Efficacy of Herbicides for Control of Duckweed (Lemna minor L.)
Research Article
Efficacy of Herbicides for Control of Duckweed (Lemna minor L.)
Bakhtiar Gul*, Saleetha Rabial and Haroon Khan
Department of Weed Science and Botany, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | An experiment was conducted in summer, 2016 at the Department of Weed Science, The University of Agriculture Peshawar to investigate the effect of herbicides on the management, growth and reproduction of duckweed (Lemna minor). The experiment was laid out in Completely Randomized Design (CRD) with three replecations and various herbicidal treatments. Duckweed was collected from fresh water bodies and transferred to the experimental units (pots). Data were recordrd on fresh biomass, budding (%), duckweed mortality (%), plant growth, root length, frond diameter, growth of microscopic algae and macrophytes associated with duckweed, and water surface coverage (%). Results revealed that herbicides had a significant effect on duckweed fresh biomass, budding % and water surface coverage. Minimum fresh biomass (2.98 g pot-1), budding (24 %) and water surface coverage (14.67 %) were recorded for glyphosate as compared to weedy check (4.45 g pot-1, 58.7 % and 22.67 %, respectively). Moreover, highest plant mortality (24.33 %) after four weeks was noted in glyphosate. Glyphosate effectively controlled the duckweed, followed by MCPA as compared to bromaxonil, isoproturon and paraquat. Therefore, glyphosate and MCPA proved suitable, economical and less laborious for the management of duckweed for large scale infestation.
Received | November 12, 2021; Accepted | June 18, 2021; Published | August 24, 2021
*Correspondence | Bakhtiar Gul, Department of Weed Science and Botany, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Gul, B., S. Rabial and H. Khan. 2021. Efficacy of herbicides for control of duckweed (Lemna minor L.). Sarhad Journal of Agriculture, 37(4): 1194-1200.
DOI | https://dx.doi.org/10.17582/journal.sja/2021/37.4.1194.1200
Keywords | Duckweed, Herbicides, Weed management, Lemna minor, Weed control
Introduction
Pakistan is rich in water resources from most of the countries and has one of the best canal irrigation systems in the world. Water is one of most important natural resource and in fact the basis of all life forms on this planet (Bhetalu et al., 2012). The rivers, streams, lakes and artificial dams further add to its aesthetic value. However, aquatic weeds infestations mars its scenic beauty and intended use of these water bodies. Presently the un-infested of the water bodies are prone to weed infestation due to lack of proper check and management. The irrigated plains of the Khyber Pakhtunkhwa are even more predisposed due to the less topography and low slope and where the slower water flow makes these areas more favourable for aquatic weeds infestation.
In many tropical and subtropical countries it is known as one of the important pan-tropical aquatic weeds (Labrada and Fornsari, 2002) and can cause serious problems. The worst invasive weeds in the world are aquatic plants and duckweed is one of them (Chambers et al., 2007). In Pakistan, duck weed is widespread; found everywhere in almost all aquatic bodies with still or slow moving waters. While in Khyber Pakhtunkhwa province, this weed is a major aquatic weed of rice fields, stagnant water ponds and temporary rain water ponds. It is a noxious weed and so far there is no use of this plant (Khan et al., 2014). It forms a dense and uniform mat covering the surface of the water. They belong to Lemnaceae a a monocot family having 28 species (Bonomo et al., 1997). The leaves and stems are not distinguishable and are fused to form the so called “fronds” (Bejarano, 2005). In suitable growing conditions their population, total fronds number and biomass doubles every 2 to 4 days (Xu et al., 2011; Harvey and Fox, 1973) and are included among those plants which have the most vigorous growth on the earth (Xu, et al., 2011).
Duckweed can be managed through mechanical, physical and cultural methods but chemical weed management is more effective for 100 % control. The use of chemicals might have some drawbacks, but could be minimized by sagacious use of herbicides. The time and method of herbicide application varies with the time, water body type and intended use of water body in which the weeds are to be controlled (Lancar and Krake, 2002). However once the infestation occurs then herbicide should be used which best suits the situation alone or in combination or in combination with other methods as integrated weed management approach (Mortensen et al., 2000). The present experiments were conducted to investigate the effect of various herbicides on the growth and reproduction of Lemna spp. and to find out a suitable herbicide for controlling the duckweed.
