorded twice a day during the experimental trail.
Growth indices
Measurement of the fish individual (Body weight and total length) were taken in the beginning of the experiment and after every 5th day throughout the experimental period. The biomass of each aquarium was determined weekly by weighting the entire stock in the aquarium. The following formulae’s were used to calculate growth performance.
WG = Fw (g) – IW (g)
WG= Weight gain; FW= Final body weight; Iw = Initial body weight.
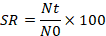
SR= Survival rate; Nt= Final Population N0= Initial Population.
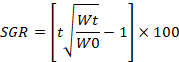
SGR= Specific growth rate (%/day); Wt= Final average weight (g); W0= Initial average weight (g).
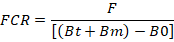
FCR = Feed conversion ratio; F = Total feed amount (g); Bt = Final biomass (g); Bm = Deceased biomass (g); B0 = Initial biomass (g).
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine the effect of different feed on the C. marulius. The significance of difference between mean was determined by Tukey`s Multiple range test (P<0.05) by using SPSS 16.0 software (Chicago, IL, USA).
Results and Discussion
Survival rate
The survival rate in C. marulius fed with chicken liver, BSF-larvae commercial feed and Beef mince for 45 days were estimated as 90%, 85%, 80% and 60%, respectively and shown in Table 2. The survival rate is observed from final and initial number of fish. Due to the aggressive behavior of target juveniles most of the fries were affected and injured by the cannibalistic behavior throughout the study trials. The cannibalistic tendency was observed at minimum level in fries fed with Chicken liver followed by BSF-larvae, commercial feed and Beef mince respectively. Similarly, the survival rate of the C. marulius is minimum due to cannibalistic behavior.
Absolute growth
Absolute growth is calculated by the standard formula. Table 2 and Figure 1 shows the absolute growth of
Table 2: Growth response of C. marulius fed with different diet.
|
Diet |
Survival rate |
Absolute growth |
Absolute growth rate |
Specific growth rate |
|||
|
Weight gained (g) |
Increase in length (cm) |
Weight gained (g/day) |
Increase in length (cm/day) |
Weight gained (% per day) |
Length (% per day) |
||
|
Chicken liver |
90% |
2.24 |
0.9 |
0.049 |
0.02 |
1.803 |
0.32 |
|
BSF-larvae |
85% |
2 |
0.8 |
0.044 |
0.01 |
1.727 |
0.35 |
|
commercial feed |
80% |
1.9 |
0.9 |
0.042 |
0.02 |
1.527 |
0.36 |
|
Beef mince |
60% |
1.86 |
0.9 |
0.041 |
0.02 |
1.712 |
0.37 |
C. marulius. The value of the absolute weight is 2.24 g on chicken liver, 2.00 g on BSF-larvae, 1.9 g on commercial diet and 1.86 on Beef mince while the value of the absolute length is 0.9 cm fed on chicken liver, 0.8cm on BSF-larvae, 0.9 cm on commercial diet and 0.9 on Beef mince.
Absolute growth rate
Table 2 and Figure 2 shows the absolute growth rate of the C. marulius. Absolute growth rate shows the growth increase in specific unit time. The weight gained on chicken liver is 0.049 g/day, 0.044 g/day on BSF-larvae, 0.042 g/day on commercial feed and 0.041 g/day on Beef mince. While the length gained was 0.02 cm/day on chicken liver, 0.01cm/day on BSF-larvae, 0.042 cm/day on commercial feed and 0.041 cm/day on beef mince.
Specific growth performance
Specific growth rate (SGR) is the fish body weight that grows in daily percentage. Table 2 and Figure 3 shows the SGR of the C. marulius fed with chicken liver, BSF-larvae, commercial feed and beef mince. The value of SGR in term of weight is 1.803% on chicken liver, 1.727% on BSF-larvae, 1.5% on commercial diet and 1.7% on beef mince. While in term of length the value of SGR on 0.32% on chicken liver, 0.35% on BSF-LARVAE, 0.36% on commercial diet and 0.37% on the beef mince.
