Effects of Fermented Cottonseed Meal and Enzymatic Hydrolyzed Cottonseed Meal on Amino Acid Digestibility and Metabolic Energy in White Leghorn Rooster
Effects of Fermented Cottonseed Meal and Enzymatic Hydrolyzed Cottonseed Meal on Amino Acid Digestibility and Metabolic Energy in White Leghorn Rooster
Xiaopeng Tang1,3, Rong Xiang1, Sijia Chen1,3, Shufen Yang1,3, Hu Liu1,3, Rejun Fang1,3,* and Aike Li2,*
1College of Animal Science and Technology, Hunan Agricultural University, Changsha, China
2Academy of State Administration of Grain, Beijing, China
3Hunan Co-Innovation Center of Animal Production Safety, Changsha, China
ABSTRACT
The aim of this study was to study the effects of fermentation and enzymatic hydrolysis treatment of cottonseed meal on crude protein (CP), water-soluble protein (WSP), amino acid (AA) and peptide fractions, and the AA digestibility and metabolic energy of fermented cottonseed meal (FCSM) and enzymatic hydrolyzed cotton seed meal (EHCSM) in white leghorn roosters. Firstly, CSM were fermented with Aspergillus niger, or hydrolyzed with alcalase and flavourzyme. Secondly, a total of 32 white leghorn roosters with similar body weight (1.95± 0.14 kg) were randomly assigned into 1 of 4 treatments, 1) starvation group, 2) untreated CSM group, 3) FCSM group, and 4) EHCSM group to determinate the Apparent metabolic energy (AME), True metabolic energy (TME) and AA digestibility. Results showed that: (1) CP content in FCSM and EHCSM increased 8.42% (P < 0.05) and 1.11% (P > 0.05), WSP content increased about 5.64 -fold (P < 0.05) and 6.39 -fold (P < 0.05), total AA content increased 8.95% and 8.95%, respectively; the peptide fractions (≤ 600 Da, 600-1800 Da and ≥1800 Da) in FCSM and EHCSM increased significantly (P < 0.05). (2) FCSM, and EHCSM has no effects on AA digestibility and AME (P > 0.05), but the TME for FCSM was significant higher than untreated CSM and EHCSM (P < 0.05). Our results suggest that the solid-state fermentation and enzymatic hydrolysis method offer effective approach to improving the quality of unconventional protein sources, such as the CSM.
Article Information
Received 19 October 2016
Revised 01 March 2017
Accepted 31 March 2017
Available online 18 April 2018
Authors’ Contribution
XT and RX designed and performed the experiments. XT wrote the paper. SC, SY and HL took part in lad analysis. AL and RF provided technical and financial support, and revised the paper.
Key words
AA digestibility, Cottonseed meal, Enzymatic hydrolysis, Fermentation, Metabolism energy.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.3.957.962
* Corresponding authors: fangrj63@126.com;LAK@chinagrain.org
0030-9923/2018/0003-0957 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introduction
Cottonseed meal (CSM), a byproduct of the process of extracting the oil from cotton seeds, is the third most widely traded protein ingredient after soybean meal (SBM) and rapeseed meal (Li et al., 2012), which considered as an attractively alternative protein source in China (Sun et al., 2012). However, the use of CSM in poultry diets is limited due to the presence of gossypol and a relative low lysine level compared to SBM (Tang et al., 2012; He et al., 2015). Free gossypol (FG) is a main anti-nutritional factor of CSM, which can bind with amino acids, mainly lysine, and therefore, reduces the availability of lysine in CSM further (Mahmood et al., 2011).
Solid-state fermentation has been reported as an effective way to reduce free gossypol (FG), and to improve AA and small-size peptides content of CSM (Zhang et al., 2007; Tang et al., 2012; Sun et al., 2012; Nie et al., 2015a). Aspergillus niger is one of the most common used strains in fermentation process due to its strong enzymes secreting ability (Adav et al., 2010). Solid-state fermentation with A. niger could improve the nutritional quality of by-products through biodegrading anti-nutritional factor, cellulose and high molecular weight protein (Yang et al., 2012; Tang et al., 2012; de Castro et al., 2014). But studies on protein biodegradation of CSM by A. niger are lacking. Enzymatic hydrolysis is another important way to breakage of high molecular weight protein into low-molecular-weight protein, small peptide and AAs (Moure et al., 2006; Chabnon et al., 2007; Sun et al., 2012).