Materials and Methods
Pots experiment was conducted during April to May 2016, in the Department of Weed Science, The University of Agriculture Peshawar to evaluate the effect of various herbicides on the management, growth and reproduction of duck weed. Duckweeds were collected from various stagnant and fresh water bodies in the vicinity of the University. Various herbicides were used to evaluate their efficacy against duckweed. Each treatment consisted of a pot having a diameter of 5 inches (12.7 cm) and 4 inches (10.16 cm) depth. Water were added to the pots and the pots were inoculated with equal number of duckweed plants (20 plants per pot) floating freely in the containers. The experiment was conducted in the well lit and ventilated laboratory conditions providing all the necessary conditions required for optimum growth of the duckweed. The experiment was arranged in Completely Randomized Design (CRD) with three repetitions and various herbicidal treatments. The details of the treatments are given below:
The following treatments were applied to the experimental units;
|
Name of treatments |
Rate (kg a.i ha-1) |
Require pot-1 in mg (experimental unit) |
|
Glyopsate |
2.00 |
2.532 |
|
Paraquat |
1.00 |
1.266 |
|
MCPA |
0.20 |
0.025 |
|
Bromoxynil |
0.20 |
0.025 |
|
Atrazine |
0.50 |
0.063 |
|
Isoproturon |
1.50 |
1.899 |
|
Weedy check |
------- |
--------- |
The data were recorded on the following parameters:
Fresh biomass before and after the treatment periodically after 4 wks
Fresh biomass of the plants was calculated before the application of the treatment as well as after four weeks after the application of the treatment with the help of a digital weighting scale.
Budding percentage of the plant and increase in the population in percent
As there were a specific number of plants in each treatment so the increase in number is due to plant budding. Budding percentage was calculated by using the following formula:
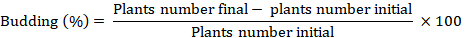
Plant mortality (%)
The mortality of plants can be calculated by subtracting the present number of plants from the original number of plants. The positive resultant figure indicates plant mortality due to herbicide or nutrient toxicity or stress. The resultant figure is divided by the original number of plant s and is multiplied by 100 to get % plant mortality. But if there is a negative figure then there no plant mortality and the plant population has increased from the original population due to budding and reproduction.
Growth rate (%)
Growth rate of L. minor was measured by weighing the initial and the final fresh biomass of the L. minor in each experimental unit (pot) at the end of experiment. By removing the water excess from the wet biomass by the water absorbent tissue paper, the percent growth rate was measured by using the following formula:
Growth Rate (%) = ((Present biomass-initial biomass) / initial biomass) x 100
Root length
The duckweed has a single very small root and is calculated in millimeter. Root length was calculated by using a transparent ruler with clear marking (mm) on it. Five representative plants were selected randomly and their root length was measured and average was calculated.
Frond diameter
The duckweed bogy consists of two leaf-like structure closely attached to each other and has a single root or root system on its lower surface. This structure is called a frond, which in no more than a few millimeters in its diameter. The frond diameter was calculated by using a transparent ruler with clear marking of millimeter on it. Five representative plants were selected randomly and the average frond diameter of 5 plants were calculated by adding their individual diameter and divided by the number of representative plants i.e. 5.
Growth of microscopic algae and other macrophytes in association with Lemna minor
Duckweed grows in natural environment where microscopic algae and higher aquatic grows naturally. These plants might be possible to grow with duckweed in the laboratory conditions. So, microscopic algae and other macrophytes were also investigated before as well as after the inoculation of L. minor plant in to the pots and after the treatment application, to investigate treatment effect on these plants.
Water surface coverage (%)
Being a free floating plant the duckweed floats on the surface of water and often covers the surface like a green carpet and the water of the entire lake or pond is not visible in many cases where the L. minor population is denser. The surface coverage of duckweed was calculated at the time of inoculation of the plants in to the experimental units i.e. pots as well as at various intervals (weeks after inoculation) during the span of experiment. The surface coverage was relative to the whole surface of the pot i.e. how much surface of the pot is covered as compared to the free or uncovered surface of the pot.