Overall growth performance
The overall growth performance of C. marulius on different diet is shows in Figure 4. A significance difference (P<0.05) was observed in the weight gain of C. marulius on chicken liver, BSF-larvae, commercial diet and beef mince. C. marulius fed with chicken liver shows the highest level of growth and the fish fed with beef mince showed lowest value. The growth rate in terms of total length observed has shown the same result in C. marulius fries fed with chicken liver, commercial feed, beef mice whereas the lowest total length was observed in C. marulius fries fed with BSF-larvae. The means values depicted with standard error bars in Figure 4.
Analysis of variance
Analysis of variance (Tukey test) was applied to estimate the significance of test variables Table 3. The mean value of the final weight, final total length and final standard length were shown. The difference in growth performance observed were highly significant (P ˂ 0.05). Similarly, the weight gain was highly significant in C. marulius fries fed on chicken liver (P ˂ 0.05) when compared to beef mince. The difference in growth performance observed was highly significant in C. marulius fries fed on chicken liver, BSF-larvae, commercial feed, and beef mince. The length gain was highly increase in fries fed with beef mince as compared to other.
Water quality parameter
The physio-chemical parameters, including temperature in the 28–30 °C range, PH 7.5–8.5, total hardness of 140-158 mgL-1, and DO 5.6–8.5 mgL-1, were recorded. Total dissolved solid (TDS) and electrical conductivity were 450mg/l and 467 μμ-mho/cm, respectively. These values were deemed normal and acceptable for the target specie.
Table 3: ANOVA (feed composition).
|
Growth parameter |
Feed composition |
Mean with St. error |
p value |
|
Final weight
|
Chicken liver |
1.91(±0.70) |
0.00 |
|
BSF-larvae |
1.73(±0.48) |
||
|
commercial feed |
1.86(±0.68) |
||
|
Beef mince |
1.68(±0.60) |
||
|
Final total length
|
Chicken liver |
2.87(±0.32) |
0.00 |
|
BSF- larvae |
2.42(±0.37) |
||
|
commercial feed |
2.42(±0.56) |
||
|
Beef mince |
2.52(±0.36) |
The present work deal with the survival rate of the C. marulius fed with chicken liver, BSFL and beef mince. The survival rate of C. marulius fries estimated in the current study was 90% and 60% fed on chicken liver and beef mince, respectively. In current observation that when size become large, the survival rate increased with practicing proper grading of the shooter. Current work on C. marulius is in line with work done by Devraj (1973) who also worked on the survival of same species. Survival rate was recorded as 19.3 %, and 1.3% for 3 months and 5 months respectively. With increase in fish size, survival rate increased up to 57.5 %. Giri et al. (2002) recorded the highest survival rate of the Wallago attu (Bloch and Schneider) larvae fed with fishmeal or meat meal-based diets in combination with plankton. Srivastava et al. (2012) also worked on the survival rate of C. stratius fed with different diet. The survival rate was recorded as 82%, 74% and 76% (p<0.05). Yadav et al. (2016) reported overall 90% survival rate during the one-year study. Kalhoro et al. (2017) recoded the survival rate 90%, 50% and 80% and significantly different (P< 0.05). Kumar et al. (2020) conducted the experiment on rainbow trout (Oncorhynchus mykiss) and found 100% survival rate. The current survival rate of C. marulius was high compared to previous work conducted on this fish, which could be corroborated to the diet formulation and proper grading of fish. During the current study, the fry of the C. marulius were found aggressive towards the feed. The feed was mostly finished with in short period which show the voracious nature of the target fish. During the experimental period the chicken liver, BSF-larvae and commercial feed were digested easily, and fry face the problem in the digestion of beef mince. The current study confirms the cannibalistic behavior of C. marulius during fry condition. This tallies the findings of Diana et al. (1985) according to whom snakehead can easily consume a smaller fish of more than half its length (Boonyaratpalin et al., 1985; Qin et al., 1996; Yadav et al., 2016; Sonawane et al., 2012) conformed the cannibalistic behavior of C. marulius.
