Effects of Biotechnologically Produced Fulvic Acid on Nutritional Status and Health Indicators of Sprague-Dawley Rats
Effects of Biotechnologically Produced Fulvic Acid on Nutritional Status and Health Indicators of Sprague-Dawley Rats
Yang Liu1, Jing-xin Mao2, Xiao-dong Wei2, Man Yi2, Xiao-long Zhang2, Ke Zheng3, Xian-xin Chen4, Guo-Ze Wang5 and Bing-bo Chen1,*
1Laboratory Animal Center, Army Medical University, Chongqing 400038, P.R. China
2College of Pharmaceutical Sciences, Southwest University, Chongqing 400715, P.R. China
3Department of Endocrine and Breast Surgery, the First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, P.R. China
4Leshan Academy of Agricultural Sciences, Leshan 614000, P.R. China
5College of Pharmacy and Biological Engineering, Chengdu University, Chengdu 610100, P.R. China
ABSTRACT
The present study was aimed at determining the efficacy and safety value of biotechnology produced fulvic acid (BFA) as a functional feed additive. Four BFA products produced by lactobacillus, bacillus, yeast and fermented BFA from above 3 mixed bacteria were added by a proportion of 1.5% to the conventional feed. Then the Sprague-Dawley rats were fed by normal feed and 4 different BFA feed for 4 weeks. It was observed that the counts of neutrophils, white blood cells, and monocytes was significantly decreased in the lactobacillus BFA group than the control group (P<0.05); neutrophil was reduced in bacillus BFA group than the control group significantly (P<0.05). Compared to the control group, total protein, albumin, and albumin/globulin ratio were increased in lactobacillus BFA group. The total bilirubin decreased significantly (P<0.05) both in lactobacillus BFA and Bacillus BFA group. Total weight gain and daily gain were increased in mixed fermentation BFA group significantly than the control group (P<0.05). For feed conversion rates in each group, the mixed fermentation BFA group had the highest feed efficiency, increased by 10.04% than the control group, followed by the yeast BFA group, with an increase by 4.14%. The mixed fermentation BFA group and lactobacillus fermentation BFA group had significantly reduced number of E. coli than the control group (P<0.05), while the number of lactobacilli was significantly increased than the control group (P<0.05). The thymus index was significantly higher in the mixed fermentation BFA group and Lactobacillus BFA group than the control group (P<0.05); while significant difference was seen in the spleen index between the mixed fermentation BFA group and the control group (P<0.01). Immu-serological indicators: the mixed fermentation group had significant increased serum levels of IgA, IgG, IgM and IL-2 compared with the control group (P<0.05); the Lactobacillus BFA group had obviously increased levels of IgA, IgG and IL-2 than the control group (P<0.05). It proves that the BFA has a positive effect on improving the health indicators and nutritional status, blood physiology and biochemistry, weight gain of rats, which can effectively promote the growth of animals and raise feed reward. It results in improving gut microbiota, promoting the digestion and absorption of nutrients, which significantly improved weight gain rate and the quality of external fur. It was suggested that BFA has a positive and effective effect on improving the nutrition of rats and promoting their healthy growth.
Article Information
Received 28 December 2017
Revised 12 May 2018
Accepted 17 July 2018
Available online 22 March 2019
Authors’ Contribution
YL performed the experiments, designed the research and wrote the paper. JXM wrote and modified parts of the paper. XDW, MY, XLZ analyzed the data. KZ, XXC and GZW provided experimental material. BBC conceived the research.
Key words
Biotechnology fulvic acid, Nutritional analysis, Physiological effect, Gut microbiota, Weight gain rate.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.3.961.970
* Corresponding author: [email protected]
0030-9923/2019/0003-0961 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Humic acid (HA) is a complex mixture, which has complex functions and structures, gets from the biological organic matter by the decomposition and transformation of microorganisms through long-term reaction and accumulation (Alvarez-Puebla et al., 2006). According to solubility and color in solvents, HA can be classified into fulvic acid, hymatomalenic acid, pyrotomalenic acid (Maryganova et al., 1995). HA is a kind of complex natural macromolecule organic weak acid, which is unstable and often exists in the form of sodium salt. It has the characteristics of hydrophilic, weak acid, complexation and adsorption (Kollist-Siigur et al., 2001; Li et al., 2012; Bose and Reckhow, 1997).