Although, enhancing CSM nutritional quality by solid-state fermentation or protease hydrolysis methods have been reported (Tang et al., 2012; Sun et al., 2012), and the fermented CSM (FCSM) and enzymatic hydrolyzed CSM (EHCSM) have been used in poultry diets (Sun et al., 2013; Nie et al., 2015a, b), but the amino acid digestibility and metabolic energy of fermented CSM and hydrolyzed CSM in broilers are lacking. So, the aim of this study was to investigate the effects of Aspergillus niger fermentation or protease hydrolysis of cottonseed meal on CP, water-soluble protein (WSP), amino acid (AA) and peptide fractions, and the amino acid digestibility and metabolic energy of fermented CSM and hydrolyzed CSM in white leghorn roosters.
Materials and Methods
Microorganism and enzyme
Aspergillus niger was purchased from Agricultural Culture Collection of China (ACCC 30786) and maintained on potato dextrose agar (PDA) slants at 4°C and transferred every three month. Before used in solid-state fermentation, a loop of A. niger on the PDA slant was transferred into a 250 mL flask containing 50 mL potato-dextrose and incubated at 28°C, 150 r/min for 24 h.
Alcalase (0.8AU-NH/g) and Flavourzyme (1000 LAPU/g) were purchased from Novozymes (China) Investment Co., Ltd. (Beijing, China).
Solid-state fermentation of CSM
The CSM was purchased from Xi’an, China. CSM (50 g) was transferred into a 500 mL Erlenmeyer flask, inoculated with 10% (v/w) of A. niger. The samples in flasks were incubated at 28°C for 48 h. After fermentation, fresh samples were dried at 65°C for 48 h, and then milled fitted with 40 -mesh screen for chemical analysis. Triplicate flasks were used for each experimental variation.
Enzymatic hydrolysis of CSM with alcalase and flavourzyme
One hundred gram CSM and 500 mL water were transferred to a 1000 mL Erlenmeyer flask, pH was adjusted to 8, and then 3% alcalase was added to CSM. The mixture was hydrolyzed at 60°C for 6 h. After inactivation, pH was adjusted to 7 and the mixture was hydrolyzed at 60°C for 6 h in the presence of 4% flavourzyme. After enzymatic hydrolysis, the samples were freeze-dried for chemical analysis. Triplicate flasks were used for each experimental variation.
Chemical analysis
Dry matter (DM), CP content of CSM, FCSM and EHCSM were determined according to AOCS (2009) method. To measure the WSP content, the samples were pretreated according to the high performance liquid chromatography (HPLC) sample preparation method, and then CP was measured according to AOCS (2009) method. AAs profile of CSM, FCSM and EHCSM were analyzed using an automatic AA analyzer (L-8800; Hitachi, Tokyo, Japan) according to the instructions of the manufacturer. Peptide fractions of CSM, FCSM and EHCSM were determined by HPLC method according to the instructions of the manufacturer.
True AA digestibility and metabolic energy determination
The experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Hunan Agricultural University (Changsha, Hunan Province, China). Emptying-force-feeding method was used to determine the true AA digestibility and metabolic energy (Sibbald, 1976). A total of thirty-two white leghorn roosters with similar weight (1.95± 0.14 kg) were divided into 4 treatment groups, 1) starvation group, 2) untreated CSM group, 3) FCSM group, and 4) EHCSM group. Each treatment had 4 replicates, and each replicate had 2 roosters. Starvation group was used to estimate metabolic and endogenous excretion (da Silva et al., 2012).
The roosters were housed in individual metabolic cages and feed commercial pelleted diets during the pre-experimental period. Before force-feeding, the feathers around cloacae were cut off, and a 4-cm bottle cap was sewed on the cloacae. After 1 week of recovery phase, birds can conduct force-feed trial. Before force-feed feedstuffs, birds were subjected to a period of fast for 48 h, to empty the digestive tract. After fasting, birds were fed 50 g CSM, FCSM and EHCSM, respectively. The birds in starvation group were kept under the same experimental conditions at fasting receiving only water for determination of the metabolic and endogenous losses (da Silva et al., 2012). Once fed, the collecting bottle was fixed on bottle cap immediately. The excreta were collected for 48 h. Water was available ad libitum during the experimental period.