Analysis of the data
The data regarding various parameters were subjected to statistical analysis packages on computer like Statistix 8.1 and Gen Stat, 2005 and the means were separated by using LSD test at 0.05 level of probability, when the F-values were significant (Steel and Torrie, 1980).
Results and Discussion
Fresh biomass
Analysis of the data regarding various herbicidal treatments for the management of L. minor had a significant effect on the fresh biomass production of the weed at different timing interval (Table 1). Means data regarding duckweed fresh biomass (g) before treatment application had a non significant variation due to the size of individual plants as the number of plants inoculated in each pot as an experimental unit were the same. However the experimental units were statistically at par. The fresh biomass is significantly affected by the application of herbicides as measured one week after application and minimum fresh biomass were observed in the glyposate treatment (3.62 g) followed by paraquat (3.58 g) and MCPA (3.71 g), while maximum fresh biomass (4.13 g pot-1) were noted in the control treatment followed by isoproturon (3.89 g) and Bromoxynil (3.84 g) as shown in the Table 1. So, after 1st week of herbicide application paraquat and glyphosate were doing well against duckweed biomass reduction in pots as compare to other treatments. But at the end of the 2nd week glyposate continued to exert its herbicidal effect on the duckweed to affect fresh biomass production negatively (3.44 g) while paraquat treated units (3.54 g) showed some recovery by lowering the biomass less aggressively as compared to glyposate. However it was statistically similar to MCPA (3.56 g) and as usual the highest biomass was recorded in the weedy check treatment (4.31 g) and followed by isoproturon (3.81 g) and Bromoxynil (3.73 g) due to its less efficacy. In the 3rd week after herbicides application, the lowest fresh biomass (2.98 g pot-1) were recorded for glyphosate followed by the rest of herbicides treatments (all at par) as compared to the highest biomass noted in the weedy check (4.45 g pot-1). There was a significance difference among the herbicides treatments and the control. Our results are in conformity with that of Reddy and DeBusk (1984) who reported the duckweeds fresh biomass were increased in the absence of control measures (herbicides) as a result of enhanced growth rate and other relative factors. Similarly the herbicides concentration in the water has a negative impact on the duckweed (Lemna spp.) growth, reproduction and subsequently fresh biomass (Wang, 1990). All the herbicides exerted negative impact on the weed but only glyposate retained its impact for longer time or permanently damaged the plants, while the rest of the herbicide either had a short term effect on the plants or the plant recovered easily as their concentration faded away. This is evident from the fresh biomass in the 3rd week as shown in the Table 1.
Budding percentage of the plant and % increase in the population
Mean data recorded after herbicides application on duckweed have significantly affected the budding (%) and plant population of duckweed as presented in Table 2. Statistical assessment of the data regarding Lemna budding (%) after various herbicides application showed that minimum budding (%) was found in the glyposate (24 %). However it was statistically similar to atrazine (25 %), followed by paraquat (32.33), Bromoxynil (32.67), MCPA (33.00) and isoproturon (34.00 %) compared to the maximum budding (58.67 %) found for weedy check. Therefore, glyphosate and atrazine showed better efficacy in inhibition of duckweed budding. Our results are similar to (Michael, 2002) who reported that growth regulators or those with multiple site of action herbicides showed best performance against aquatic weeds control as compared to some other herbicides lacking these characteristics. The effective herbicides were able to disrupt the plant hormonal balance and protein synthesis, like the use of glyphosate which is an amino acid biosynthesis inhibitor that interfere with protein synthesis and subsequently inhibits budding, root and shoots growth.
Plant mortality (%)
The data regarding Lemna mortality (%) after treatments application is significantly affected as presented in Table 2. Statistical Analysis of the data regarding Lemna mortality (%) after treatments application revealed that least mortality (1.33 %) were recorded for control, followed by Isoproturon (14.00 %), while highest plant mortality (24.33 %) was observed for glyphosate. Our results are inline with the work of Peltier and Weber (1985) who investigated the translocated herbicide at LD50 level show more than 50 % mortality of duck weed as root elongation and seed inhibition. Castro et al., (2015) reported that glyphosate negatively affects Lemna and others aquatic weeds by changing chlorophyll contents that resulted in plant mortality.