Biotechnologically produced fulvic acid (BFA), a compound substance which used in this study, is made from fermented raw materials such as straw or wood, supplemented with soybean meal and wheat bran, which are fermented by a variety of microorganisms. The BFA used in this study is standardized derived from: Liquor’s grains as the main raw material, the use of fermentation engineering and enzyme engineering core technology, combined with excellent probiotic strain, the probiotics-composite enzyme coupled biological transformation method, auxiliary physical and chemical processing. BFA was prepared through such innovative process line. We have also done a lot of research on the content detection or quality standard of BFA which are widely used at present. The results show that the quality detection studies are mainly focused on: sensory detection, humic acid content, microbiology hygiene, probiotics activity, and product harmlessness (Ye et al., 2013). Among them, the content of humic acid and the number of probiotics live bacteria are the key functional components to play the biological role. Combined with Li (2011), quality research report on BFA. The basic indicators of BFA quality qualification used in this study are: FA content ≥ 16%, the number of probiotics alive ≥ 2×108cfu/g. Strict control on the stability of BFA source, to provide standardized samples for BFA efficacy study.
It is reported that BFA is weakly acidic and contains about 50% fulvic acid, 15% of ribonucleic acid and 9% of amino acids, inositol, vitamin, and sugar (Lv et al., 2010; Liu et al., 2013). BFA contains a large number of probiotics and fulvic acids, which combine with the function of HA and probiotics to show better functional activity. It was showed that probiotics exerts a competitive repulsion against harmful bacteria (Collins et al., 1999). Therefore, it can regulate the balance of gut microbiota, establish a microbiota that is beneficial to the host gut. In addition, it also proved that probiotics can improve immunity, prevent diarrhea, improve gastrointestinal digestion and absorption (Bengmark, 2002). All these make the BFA rich in probiotics has the functions of improving the intestinal function, animal growth performance and the utilization rate of feed. At the same time, BFA contains a variety of amino acids, nucleic acid, inositol, multi-vitamins, sugar and other substances, which may involving in the multiple reactions of body’s metabolism. These beneficial substances are essential nutrients for animal growth, and effectively promote the healthy growth of animals (Mcmurphy et al., 2009; Arif et al., 2016; Naila et al., 2019). BFA can be used to convert large molecular nutrients in feed to small molecule nutrients, which are beneficial for gastrointestinal digestion and absorption. BFA contains quinones, which can enter multiple oxidation and reduction processes of the body and promote metabolism (Scott et al., 1998).
At present, various feed additives have been widely used in various fields of agriculture. It was found that the addition of complex probiotics had positive effects on the growth performance, nutrient digestibility of growing pigs (Liu et al., 2017). At the same time, the positive effects on finishing pigs in the study of Scutellaria baicalensis and Lonicera japonica extract mixture were studied (Liu et al., 2016). In addition, HA can also be used as feed additive (Wei et al., 2017), soil conditioner (Guo et al., 2016), plant growth regulator (Adiloglu et al., 2018), and organic fertilizer fermentation (Li et al., 2018). However, there are not many studies on the application of BFA in efficient ecological agriculture research.
In this paper, Sprague-Dawley rats were taken as the research object, and the BFA feed products were added into the regular feed of Sprague-Dawley rats as functional nutritional feed additives. After feeding a certain period, we observed the influence of BFA on the typical life state of Sprague-Dawley rats, then analyzed the effects of BFA on blood indexes, growth performance, quantitative analysis of gut microbiota and immune indices in Sprague-Dawley rats. We have studied the safety and efficacy of BFA as a feed nutrient additive, providing theoretical and data support for its subsequent development and application.
Materials and methods
Animal care
The experimental procedure was approved by the Laboratory Animal Center at Army Medical University, Chongqing .We selected 100 Specific Pathogen Free (SPF) female Sprague-Dawley rats, 3-4 weeks old and weight 70-90g, and all the animals were provided by the Laboratory Animal Center of Army Medical University (NO:SCXK (YU) 2017-0002).
Instrument and equipment
Automatic blood cell analyzer (Sysmex XT-2000i, USA), Centrifuge (Anke TDL-5-A, USA), Automatic biochemical analyzer (Hitachi 7600-010, Japan), Microscope (Olympus, CX21FS1, Japan), Constant temperature magnetic stirrer (Zhen Rong, 90-2, China), Analytical balance (Guang Zheng, 562-1, China), Whirlpool mixer (QL-901, China), Anaerobic glove incubator (Thermo-1029, China), Incubator (Yuejin HH-B11, China).
Main reagents and materials
Pentobarbital, normal saline, EDTA·K2, disposable vacuum blood collection tube, disposable vacuum serum collecting tube, EMB, MRS, IL-2 ELISA kitˎ IL-4 ELISA kitˎ IL-12 ELISA kitˎ IgA ELISA kitˎ IgM ELISA kitˎ IgG ELISA kitˎ C3 ELISA kit.