The excreta samples were dried at 65°C for 48 h, and then milled fitted with 40 -mesh screen for DM, AA and energy analysis. DM was determined according to the AOCS (2009) method. AA was analyzed using an automatic AA analyzer (L-8800; Hitachi, Tokyo, Japan). Energy was measured according to ISO-9831 (1998).
Calculations
The data were used to calculate AME, TME and True amino acid digestibility (TAAD) values according to the following formulae:
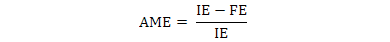
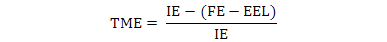
Where, IE is ingested energy; FE is fecal energy voided by the fed birds; EEL is endogenous energy loss determined by starvation group.

Where, EAAL is endogenous AA loss determined by starvation group.
Statistical analysis
Results were expressed as mean ± SE (except AA). Results were analyzed by One-way ANOVA using the SPSS 21.0 programs. Differences among treatment mean were determined using Duncan’s multiple comparison test, P < 0.05 was considered significant.
Results
CP and WSP content in CSM, FCSM and EHCSM
Analyzed nutrient contents in the CSM, FCSM and EHCSM are presented in Table I. Compared to CSM, the CP and WSP content in FCSM and EHCSM increased all. The CP content in FCSM increased 8.42% (P < 0.05), and WSP content increased about 5.64-fold (P < 0.05). Enzymatic hydrolysis has no effect on CP content improvement (P > 0.05), but the WSP content increased 6.39-fold compared to CSM (P < 0.05), and increased 9.12% compared to FCSM (P < 0.05).
Table I.- CP and WSP contents in CSM, FCSM and EHCSM (dry matter basis /%).
| Item |
Treatments |
||
|
CSM |
FCSM |
EHCSM |
|
| CP |
54.01±0.13a |
58.56±0.30b |
54.61±0.03a |
| WSP |
4.68±0.48a |
31.07±0.40b |
34.59±0.16c |
Values are presented as mean ± SE; n, 3; a,b,c, Means within rows with different letters differ significantly (P < 0.05). CSM, cottonseed meal; CP, crude protein; EHCSM, enzymatic hydrolyzed cottonseed meal; FCSM, fermented cottonseed meal; WSP, water-soluble protein.
Table II.- Content of peptide fractions in CSM, FCSM and EHCSM (dry matter basis /%).
| Item | < 600 Da | 600-1800 Da | >1800 Da |
| CSM |
4.56±0.23a |
0.52±0.01a |
1.03±0.12a |
| FCSM |
26.94±0.92b |
3.33±0.02b |
1.65±0.07b |
| EHCSM |
24.96±0.43b |
6.58±0.10c |
3.12±0.01c |
For abbreviations and statistical details, see Table I.
Content of peptide fractions in CSM, FCSM and EHCSM
The peptide fractions of CSM, FCSM and EHCSM are presented in Table II. Both fermentation and enzymatic hydrolysis treatment on CSM can increase the under 600 Da peptide (< 600 Da), 600-1800 Da peptide and above1800 Da peptide (> 1800 Da) content (P < 0.05). Compared to untreated CSM, the < 600 Da peptide content in FCSM increased 4.91-fold (P < 0.05), and the < 600 Da peptide content in EHCSM increased 4.47-fold (P < 0.05). Compared to untreated CSM, the 600-1800 Da peptide content in EHCSM increased 97.60% (P < 0.05), and > 1800 Da peptide content increased 89.09% (P < 0.05).
AA content in CSM, FCSM and EHCSM
The AA content in CSM, FCSM and EHCSM are presented in Table III. Compared to untreated CSM, the total AA content in FCSM and EHCSM increased 8.95% and 7.24%, respectively. Especially, the Lys content in FCSM and EHCSM increased 6.83% and 10.44%, respectively. The Tyr content in FCSM was increased 32.02%, and the Val content in EHCSM increased 32.64%.