The highest growth performance was attained on with the chicken liver while the lowest weight gain was attained by the C. marulius fed with beef mince. In this experiment, Specific growth rate and weight gain enhanced in the fry fed with chicken liver and the specific growth rate decrease with commercial feed. The maximum growth observes in our study in the fry fed with chicken liver and minimum in fry fed with the beef mince. The minimum growth rate on beef mince is due to the digestion of beef mince by C. marulius. In a related investigation, Zhao et al. (2021) supplied extruded soybean meal to the Epinephelus coioides in place of the fish meal that was normally consumed. The study showed that the growth and feed utilisation of different diets were significantly impacted by replacement. This suggested that grouper development retardation could result from greater dietary ESBM inclusion levels at levels above 45%. In studies similar to this one, other fish species including Dolly varden (Meng et al., 2020), rainbow trout (Yang et al., 2011), and Japanese flounder Saitoh et al. (2003) were able to tolerate up to 50%, 40.8%, and 32% soyabean meal substitution for fish meal protein, respectively, without affecting growth utilisation.
In the current study, BSFL provided better weight growth than chicken liver. In comparison to fishmeal and other protein sources, dried, defatted, and chitin-reduced BSFL meal (60%) combined with BSFL oil (4.8%) and dried BSFL meal (10-20%) resulted in similar growth performance in Atlantic salmon pre-smolts, according to the findings of (Belghit et al., 2018) and (Fisher and Romano, 2020). When feeding partially defatted, dried BSFL meal (5-15%) to Atlantic salmon post-smolts, similar results have also been seen (Belghit et al., 2019). The findings of Kumar et al. (2020) are consistent with this study in which the significant (p<0.05) effect of different diet on the growth performance of O. mykiss was observed. Kalhoro et al. (2017) conducted the experiment on C. marulius fed with different diet containing squid, Tubifex and Pellet feed. From the study increase in length and weight was found with fries fed with squid and pellet feed. In present study as compared to the commercial feed and beef mince the BSF-larvae show significant SGR result. This finding is in contrast with the results of previous work done. In previous research, young turbot fed BSFL meal containing chitin (1.6–7.3%) were found to have lower SGR (Kroeckel et al., 2012). The outcomes of formula-fed fry in the current investigation with regard to growth were not noteworthy. In a related investigation, Mohanty and Samantaray (1996) found that C. striata fry fed a formula diet comprising 550 g kg-1 protein showed the highest growth performances (energy 4320 kcal kg-1). Similar observations have also been obtained regarding young Sarotherodon mossambicus, Ictaluruspunctatus, and Cyprinus carpio (Ogino and Saito 1970), as well as I. punctatus and C. carpio (Jauncey, 1982). The diet that contained 13.54% fat and 49.72% protein in the feed was ideal for C. striatus’s better growth. Kalhoro et al. (2017) observed that the SGR value was highly significant (P< 0.01). Devraj (1973) reported the 5.0-7.9 cm increase in length of C. marulius fed with fish liver in 5-months.
Conclusions and Recommendations
It is, therefore determined that C. marulius can be culture on animal-based protein diet for profitable farming. When the shooters were properly graded, cannibalism gradually came to an end, and in comparison to cultures based on predator-prey systems or supplemental feeding with fish and animal meat, the shooters had a comparatively greater survival rate and better production. By creating more enticing and delectable artificial feed, changing feeding strategy, and making more attractive artificial feed, additional study is required to reduce the generation of shooters at juvenile stages.
Acknowledgements
The authors are grateful to the Chairman Department of Zoology, Abdul Wali Khan University Mardan for providing laboratory for experimental work.
Novelty Statement
Channa marulius can be culture on animal-based protein diet for profitable farming. By creating more enticing and delectable artificial feed, changing feeding strategy, and making more attractive artificial feed, additional study is required to reduce the generation of shooters at juvenile stages.
Author’s Contribution
Muhammad Khubaib: This paper is a part MPhil study of 1st author, performed this research study.
Muhammad Salman Khan: Helped in experimental setup and data collection.
Zia Ur Rahman: Data analysis.
Nafees Ahmad and Muhammad Atif Haider: Performed the field work collection of fish.