Feed formulation and experimental grouping
During the experiments, rats were fed with regular diet from the Laboratory Animal Center of Army Medical University. BFA products were provided by the Micro-ecological Research and Development Center, Southwest Hospital affiliated to Army Medical University. 100 female Sprague-Dawley rats were randomly divided into 5 groups, and each group was fed with a recipe shown in Table I.
Table I.- Groups of the rats and feed formulations.
|
Groups |
BFA |
Normal feeding stuff (%) |
|
Lactobacillus group |
1.5% Lactobacillus fermentation |
98.5 |
|
Bacillus group |
1.5% Bacillus fermentation |
98.5 |
|
Yeast group |
1.5% Yeast fermentation |
98.5 |
|
Mixed group |
1.5% Mixed fermentation |
98.5 |
|
Control group |
0 |
100 |
Experimental method
The 100 female Sprague-Dawley rats were randomly divided into 5 groups, Lactobacillus group, Bacillus group, yeast group, mixed group and control group (routine feed), each with 20 rats. Rats were reared in the SPF room of Laboratory Animal Center, Army Medical University, for 4 weeks at 20-25°C and 40%-70% humidity. All groups were provided with food and water ad libitum. Throughout the experimental period, the feeding status, activity, consciousness and morbidity of Sprague-Dawley rats were observed and recorded. At the same time, the daily feed consumption and the weekly weight of rats was recorded. Blood, cecum contents, spleen and thymus were collected at the end of the feeding cycle, and cryopreserved for subsequent hematometry, quantitative analysis of gut microbiota and detection of immune indices.
Hematometry
The Sprague-Dawley rats were anesthetized with pentobarbital at a concentration of 3%, and blood samples were taken from femoral vein. Blood cell count was done by SysmexXT-20001 automatic blood corpuscle instrument. Liver and renal functions were checked by seperating serum from blood and then analyzed by Hitachi 7600-010 automatic biochemical analyzer.
Blood sera were used for estimation of IL-2, IL-4, IL-12, IgA, IgM, IgG, IgG, and C3 of the rats, according to the instructions of the kits (American R&D Company).
The thymic and the spleen indices
After killing the rats, the spleen and thymus gland were removed, weighed and there indices were calculated as follows:
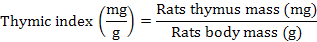

Rats weight gain and feed conversion rate
The rats were weighed on day 1 and day 7 of the week. The fasting weight of each Sprague-Dawley rats was also recorded. Average weekly weight gain was calculated.
Feed conversion rate:
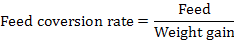
Analysis of gut microbiota
The contents of the rat cecum were taken out aseptically. 0.2g of specimen with 9 times the amount of diluent and 3 sterilized glass beads were sonicated to make it all pulp. The specimens were diluted in series from 10-1 to 10-9 with the original liquid to make 10 dilutions. Diluted specimens were inoculated on deMan, Rogosa, Sharpe (MRS) agar and eosin-methylene blue (EMB) agar.
Lactobacillus colonies was counted after 48h of anaerobic culture, and the E. coli colonies were counted after 24h of aerobic culture.
Statistical analysis
The experimental data are expressed by mean ± standard deviation (Mean±SD), and the SPSS 19.0 software was used for statistical analysis. Using the single factor variance analysis to analyze the population mean of multiple groups, the comparison of the mean of multiple samples was analyzed using LSD test method, P<0.05 showed significant difference, and P<0.01 was very significant difference.
Results
Blood indices
Figure 1 shows effect of feeding BFA for four weeks, on the white blood cells (WBC), lymphocytes (Lymph) and monocytes (Mon) in the Lactobacillus group and the mixed group were significantly higher than the control group. The WBC of yeast group was higher than that of control group. There were no significant differences in the other blood indices of the other three groups. There were no significant differences between the blood indices of the Bacillus group and the control group.
Blood biochemical indices
Figure 2 shows the total protein (TP) , albumin (Alb), albumin/globulin ratio (A/G) of the Lactobacillus group were higher than the control group. The total bilirubin (TBIL) of the Lactobacillus group and the Bacillus group were significantly lower than the control group.
Immune indices
Table II shows that IgA, IgG and IL-2 of Lactobacillus group were significantly higher than those in the control group after the BFA feeding for four weeks. IgA, IgG, IgM and IL-2 were significantly higher in the mixed group than the control group.