Table III.- Content of amino acids in CSM, FCSM and EHCSM (dry matter basis /%).
| Amino acids |
Treatments |
||
|
CSM |
FCSM |
EHCSM |
|
| Asp |
5.63 |
5.88 |
6.03 |
| Thr |
1.73 |
1.93 |
1.90 |
| Ser |
2.58 |
3.25 |
2.71 |
| Glu |
10.47 |
10.77 |
10.33 |
| Gly |
2.02 |
2.25 |
2.44 |
| Ala |
2.07 |
2.20 |
2.27 |
| Cys |
1.48 |
1.78 |
1.64 |
| Val |
2.39 |
2.74 |
3.17 |
| Met |
0.77 |
0.91 |
0.76 |
| Ile |
1.46 |
1.67 |
1.57 |
| Leu |
3.32 |
3.51 |
3.45 |
| Tyr |
2.53 |
3.34 |
2.86 |
| Phe |
3.21 |
3.79 |
3.23 |
| Lys |
2.49 |
2.66 |
2.75 |
| His |
1.52 |
1.58 |
1.72 |
| Arg |
6.67 |
6.95 |
6.88 |
| Pro |
1.75 |
1.63 |
1.87 |
| Trp |
0.66 |
0.52 |
0.88 |
| Total |
52.65 |
57.36 |
56.46 |
For abbreviations and statistical details, see Table I.
Amino acid digestibility of CSM, FCSM and EHCSM
The results of true AA digestibility are shown in Table IV. True AA digestibility of CSM ranged from 0.46 for Met to 0.94 for Glu and Arg, true AA digestibility of FCSM ranged from 0.54 for Met to 0.95 for Arg, true AA digestibility of EHCSM ranged from 0.56 for Met to 0.95 for Met and Arg. In total, the FCSM and EHCSM had a higher true AA digestibility than CSM, but the difference was not significant (P > 0.05).
Table IV.- The true amino acid digestibility of CSM, FCSM and EHCAM in white leghorn roosters.
| Amino acids |
Treatments |
||
|
CSM |
FCSM |
EHCSM |
|
| Asp |
0.91±0.02 |
0.92±0.03 |
0.93±0.02 |
| Thr |
0.83±0.03 |
0.83±0.05 |
0.87±0.02 |
| Ser |
0.87±0.05 |
0.89±0.02 |
0.91±0.05 |
| Glu |
0.94±0.04 |
0.93±0.03 |
0.95±0.06 |
| Gly |
0.67±0.03 |
0.65±0.02 |
0.75±0.02 |
| Ala |
0.84±0.03 |
0.86±0.05 |
0.87±0.10 |
| Cys |
0.91±0.03 |
0.92±0.05 |
0.93±0.07 |
| Val |
0.84±0.02 |
0.85±0.03 |
0.90±0.08 |
| Met |
0.46±0.03 |
0.54±0.05 |
0.56±0.06 |
| Ile |
0.84±0.05 |
0.84±0.03 |
0.88±0.08 |
| Leu |
0.89±0.04 |
0.91±0.05 |
0.91±0.02 |
| Tyr |
0.84±0.03 |
0.91±0.02 |
0.90±0.05 |
| Phe |
0.91±0.07 |
0.92±0.05 |
0.90±0.06 |
| Lys |
0.85±0.05 |
0.88±0.03 |
0.94±0.04 |
| His |
0.84±0.03 |
0.85±0.05 |
0.80±0.06 |
| Arg |
0.94±0.03 |
0.95±0.04 |
0.95±0.05 |
| Pro |
0.88±0.05 |
0.85±0.08 |
0.87±0.02 |
| Try |
0.79±0.07 |
0.63±0.06 |
0.84±0.04 |
For abbreviations and statistical details, see Table I.
Table V.- Metabolic energy for CSM, FCSM and EHCSM in white leghorn roosters.
| Item |
Treatment |
||
|
CSM |
FCSM |
EHCSM |
|
| AME (Mcal/kg) |
2.08±0.26 |
2.17±0.26 |
2.05±0.16 |
|
TME (Mcal/kg) |
2.34±0.24a |
2.43±0.19b |
2.31±0.21a |
n, 4; For abbreviations and statistical details, see Table I.