Plant growth (%)
Statistical analysis of the means data regarding Lemna sp. growth as affected by various treatments is presented in the Table 2. Effect of treatments application on duckweed plant growth revealed that highest plant growth (18.33 %) were recorded for control, followed by (3.36 %) Bromoxynil. Meanwhile, lowest plant growth rate (2.78 %) was recorded for glyphosate. Our results are supported by Wang (1990) who observed the growth of duckweed has a negative relationship to chemical concentration, no significance, but chlorophyll a/b decrease when chemical rates are increased, and the chlorophyll decreasing also affected the growth of Lemna species.
Root length (mm)
Statistical analysis of mean data relating to Lemna species root length (mm) as affected after treatments application is non-significant. However, the means data regarding duckweed root length (mm) after treatments application showed, the maximum root length (10.67 mm) was noted for Isoproturon, followed by atrazine (9.00 mm) and weedy check (8.67 mm). Whereas, the minimum duckweed root length (7.33 mm) was recorded for glyphosate as shown in the Table 3. Therefore, isoproturon and control (tape water)
Table 1: Effects of herbicides before and after application on duckweed fresh biomass (g) at various interval.
|
Treatments |
Fresh biomass (g) |
Fresh biomass (g) after 1st week |
Fresh biomass (g) after 2nd week |
Fresh biomass (g) after 3rd week |
|
Glyphosate |
3.94 |
3.62 f* |
3.44 f |
2.98 c |
|
Paraquat |
3.93 |
3.58 g |
3.54 e |
3.53 b |
|
MCPA |
3.93 |
3.71 e |
3.56 e |
3.43 b |
|
Bromoxynil |
3.94 |
3.84 c |
3.73 c |
3.64 b |
|
Atrazine |
3.94 |
3.74 d |
3.64 d |
3.56 b |
|
Isoproturon |
3.94 |
3.89 b |
3.81 b |
3.73 b |
|
Weedy check |
3.94 |
4.13 a |
4.31 a |
4.45 a |
|
LSD (α =0.05) |
0.0206 |
0.0286 |
0.0452 |
0.3712 |
* Means followed by different letters are significantly different from each other.
is recorded as maximum root length due to less inhibition while other herbicides including glyphosate showed good response in root length inhibition and subsequently the overall growth and performance of the plant. Our research findings are in conformity with the findings of Sikorski et al. (2019) who reported the Glyphosate accumulation in plant tissues exerted toxic effects on duckweed by decreasing its growth and yield, inhibiting the synthesis of chlorophyll a and b and carotenoids, and decreasing the photochemical activity of photosystem II (PSII) that inhibited root growth of duckweed and greatly disrupted the metabolism and final biomass of the Lemna roots.
Table 2: Effects of herbicides application on duckweed Budding (%), plant mortality (%) and plant growth rate (%).
|
Treatments |
Budding (%) |
Plant mortality (%) |
Plant growth (%) |
|
Glyposate |
24.00 c* |
24.33 a |
2.78 b |
|
Paraquat |
32.33 b |
20.00 ab |
2.82 b |
|
MCPA |
33.00 b |
18.33 ab |
3.04 b |
|
Bromoxynil |
32.67 b |
21.00 a |
3.36 b |
|
Atrazine |
25.00 c |
20.00 ab |
2.92 b |
|
Isoproturon |
34.00 b |
14.00 b |
3.15 b |
|
Weedy check |
58.67 a |
1.33 c |
18.33 a |
|
LSD (α =0.05) |
7.3109 |
6.2443 |
1.5147 |
* Means followed by different letters are significantly different from each other.