Table II.- The effect of BFA on immune indices in the Sprague-Dawley rats.
|
Group |
C3 (µg/ml) |
IgA (µg/ml) |
IgG (µg/ml) |
IgM (µg/ml) |
IL-2 (ng/l) |
IL-4 (ng/l) |
IL-12 (ng/l) |
|
Lactobacillus group |
28.28±4.42 |
1.95±0.56* |
1.68±0.13* |
1.69± 0.15 |
343.78± 44.94* |
13.00± 7.52 |
6.83± 1.86 |
|
Bacillus group |
25.36±3.84 |
1.47±0.36 |
1.52±0.12 |
1.66± 0.20 |
289.82± 18.73 |
13.14± 6.74 |
7.01± 3.68 |
|
Yeast group |
23.71±5.40 |
1.58±0.36 |
1.66±0.19 |
1.63± 0.21 |
305.97± 17.84 |
13.11± 8.81 |
7.26± 3.33 |
|
Mixed group |
25.73±4.75 |
2.94±0.49** |
1.85±0.12** |
1.89± 0.11* |
390.94± 48.44** |
16.50± 6.51 |
7.72± 1.23 |
|
Control group |
23.39±3.12 |
1.22±0.42 |
1.55±0.08 |
1.63± 0.22 |
292.28± 22.17 |
9.28± 1.85 |
4.48± 2.05 |
The data is represented as mean ± SD (n = 20). *P < 0.05, **P < 0.01 vs control group.
Table III.- The effect of BFA on thymus index and spleen index in the SD rats.
|
Group |
Thymus index |
Spleen index |
|
Lactobacillus group |
2.06±0.34* |
2.56±0.24 |
|
Bacillus group |
1.89±0.28 |
2.50±0.29 |
|
Yeast group |
1.93±0.37 |
2.54±0.49 |
|
Mixed group |
2.27±0.30** |
2.85±0.21** |
|
Control group |
1.73±0.28 |
2.33±0.33 |
The data is represented as mean ± SD (n = 20). *P< 0.05, **P< 0.01 vs control group.
Table IV.- The effect of BFA on feed conversion in the SD rats.
|
Group |
Total weight gain |
Total feed consumption |
Daily weight gain |
Daily feed consumption |
Feed conversion |
|
Lactobacillus group |
124.17±1.23 |
16622±3.89 |
4.43±0.53 |
29.68±1.34 |
6.70 |
|
Bacillus group |
121.22±2.13 |
16334.5±2.95 |
4.33±0.55 |
29.17±1.56 |
6.74 |
|
Yeast group |
122.77±1.34 |
15922.5±3.96 |
4.38±0.78 |
28.43±1.68 |
6.49 |
|
Mixed group |
131.63±1.02* |
16024±2.86 |
4.70±0.43* |
28.61±1.23 |
6.09 |
|
Control group |
118.27±2.25 |
16004.5±3.97 |
4.22±0.62 |
28.58±1.78 |
6.77 |
The data is represented as mean ± SD (n = 20). *P < 0.05 vs control group.
Table III shows that the thymic indices of four BFA fermentation groups were significantly higher than those in the control group after the BFA fermentation products were fed to Sprague-Dawley rats. The Lactobacillus group and the mixed group were significantly higher than the control group (P<0.05, P<0.01).
Body weight gain
Figure 3 shows that the weight of Sprague-Dawley rats increased during the first weeks and then decreased gradually.
Feed consumption and feed conversion
Table IV shows that the total weight gain and daily weight gain of the mixed group were significantly higher after the BFA feeding than that in the control group (P<0.05). The feed conversion of each group were: mixed group (6.09), yeast group (6.49), Lactobacillus group (6.70), Bacillus group (6.74), and control group (6.77), with the highest feed conversion of the mixed group, 10.04% higher than the control group. The yeast group took the second place, the feed conversion was 4.14% higher than that in the control group.
Table V.- The impact of BFA feed on E. coli and Lactobacillus in the SD rats.
|
Groups |
E. coli |
Lactobacillus |
|
Lactobacillus group |
7.25±0.52* |
10.12±0.60* |
|
Bacillus group |
7.93±0.87 |
9.69±0.75 |
|
Yeast group |
8.20±0.84 |
9.56±0.91 |
|
Mixed group |
6.91±0.66* |
9.89±0.81* |
|
Control group |
8.53±1.01 |
8.92±0.66 |
The data is represented as mean ± SD (n = 20). *P < 0.05 vs control group.