AME and TME value for CSM, FCSM and EHCSM
Data on AME and TME values for CSM, FCSM and EHCSM are shown in Table V. The AME value for CSM, FCSM and EHCSM was 2.08 Mcal/kg, 2.17 Mcal/kg and 2.05 Mcal/kg, respectively. No significant difference was found among CSM, FCSM and EHCSM. The TME value for CSM, FCSM and EHCSM was 2.34 Mcal/kg, 2.43 Mcal/kg and 2.31 Mcal/kg, respectively. The TME value for FCSM was significantly higher than CSM and EHCSM (P < 0.05). There was no significant difference between CSM and EHCSM.
Discussion
In the present study, the content of CP, WSP, AA, and peptide fractions increased after fermentation by A. niger, which was similar to previous reports (Tang et al., 2012; Sun et al., 2012; Nie et al., 2015a). This may be partly due to the use of carbon sources in CSM during the fermentation process, leading to the concentration of other nutrients (Khalaf and Meleigy 2008; Sun et al., 2012). What important, A. niger has a strong enzymes secreting ability during fermentation (Dinu et al., 2007; Adav et al., 2010; Shi et al., 2015). The secreted protease can degrade the high molecular weight protein into small peptide and free AA, thus resulted an increased WSP, AA and peptide fractions. What else, the growth and propagation of A. niger can produce a large number of bacteria protein also contribute to the elevated level of WSP and AA.
Enzymatic hydrolysis was another important way to break high molecular weight protein into low molecular weight protein, peptide fractions and amino acids. In the present study, the CP contents increased a little, WSP increased 6.39-fold, total AA increased 7.24%, < 600 Da peptide increased 4.47-fold after hydrolyzed with alcalase and flavourzyme. The increase of CP is mainly due to the additional added of enzymes (alcalase and flvourzyme), because the enzyme itself was a kind of protein, thus resulted an increase of total protein. Alcalase and flavourzyme can degrade the high molecular weight protein into small peptide and free AA, thus resulted an increased WSP, AA and peptide fractions (Ordonez et al., 2008; Berends et al., 2014).
In the the present study, the FCSM and EHCSM had a higher true AA digestibility than CSM. The following reasons may explain why FCSM and EHCSM had a higher AA digestibility. First, fermentation and enzymatic hydrolysis treatment on CSM can degrade the high molecular weight protein into small peptide and free AA, the utilizable CP was increased (Sun et al., 2012). Second, some AA and small peptides would adsorb in cellulose, that make this part AA which can not fully contact with the digestive enzymes, so, reducing the AA digestibility. While, during fermentation process, Aspergillus niger can secret celluase to degrade cellulose (Tang et al., 2012; Shi et al., 2015), and adsorbed AA would release from cellulose and fully contact with the digestive enzymes, which resulted an increased AA digestibility. Third, FG is the main anti-nutritional factor of CSM, which can bind with amino acids, thereby reduce the availability of AA (Arinbasarova et al., 2012; He et al., 2015), while fermentation treatment can degrade FG in CSM (Tang et al., 2012; Sun et al., 2013), and enzymatic hydrolysis treatment can produce antioxidant activity peptide to reduce the toxicity of FG (Gao et al., 2010), thus increasing the amino acid digestibility.
Previous studies have reported that the ME ranged from 1860 kcal/kg to 2963 kcal/kg for CSM (Panigrahi et al., 1989; NRC, 1994, 2012; Reddy et al., 1998; Salas et al., 2013). In the present study, the AME value for CSM, FCSM, EHCSM was 2080 kcal/kg, 2170 kcal/kg, 2050 kcal/kg, respectively, the TME value for CSM, FCSM, EHCSM was 2340 kcal/kg, 2430 kcal/kg, 2310 kcal/kg, respectively, which was within the range of previously reported values (Panigrahi et al.,1989; NRC, 1994; Reddy et al., 1998; NRC, 2012; Salas et al., 2013). No significant difference of AME was found among CSM, FCSM and EHCSM, but the TME value for FCSM was significant higher than CSM and EHCSM. Because, during the fermentation process, A. niger can secret cellulase and amylase to degrade cellulose and starch (Dinu et al., 2007; Adav et al., 2010; Dojnov et al., 2015), thus resulting in increased available energy.