Mushtaq Ahmad Khan: Reviewed and edited the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Ali, A., N. Dahanukar and R. Raghavan. 2013. Length-weight and length-length relationship of three species of snakehead fish, Channa diplogramma, C. marulius and C. striata from the riverine reaches of Lake Vembanad, Kerala, India. J. Threat. Taxa, 5(13): 4769-4773. https://doi.org/10.11609/JoTT.o3353.4769-73
Belghit, I., N.S. Liland, R. Waagbø, I. Biancarosa, N. Pelusio, Y. Li, A. Krogdahl and E.J. Lock. 2018. Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture, 491: 72-81. https://doi.org/10.1016/j.aquaculture.2018.03.016
Belghit, I., N.S., Liland, P. Gjesdal, I. Biancarosa, E. Menchetti, Y. Li, R. Waagbø, A. Krogdahland E.J. Lock. 2019. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture, 503: 609-619. https://doi.org/10.1016/j.aquaculture.2018.12.032
Boonyaratpalin, M., E.W. McCoy and T. Chittapalapong. 1985. Snakehead culture and its socio-economics in Thailand.
Britz, R., E. Adamson, R. Raghavan, A. Ali and N. Dahanukar. 2017. Channa pseudomarulius, a valid species of snakehead from the Western Ghats region of peninsular India (Teleostei: Channidae), with comments on Ophicephalus grandinosus, O. theophrasti and O. leucopunctatus. Zootaxa, 4299(4): 529-545. https://doi.org/10.11646/zootaxa.4299.4.4
Chowdhury, A.A., M.Y. Hossain, Z. Mawa, D. Khatun, M.A. Islam, M.A. Rahman, M.R. Hasan, O. Rahman, R.H. Konok, M.A. Rahmanand and M.F. Parvin. 2021. Some biological aspects of the spotted snakehead Channa punctata (Bloch, 1793) in the wetland ecosystem, Gajner Beel, North-western Bangladesh. Indian J. Fish, 68(3): 17-26. https://doi.org/10.21077/ijf.2021.68.3.98724-03
Courtenay, W.R. and J.D. Williams. 2004. Snakeheads (Pisces, Channidae): A biological synopsis and risk assessment (No. 1251). US Geological Survey. https://doi.org/10.3133/cir1251
Devraj, M., 1973. Biology of the large snakehead, Ophiocephalus marulius (Ham.) in Bhavanisagar water. Indian J. Fish, 20(10): 139-147.
Diana, J.S. and W.Y.B. Chang. 1985. Production systems for commonly cultured freshwater fishes of Southeast Asia. No. 36224 (722). https://doi.org/10.5962/bhl.title.58512
Dua, A. and K. Kumar. 2006. Age and growth patterns in Channa marulius from Harike Wetland (A Ramsar site), Punjab, India. J. Environ. Biol., 27(2): 377.
FAO, 2010. International scientific symposium biodiversity and sustainable diets united against hunger.
FAO, 2020. The state of world fisheries and aquaculture. Sustainability in action, Rome.
Fischer, H. and N. Romano. 2020. Black soldier fly larval production in a stacked production system. Glob. Aquacult. Advocate, 16: 1-8.
Gangadhar, B., B.A. Kumar, M.R. Raghunath and N. Sridhar. 2018. Pre-pupae (larvae) of black soldier fly-a potential alternate protein source for aquaculture feeds. Aquacult. Asia Magaz., 22(1): 11-15.
Giri, S.S., S.K. Sahoo, B.B. Sahu, A.K. Sahu, S.N. Mohanty, P.K. Mukhopadhyay and S. Ayyappan. 2002. Larval survival and growth in Wallago attu (Bloch and Schneider): Effects of light, photoperiod and feeding regimes. Aquaculture, 213(1-4): 151-161. https://doi.org/10.1016/S0044-8486(02)00012-1
Habib, M., W.S. Lakra, V. Mohindra, P. Khare, A.S. Barman, A. Singh, K.K. Lal, P. Punia and A.A. Khan. 2011. Evaluation of cytochrome b mtDNA sequences in genetic diversity studies of Channa marulius (Channidae: Perciformes). Mol. Biol. Rep., 38: 841-846. https://doi.org/10.1007/s11033-010-0175-2
Hamilton, F., 1822. An account of the fishes found in the river ganges and its branches (Vol. 1). Archibald Constable.