E. coli and Lactobacillus
Table V shows quantitative analysis of the representative gut microbiota of rats, E. coli and Lactobacillus after being fed by BFA for four weeks. The results showed that the number of E. coli in mixed group and Lactobacillus group were significantly lower than that in the control group (P<0.05), while the number of Lactobacillus was significantly higher than that in the control group (P<0.05). There was no significant difference in the other groups.
Discussion
The BFA is a type of composite fermentation products, which use straw or sawdust, supplemented with soybean meal, wheat bran and other fermented raw materials, and is fermented by a variety of microorganisms (Liu et al., 2013). BFA contains fulvic acid, probiotics, amino acids, vitamins, sugar, and inositol. The most remarkable characteristic of BFA is that it has the function of fulvic acid and probiotics. Fulvic acid has the function of antibiosis and antiphlogistic, it can improve gastrointestinal function, promote metabolism, promote growth and development of animals, enhance immunity and disease resistance (Visser, 1973; Stefano, 2017). Probiotics can regulate gut microbiota, auxiliary to establish beneficial to the host animals gastrointestinal microflora, adjust the gastrointestinal function, prevent diarrhea, enhance immunity, improve nutrient digestion and absorption (Collins et al., 1999; Mcfarland, 2006). This study showed that the BFA improves the gut microbiota, promote nutrient digestion and absorption, enhance immunity, promote metabolism, promote cell proliferation, accelerating growth has a positive role in such aspects. During experimentation, eating, movement, consciousness, was all normal in the experimental group and control group. After observation, four experimental rats that were fed BFA, fur more smooth, point out BFA can significantly promote the absorption and utilization of nutrients in rats, thereby significantly improving animal health and appearance, including fur quality.
Changes in blood biochemical indicators can reflect the health of the body. The liver and kidneys are the primary sites for the transformation and metabolism of allogenic substances in the body. In this study, after the Sprague-Dawley rats were fed by BFA for four weeks. There was no significant differences in ALT, AST, Scr and UN in the four BFA fermentation groups (P>0.05), suggesting that BFA products did not have adverse effects on liver and kidney function of Sprague-Dawley rats. Serum TP consists of Alb and Glb. Its main physiological function is the maintenance of the colloid osmotic pressure, and also have the effect of immunity, transporting, repairing tissue and buffering (Szabó et al., 2017). In this experiment, the Alb, TP, A/G in Lactobacillus group were significantly increased compared with control subjects (P<0.05), it is suggested that Lactobacillus group can enhance the capacity of Sprague-Dawley rat liver protein synthesis, improve liver protein metabolism, promote production and utilization of protein. At the same time, TBIL in the Lactobacillus group and Bacillus group was lower than the control group, and the difference was significant (P<0.05, P<0.01). It is indicated that with the increase of hepatic synthesis function and the increase of serum albumin, the binding, carrying and metabolic capacity of albumin to bilirubin have also been increased. Cagin et al. (2016) designed to test chelator effect of HA on as well as its anti-oxidant effect against the iron-induced hepatotoxicity and cardiotoxicity. They found that no significant differences were observed in the serum biochemical values and the histopathological results among the HA groups in the liver tissue but not in the heart tissue. The protective effects of HA against iron-induced cardiotoxicity were shown but not against hepatotoxicity in their study (Cagin et al., 2016). Observation of the blood indicators suggest that four weeks after feeding with BFA, the WBC, lymphocyte (Lymph) and mononuclear cells (Mon) of Lactobacillus group and mixed group were higher than the control group, with significant difference (P<0.05); The WBC of the yeast group were higher than the control, and the difference was significant (P<0.05). There was no significant difference in blood indices of Bacillus group. It was suggested that the Lactobacillus group and mixed group could have a more obvious effect on hematological indices. It was found that no significant differences were noted with lymphocyte stimulation test. Diameter of the ‘B’-dependent lymphoid tissues in the ileum and spleen were significantly (P< 0.05) larger in fulvic acid-treated animals, fulvic acid supplementation resulted in strong humoral immune stimulation (Vucskits et al., 2010).