Conclusion
The fermentation of CSM by Aspergillus niger effectively increased CP, WSP, AA and peptide fractions. Enzymatic hydrolysis treatment on CSM can increase WSP, AA and peptide fractions. Both fermentation and enzymatic hydrolysis treatment on CSM can improve AA digestibility. TEM for CSM was significantly improved after fermented by Aspergillus niger. Our results suggest that the solid-state fermentation and enzymatic hydrolysis method offer effective approach to improving the quality of unconventional protein sources, such as the CSM.
ACKNOWLEDGMENTS
This work was funded by the National Twelfth Five-Year Science and Technology Plan Program of Rural Areas (2011BAD26B01-3), and the National Special Basic Project of Science and Technology (2014FY111000-3-4).
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Adav, S.S., Li, A.A., Manavalan, A., Punt, P. and Sze, S.K., 2010. Quantitative iTRAQ secretome analysis of Aspergillus niger reveals novel hydrolytic enzymes. J. Proteome Res., 9: 3932–3940. https://doi.org/10.1021/pr100148j
Arinbasarova, A.Y., Medentsev, A.G. and Krupyanko, V.I., 2012. Gossypol inhibits electron transport and stimulates ROS generation in yarrowia lipolytica mitochondria. Open Biochem. J., 6: 11-15. https://doi.org/10.2174/1874091X01206010011
Chabanon, G., Chevalot, I., Framboisier, X., Chenu, S. and Marc, I., 2007. Hydrolysis of rapeseed protein isolates: Kinetics, characterization and functional properties of hydrolysates. Process Biochem., 42: 1419–1428. https://doi.org/10.1016/j.procbio.2007.07.009
da Silva, E.A., Albino, L.F.T., Rostagno, H.S., Vieira, R.A., Junior, V.R. and Pereira, J.P.L., 2012. Determination of true digestible amino acids of feedstuffs utilizing cecectomized roosters. Rev. Bras. Zootec., 41: 2070-2078. https://doi.org/10.1590/S1516-35982012000900015
de Castro, R.J.S., Nishide, T.G. and Sato, H.H., 2014. Production and biochemical properties of proteases secreted by Aspergillus niger under solid state fermentation in response to different agroindustrial substrates. Biocatal. agric. Biotechnol., 3: 236-245. https://doi.org/10.1016/j.bcab.2014.06.001
Dinu, D., Nechifor, M.T., Stoian, G., Costache, M. and Dinischiotu, A., 2007. Enzymes with new biochemical properties in the pectinolytic complex produced by Aspergillus niger MIUG 16. J. Biotechnol., 131: 128-137. https://doi.org/10.1016/j.jbiotec.2007.06.005
Dojnov, B., Grujic, M. and Vujcic, Z., 2015. Highly efficient production of Aspergillus niger amylase cocktail by solid state fermentation using triticale grains as a well-balanced substrate. J. Serb. chem. Soc., 80: 1375-1390. https://doi.org/10.2298/jsc150317041d
Gao, D., Cao, Y. and Li, H., 2010. Antioxidant activity of peptide fractions derived from cottonseed protein hydrolysate. J. Sci. Fd. Agric., 90: 1855-1860. https://doi.org/10.1002/jsfa.4024
He, T., Zhang, H.J., Wang, J., Wu, S.G., Yue, H.Y. and Qi, G.H., 2015. Application of low-gossypol cottonseed meal in laying hens’ diet. Poult. Sci., 94: 2456–2463. https://doi.org/10.3382/ps/pev247
Khalaf, M.A. and Meleigy, S.A., 2008. Reduction of free gossypol levels in cottonseed meal by microbial treatment. Int. J. Agric. Biol., 10: 185–190.
Li, J.T., Li, D.F., Zang, J.J., Yang, W.J., Zhang, W.J. and Zhang, L.Y., 2012. Evaluation of energy digestibility and prediction of digestible and metabolizable energy from chemical composition of different cottonseed meal sources fed to growing pigs. Asian Austral. J. Anim. Sci., 25: 1430-1438. https://doi.org/10.5713/ajas.2012.12201
Mahmood, F., Khan, M.Z., Khan, A., Muhammad, G. and Javed, I., 2011. Lysine induced modulation of toxico-pathological effects of cottonseed meal in broiler breeder males. Pakistan J. Zool., 43: 357–365.