Hossain, M.A., S.M. Almatar and C.M. James. 2010. Optimum dietary protein level for juvenile silver pomfret, Pampus argenteus (Euphrasen). J. World Aquacult. Soc., 41(5): 710-720. https://doi.org/10.1111/j.1749-7345.2010.00413.x
Jauncey, K., 1982. The effects of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture, 27(1): 43-54. https://doi.org/10.1016/0044-8486(82)90108-9
Kalhoro, H., A. Malik, G. Abbas, I.B. Kalhoro, S.A. Shah and H. Kalhoro. 2017. Evaluation of the growth performance, body composition and survival rate of juvenile snakehead (Channa marulius) fed on different feeds. Pak. J. Zool., 49(5): 1871-1877. https://doi.org/10.17582/journal.pjz/2017.49.5.1871.1877
Khan, M.A., S. Khan and K. Miyan. 2012. Length–weight relationship of giant snakehead, Channa marulius and stinging catfish, Heteropneustes fossilis from the River Ganga, India. J. appl. Ichthyol., 28(1): 154-155. https://doi.org/10.1111/j.1439-0426.2011.01901.x
Kroeckel, S., A.G. Harjes, I. Roth, H. Katz, S. Wuertz, A. Susenbeth and C. Schulz. 2012. When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture, 364: 345-352. https://doi.org/10.1016/j.aquaculture.2012.08.041
Kumar, V., S. Lee, B.M. Cleveland, N. Romano, R.S. Lalgudi, M.R. Benito, B. McGraw and R.W. Hardy. 2020. Comparative evaluation of processed soybean meal (EnzoMealTM) vs. regular soybean meal as a fishmeal replacement in diets of rainbow trout (Oncorhynchus mykiss): Effects on growth performance and growth-related genes. Aquaculture, 516: 734652. https://doi.org/10.1016/j.aquaculture.2019.734652
Lee, S.M., I.G. Jeon and J.Y. Lee. 2002. Effects of digestible protein and lipid levels in practical diets on growth, protein utilization and body composition of juvenile rockfish (Sebastes schlegeli). Aquaculture, 211(1-4): 227-239. https://doi.org/10.1016/S0044-8486(01)00880-8
Li, X., P. Musikasinthorn and Y. Kumazawa. 2006. Molecular phylogenetic analyses of snakeheads (Perciformes: Channidae) using mitochondrial DNA sequences. Ichthyol. Res., 53: 148-159. https://doi.org/10.1007/s10228-005-0321-3
Meng, F., B. Li, Y. Xie, M. Li. and R. Wang. 2020. Substituting fishmeal with extruded soybean meal in diets did not affect the growth performance, hepatic enzyme activities, but hypoxia tolerance of Dolly Varden (Salvelinus malma) juveniles. Aquacult. Res., 51(1): 379-388. https://doi.org/10.1111/are.14385
Mirghaed, A.T., S.M. Hoseini and M. Ghelichpour. 2018. Effects of dietary 1, 8-cineole supplementation on physiological, immunological and antioxidant responses to crowding stress in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol., 81: 182-188. https://doi.org/10.1016/j.fsi.2018.07.027
Mohanty, S.S. and K. Samantarayl. 1996. Effect of varying levels of diet ARY protein on the growth performance of channa striatus fry under different culture conditions. J. Aquacult., 4: 37-46. https://doi.org/10.61885/joa.v4.1996.177
National Research Council, 2011. Nutrient requirements of fish and shrimp. National academies press.