Thymus and spleen are important immune organs in the body, and thymus is the central lymphatic organ, which is the site of differentiation, development and maturation of T cells (Mao et al., 2017). The spleen is the peripheral lymphatic organ, which is closely related to humoral immunity and cellular immunity, and contains numerous lymphocytes (including T lymphocytes, B lymphocytes) and macrophages (Xu et al., 2016). In the experiment, the thymus index, spleen index, IgA, IgG, IgG, IgM and IL-2 in the mixed group were significantly higher than those in the control group, and the difference was significant (P<0.05). The thymus index, IgA, IgG and IL-2 of lactobacillus group were significantly higher than the control group, and the difference was significant (P<0.05). Preliminary results show that BFA has a positive impact on the development of central and peripheral lymphatic organs. Thus, by increasing the number of T lymphocytes and B lymphocytes in the immune organs of Sprague-Dawley rats, improve the body’s humoral immunity and cellular immunity and improve the body’s overall immunity (Marek et al., 2016). The IL-4 and IL-12 of the four BFA groups were higher than the control group, but no statistical significance was found. This may be due to the differences between individual Sprague-Dawley rats, and further research is needed. It was showed that the young bifidobacterium stimulates the abdominal macrophages of mice to produce a large amount of IL-1, IL-6 and TNF-α (Nicaise et al., 1993). It was showed that the baby bifidobacterium was able to activate the mice abdominal macrophages and enhance the mRNA expression of IL-1 and IL-6 (Sekine et al., 1995). It also indicated that fulvic acid improves growth performance and intestinal health condition of loach, indicates that fulvic acid could be used as an immunoenhancer in loach culture (Gao et al., 2017).
It can be seen from the weight gain curve of Sprague-Dawley rats that the weight of Sprague-Dawley rats were increased with the increase of the age of week, and two rapid growth periods were occurred (week 1 to week 2 and week 4 to week 5, respectively). Then with the increase of the age of week, the weight gain gradually slowed down and finally stabilized. It showed that in the four BFA groups, the weight gain of the mixed group was the highest in the two rapid growth periods, and the total weight gain and the daily weight gain were significantly higher than those in the control group. The result is consistent with the literature, BFA has the effect of promoting the digestion and absorption of nutrients, boosting metabolism, promoting cell proliferation, accelerating growth (Wang et al., 2008; Ozturk et al., 2014). The experimental results of feed conversion showed that the mixed group was better and the yeast group was inferior, while the control group was worse. The results showed that the mixing group increased the feed conversion rate by 10.04% than the control group, and the yeast group increased the feed conversion rate by 4.14% compared with the control group. It is suggested that the BFA can effectively convert the large molecule nutrients of the feed into small molecular nutrients, which are better for gastrointestinal digestion and absorption. At the same time, the active ingredient of BFA contains quinone, that can be used to enter multiple oxidation and reduction processes of the body, promote metabolism, and play a positive and effective role in promoting the growth of animals and improving the utilization rate of feed. Quinones are also responsible for the formation of reactive oxygen species (ROS) in humic acids, which are useful for wound healing and have bactericidal properties (Melo et al., 2016).
The results showed that the number of E. coli in mixed group and lactobacillus group were significantly lower than that in the control group after the BFA feeding, while the number of live Lactobacillus was significantly higher than that in the control group. It is indicated that BFA can effectively inhibit the growth of E. coli in rats and promote the proliferation and activity of Lactobacillus. The mechanism may be that the Lactobacillus group and the mixed group BFA contain a large number of beneficial lactobacillus and other acid bacteria, these live bacteria have the effect of lowering the PH value in the cecal and ileum contents, by changing the acidity of the digestive tract, competition inhibits the reproduction of harmful bacteria such as E. coli and Salmonella, while promoting the growth of anaerobic probiotics such as Lactobacillus (Gibson et al., 1994).
Conclusion
In present study, it was proved that BFA has a positive impact on improving the health indicators and nutritional status, blood physiology and biochemistry, weight gain of rats, which can effectively promote the growth of animals and raise feed reward. At the same time, the BFA results in improving gut microbiota, then promote the digestion and absorption of nutrients, which significantly improved weight gain rate and the quality of external fur. It is suggested that BFA has a positive and effective effect on improving the nutrition of rats and promoting their healthy growth.
Acknowledgment
This work was supported by the key project of Chongqing Science and Technology Plan, China (NO: CSTC, 2011AB1045; CSTC, 2011ggB10014).
Statement of conflict of interest
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
Alvarez-Puebla, R.A., Valenzuela-Calahorro, C. and Garrido, J.J., 2006. Theoretical study on fulvic acid structure, conformation and aggregation: A molecular modelling approach. Sci. Total Environ., 358: 243-254. https://doi.org/10.1016/j.scitotenv.2004.11.026
Adiloglu, A., Adiloglu, S., Karaman, M.R., Solmaz, Y. and Açikgöz, F.E., 2018. The Effect of increasing humic acid applications on some nutrient contents of cress (Lepidium sativum L.) plant. Turk. J. Agric., 6: 199-202.
Arif, M., Rehman, A. and Saeed, M., 2016. Impacts of dietary humic acid supplementation on growth performance, some blood metabolites and carcass traits of broiler chicks. Indian J. Anim. Sci., 86: 1073-1078.