Moure, A., Sineiro, J., Dominguez, H. and Parajo, J.C., 2006. Functionality of oilseed protein products: A review. Fd. Res. Int., 39: 945–963. https://doi.org/10.1016/j.foodres.2006.07.002
Nie, C., Zhang, W., Wang, Y., Liu, Y., Ge, W. and Liu, J., 2015a. Tissue lipid metabolism and hepatic metabolomic profiling in response to supplementation of fermented cottonseed meal in the diets of broiler chickens. J. Zhejiang Univ. Sci. B., 16: 447–455. https://doi.org/10.1631/jzus.B1400255
Nie, C.X., Zhang, W.J., Ge, W.X., Liu, Y., Wang, Y.Q. and Liu, J.C., 2015b. Effect of cottonseed meal fermented with yeast on the lipid-related gene expression in broiler chickens. Braz. J. Poult. Sci., 17: 57-64. https://doi.org/10.1590/1516-635xspecialissuenutrition-poultryfeedingadditives057-064
NRC, 1994. Nutritional requirements of poultry, 9th edn. National Academy Press, Washington, DC.
NRC, 2012. Nutrient requirements of swine, 11th edn. National Academies Press, Washington, DC.
Ordonez, C., Benitez, C. and Gonzalez, J.L., 2008. Amino acid production from a sunflower whole meal protein concentrate. Bioresour. Technol., 99: 4749–4754. https://doi.org/10.1016/j.biortech.2007.09.061
Panigrahi, S., Plumb, V.E. and Machin, D.H., 1989. Effects of dietary cottonseed meal with and without iron treatment on laying hens. Br. Poult. Sci., 30: 641-651. https://doi.org/10.1080/00071668908417187
Reddy, G.P.K., Narasimha, G., Kumar, K.D., Ramanjaneyulu, G., Ramya, A., Kumari, B.S.S. and Reddy, B.R., 2015. Cellulase production by Aspergillus niger on different natural lignocellulosic substrates. Int. J. Curr. Microbiol. appl. Sci., 4: 835-845.
Reddy, P., Reddy, P.S., Reddy, P.V.V. and Rao, D.S., 1998. Influence of cotton seed cake on the performance of broilers. Indian J. Anim. Nutr., 15: 188-193.
Salas, C., Ekmay, R., England, J., Cerrate, S. and Coon, C.N., 2013. The TMEn, proximate analysis, amino acid content and amino acid digestibility of glandless and commercial cottonseed meal for broilers. Int. J. Poult. Sci., 12: 212-216. https://doi.org/10.3923/ijps.2013.212.216
Shi, C., He, J., Yu, J., Yu, B., Huang, Z., Mao, X., Zheng, P. and Chen, D., 2015. Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J. Anim. Sci. Biotechnol., 6: 340-346. https://doi.org/10.1186/s40104-015-0015-2
Sibbald, I.R., 1976. A bioassay for true metabolizable energy in feeding stuffs. Poult. Sci., 55: 303-308. https://doi.org/10.3382/ps.0550303
Sun, H., Tang, J.M., Yao, X.H., Wu, Y.F., Wang, X. and Feng, J., 2012. Improvement of the nutritional quality of cottonseed meal by Bacillus subtilis and the addition of papain. Int. J. Agric. Biol., 14: 563–568.
Sun, H., Tang, J.W., Yao, X.H., Wu, Y.F., Wang, X. and Feng, J., 2013. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop. Anim. Hlth. Prod., 45: 987-993. https://doi.org/10.1007/s11250-012-0322-y
Tang, J.W., Sun, H., Yao, X.H., Wu, Y.F., Wang, X. and Feng, J., 2012. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian Austral. J. Anim. Sci., 25: 393–400. https://doi.org/10.5713/ajas.2011.11381
Yang, X., Sun, J.Y., Guo, J.L. and Weng, X.Y., 2012. Identification and proteomic analysis of a novel gossypol-degrading fungal strain. J. Sci. Fd. Agric., 92: 943-951. https://doi.org/10.1002/jsfa.4675
Zhang, W.J., Xu, Z.R., Zhao, S.H., Sun, J.Y. and Yang X., 2007. Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Anim. Feed Sci. Technol., 135: 176–186. https://doi.org/10.1016/j.anifeedsci.2006.06.003
To share on other social networks, click on any share button. What are these?