Ogino, C. and Saito, K., 1970. Protein nutrition in fish. 1. The utilization of dietary protein by young carp. Japan. Soc. Fisher. Sci., 36: 250-254 https://doi.org/10.2331/suisan.36.250
Qin, J. and A.W. Fast. 1996. Size and feed dependent cannibalism with juvenile snakehead Channa striatus. Aquaculture, 144(4): 313-320. https://doi.org/10.1016/0044-8486(96)01299-9
Qin, J.G. and A.W. Fast. 1997. Food selection and growth of young snakehead Channa striatus. J. Appl. Ichthyol., 13(1): 21-25. https://doi.org/10.1111/j.1439-0426.1997.tb00093.x
Rahman, M.A., Arshad, A., Amin, S.M.N. and N.S. Mariana. 2013. Growth and survival of fingerlings of a threatened snakehead, Channa striatus (Bloch) in earthen nursery ponds. Asian J. Anim. Vet. Adv., 8(2): 216-226. https://doi.org/10.3923/ajava.2013.216.226
Raizada, S., P.P. Srivastava, P. Punia, K.C. Yadav, V. Sahu, S. Chowdhary and J. Jena. 2012. Dietary protein requirement of giant snakehead, Channa marulius (Ham., 1822) fry and impact on growth indices. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci., 82: 489-496. https://doi.org/10.1007/s40011-012-0060-y
Rumpold, B.A. and O.K. Schlüter. 2013. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res., 57(5): 802-823. https://doi.org/10.1002/mnfr.201200735
Sagada, G., J. Chen, B. Shen, A. Huang, L. Sun, J. Jiang and C. Jin. 2017. Optimizing protein and lipid levels in practical diet for juvenile northern snakehead fish (Channa argus). Anim. Nutr., 3(2): 156-163. https://doi.org/10.1016/j.aninu.2017.03.003
Sekino, M., K. Saitoh, T. Yamada, A. Kumagai, M. Hara and Y. Yamashita. 2003. Microsatellite-based pedigree tracing in a Japanese flounder Paralichthys olivaceus hatchery strain: Implications for hatchery management related to stock enhancement program. Aquaculture, 221(1-4): 255-263. https://doi.org/10.1016/S0044-8486(02)00667-1
Sonawane, S., A. Gedam, S. Anand and S. Pawar. 2012. Food and feeding habits of Channa punctatus from Kaigaon Toka Dist. Aurangabad (MS) in relation to biochemical studies. J. Exp. Sci., 3(8): 7-13.
Srivastava, P.P., R. Dayal, S. Chowdhary, J.K. Jena, S. Raizada and P. Sharma. 2012. Rearing of fry to fingerling of Saul (Channa Striatus) on artificial diets. Online J. Anim. Feed Res., 02(02)
Van Huis, A., J. Van Itterbeeck, H. Klunder, E. Mertens, A. Halloran, G. Muir and P. Vantomme. 2013. Edible insects: Future prospects for food and feed security (No. 171). Food and agriculture organization of the United Nations.
Wallady, A.A., B.S. Rahardja and H. Kenconojati. 2022. Dietary combination of maggot and commercial feed enhance the growth rate and feed conversion ratio of snakehead fish (Channa striata). IOP Conf. Ser. Earth Environ. Sci., 1036(1): 012085. https://doi.org/10.1088/1755-1315/1036/1/012085
Yadav, K.C., A. Mishra, S. Raizada, V. Sahu and P.P. Srivastava. 2016. Influence of formulated diet on survival and growth of giant-snakehead, Channa marulius reared in pond condition. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci., 86(1): 97-103. https://doi.org/10.1007/s40011-014-0414-8
Yang, Y.H., Y.Y. Wang, Y. Lu and Q.Z. Li. 2011. Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion on rainbow trout (Oncorhynchus mykiss). Aquacult. Int., 19(3): 405-419. https://doi.org/10.1007/s10499-010-9359-y
Zhao, X., Y. Wang, X. Wang and J. Ye. 2021. Growth performance, plasma components, and intestinal barrier in grouper (Epinephelus coioides) are altered by dietary fish meal replacement with extruded soybean meal. Aquacult. Rep., 21: p.100863. https://doi.org/10.1016/j.aqrep.2021.100863
To share on other social networks, click on any share button. What are these?