Bengmark, S., 2002. Gut microbial ecology in critical illness: Is there a role for prebiotics, probiotics, and synbiotics? Curr. Opin. Crit. Care, 8: 145-151. https://doi.org/10.1097/00075198-200204000-00010
Bose, P. and Reckhow, D.A., 1997. Modeling pH and ionic strength effects on proton and calcium complexation of fulvic acid: A tool for drinking water-nom studies. Environ. Sci. Technol., 31: 765-770. https://doi.org/10.1021/es9604469
Chand, N., Shamsullah., Rafiullah., Khan, R.U. and Mobashar, M., 2019. Mannanoligosaccharide (MOS) in broiler ration during the starter phase: Growth performance and intestinal histomorpholgy. Pakistan J. Zool., 51: 173-176.
Collins, M.D. and Gibson, G.R., 1999. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am. J. clin. Nutr., 69: 1052S-1057S. https://doi.org/10.1093/ajcn/69.5.1052s
Cagin, Y.F., Sahin, N., Polat, A., Erdogan, M.A. and Atayan, Y., 2016. The acute effect of humic acid on iron accumulation in rats. Biol. Trace Elem. Res., 171: 145-155. https://doi.org/10.1007/s12011-015-0507-0
Guo, J., Wang, Y., Chen, J., Zhang, R. and Guo, W., 2016. Effects of biochemical fulvic acid on degradation of cyanide and speciation of heavy metal during composting. Environ. Sci. Technol., 38: 1-8.
Gao, Y., He, J., He, Z.L., Li, Z.W. and Chu, Z.J., 2017. Effects of fulvic acid on growth performance and intestinal health of juvenile loach Paramisgurnus dabryanus (Sauvage). Fish Shellf. Immunol., 62: 47-56.
Gibson, G.R. and Wang, X., 1994. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. Appl. Bact., 77: 412-420. https://doi.org/10.1111/j.1365-2672.1994.tb03443.x
Hu, L., Yang, W. and Xu, J., 2014. Effect of dazomet and biotechnology fulvic acid treatment on fungal diversity in greenhouse soil. J. Shenyang Agric. Univ., 45: 217-220.
Kollist-Siigur, K., Nielsen, T., Gron, C., Hansen, P.E. and Helweg, C., 2001. Sorption of polycyclic aromatic compounds to humic and fulvic acid HPLC column materials. J. environ. Qual., 30: 526-537. https://doi.org/10.2134/jeq2001.302526x
Li, J., Zhang, S., Chen, C., Zhao, G. and Yang, X., 2012. Removal of Cu (II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS appl. Mater. Interf., 4: 4991-5000. https://doi.org/10.1021/am301358b
Li, R.B., 2011. Biological humic acid and ecological agriculture. Chemical Press, pp. 18-20.
Lv, Z.W. and Hu, X.M., 2010. Production of biotechnology fulvic acid from fermented corn straw by microbial community LCM9 and its application effect. Agric. Sci. Technol., 11: 14-16.
Liu, W.C., Devi, S., Park, J. and Kim, I., 2017. Effects of complex probiotic supplementation in growing pig diets with and without palm kernel expellers on growth performance, nutrient digestibility, blood parameters, fecal microbial shedding and noxious gas emission. Anim. Sci. J., 89: 552-556. https://doi.org/10.1111/asj.12965
Liu, W.C., Pi, S.H. and Kim, I.H., 2016. Effects of Scutellaria baicalensis and Lonicera japonica extract mixture supplementation on growth performance, nutrient digestibility, blood profiles and meat quality in finishing pigs. Italian J. Anim. Sci., 15: 446-452. https://doi.org/10.1080/1828051X.2016.1202736
Liu, Y., Wu, L.K., Zhang, J. and Chen, B.B., 2013. Exploration of the value of biotechnology fulvic acid (BFA) as nutritional additives in functional feed. Acta Lab. Anim. Sci. Sin., 21:50-55.
Li, P.C., Su, X.D. and Wang, J.J., 2018. Effects of humic acid fertilizer and biological fertilizer on soil properties and grape yield and quality. Soil Fertil. Sci. China, 13: 31-35.
Maryganova, V.V., Bambalov, N.N., Strigutsky, V.P. and Rykov, S.V., 1995. Peat humic and fulvic acid structure analysis by 13C-NMR-spectroscopy method. Fuel Abstr. Curr. Titles, 38: 52-55.
Mcmurphy, C.P., Duff, G.C., Harris, M.A., Chirase, N.K. and Bailey, C.R., 2009. Effect of humic/fulvic acid in beef cattle finishing diets on animal performance, ruminal ammonia and serum urea nitrogen concentration. J. appl. Anim. Res., 35: 97-100. https://doi.org/10.1080/09712119.2009.9706995
Mirzamasoumzadeh, B., Salami, M., Ghalichechi, S. and Rouhi, N., 2012. A comparison study on humic acid fertilizers effect on initial growth stages on four wheat cultivars. Annls. biol. Res., 3: 4747-4750.
Mcfarland, L.V., 2006. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol., 101: 812-822. https://doi.org/10.1111/j.1572-0241.2006.00465.x
Marek, S. and John, E.B., 2016. Progress in the use of swine in developmental immunology of B and T lymphocytes. Dev. Comp. Immunol., 58: 1-17. https://doi.org/10.1016/j.dci.2015.12.003
Melo, B.A., Motta, F.L. and Santana, M.H., 2016. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Engin. C, 62: 967-974. https://doi.org/10.1016/j.msec.2015.12.001
Mao, J.X., Wang, G.W., Huang Y.S., Wang, R., Wang, G.Z., Zeng, B. and Zuo, F.Y. 2018. Comparative study on BPI gene expression in various tissues between Rongchang pig and landrace [J]. Pakistan J. Zool., 50: 7-13. https://doi.org/10.17582/journal.pjz/2018.50.1.7.13
Nicaise, P., Gleizes, A. and Forestier, F., 1993. Influence of intestinal bacterial flora on cytokine (IL-1β-IL-6 and IFN-γ) production by mouse peritoneal macrophages. Eur. Cytok. Netw., 4: 133-138.
Ozturk, E., Coskun, I. and Ocak, N., 2014. Performance, meat quality, meat mineral contents and caecal microbial population responses to humic substances administered in drinking water in broilers. Br. Poult. Sci., 55: 668-674. https://doi.org/10.1080/00071668.2014.960807
Scott, D.T., Mcknight, D.M., Blunt-Harris, E.L., Kolesar, S.E. and Lovley, D.R., 1998. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol., 32: 372-373. https://doi.org/10.1021/es980272q
Stefano, S., 2017. New insights into the interaction mechanism of humic acids with phillipsite. Reac. Kinet. Mech. Cat., 120: 735-752. https://doi.org/10.1007/s11144-017-1158-2
Szabó, J., Vucskits, A.V., Berta, E., Andrásofszky, E. and Bersényi, A., 2017. Effect of fulvic and humic acids on iron and manganese homeostasis in rats. Acta Vet. Hung., 65: 66-80. https://doi.org/10.1556/004.2017.007
Sekine, K., Toida, T. and Pnishi, M., 1995. Analysis of antitumor propertiers of effector cells stimulated with a cell wall preparation of bifidobacterium infantis. Biol. Pharm. Bull., 18: 148-153. https://doi.org/10.1248/bpb.18.148
Visser, S.A., 1973. Some biological effects of humic acids in the rat. Acta biol. med. Ger., 31: 569-581.
Vucskits, A.V., Hullár, I., Bersényi, A., Andrásofszky, E. and Kulcsár, M., 2010. Effect of fulvic and humic acids on performance, immune response and thyroid function in rats. J. Anim. Physiol. Anim. Nutr., 94: 721-728. https://doi.org/10.1111/j.1439-0396.2010.01023.x
Wang, Q., Chen, Y.J. and Yoo, J.S., 2008. Effects of supplemental humic substances on growth performance, blood characteristics and meat quality in finishing pigs. Livest. Sci., 117: 270-274. https://doi.org/10.1016/j.livsci.2007.12.024
Wei, L., Rui, L.I. and Pan, J., 2017. Biological function of humic acid substances and its application in livestock production. China Feed, 12: 23-28.
Xi, X., Sun, S. and Han, L., 2010. Study on the effect of biochemical fulvic acid on somatic cell count and milk performance of dairy cows. China Dairy Cattle, 36: 32-34.
Xu, H., McClain, S., Medina, S., Lauer, F.T. and Burchiel, S.W., 2016. Differential sensitivities of bone marrow, spleen and thymus to genotoxicity induced by environmentally relevant concentrations of arsenite. Toxicol. Lett., 262: 55-61. https://doi.org/10.1016/j.toxlet.2016.09.008
Ye, Q. and Yu, X.S., 2013. Detection method and case analysis of quality index of biological humic acid. Mod. Chem. Indust., 11: 127-129.
To share on other social networks, click on any share button. What are these?