Table 3: Effects of herbicides application on duckweed root length, frond diameter and growth of other associated microscopic algae and macrophytes.
|
Treatments |
Root length (mm) |
Frond diameter (mm) |
Other microscopic algae growth |
|
Glyphosate |
7.333 |
6.33 |
1.33 c* |
|
Paraquat |
7.667 |
5.66 |
2.33 b |
|
MCPA |
7.000 |
4.66 |
1.00 c |
|
Bromoxynil |
8.000 |
4.66 |
1.67 bc |
|
Atrazine |
9.000 |
4.66 |
1.67 bc |
|
Isoproturon |
10.67 |
6.33 |
1.67 bc |
|
Weedy check |
8.667 |
6.66 |
3.33 a |
|
LSD (α =0.05) |
NS |
NS |
0.9361 |
* Means followed by different letters are significantly different from each other; NS: non-significant.
Frond diameter (mm)
Statistical analysis of the means data pertaining duckweed frond diameter (mm) showed that the application various treatments affected frond diameter non-significantly as indicated in the Table 3. However, the highest duckweed frond diameter (6.66 mm) were recorded for weedy check followed by glyphosate and isoproturon (6.33 mm each) while, the least duckweed frond diameter was noted for MCPA, Bromoxynil and atrazine (4.66 mm each) and followed by paraquat (5.66 mm). Our observations are similar to (Wang, 1990; Jones et al., 1986) they reported the atrazine different concentration against the inhibition of duckweed and other aquatic weed frond diameter and fresh biomass.
Growth of microscopic algae and other macrophytes in association with L. minor L.
Statistical analysis means data regarding the growth of algae and other associated macrophytes is significantly affected by different treatments as presented in the Table 3. The data regarding the growth of other microscopic algae and macrophytes association with Lemna, revealed that the least number of other species (1.00) were noted for MCPA followed by glyphosate (1.33), then Bromoxynil, atrazine and isoproturon (1.67 each) and paraquat (2.33) as compared to weedy check(3.33) as shown in the Table 3. It is evident from the data that among the herbicides MCPA being the broadleaf killer controlled the algae and other macrophytes growing in association with the Lemna in the culture medium, followed by glyphosate in performance. While the rest of the herbicides remained ineffective comparatively. These results are in conformity with the results of Lytle and Lytle (2001) and Newman (2004) who investigated the application of certain herbicides against macrophytes and algae associated with duckweeds may disrupt their growth and reproduction differently and it is so good for long term effects while in case of repetition of the same chemical in the aquatic environments is more prone to development of resistance in terms of resistant weeds species.
Water surface coverage (%)
Statistical analysis of the means data pertaining to the duckweeds water surface coverage (%) after treatment application at various timings interval has shown significant effect as presented in Table 4. As similar number of plants have been put in various treatments and the effect of these treatment were not much pronounce at the first week. Therefore all the means regarding the surface coverage were at par during the first week. At during the 2nd week least surface coverage (17.33%) were noted for glyphosate followed by MCPA (17.67 %), paraquat and bromoxynil (18.67 each) then atrazine (19.00%) and isoproturon (19.33%) as compared to weedy check (20.67 %).
Table 4: Effects of herbicides application on duckweed water surface coverage (g) at various intervals.
|
Treatments |
WSC (%) after 1st week |
WSC (%) after 2nd week |
WSC (%) after 3rd week |
WSC (%) after 4th week |
|
Glyphosate |
19.33 |
17.33 d* |
16.33 d |
14.67 d |
|
Paraquat |
20.00 |
18.67 bc |
17.33 cd |
16.33 bc |
|
MCPA |
19.33 |
17.67 cd |
16.67 cd |
15.67 cd |
|
Bromoxynil |
20.00 |
18.67 bc |
17.67 bc |
16.67 bc |
|
Atrazine |
20.33 |
19.00 b |
17.67 bc |
16.67 bc |
|
Isoproturon |
20.33 |
19.33 b |
18.67 b |
17.33 b |
|
Weedy check |
20.00 |
20.67 a |
21.67 a |
22.67 a |
|
LSD (α =0.05) |
NS |
1.146 |
1.011 |
1.011 |
*Means followed by different letters are significantly different from each other; NS: non-significant; WSC (%) = Water Surface Coverage Percentage.
While during the 3rd week of treatments application the difference among the various treatments became much pronounced. The effectiveness of the translocated herbicides and their slow working ability further decreased the number of plants causing a decrease in the surface coverage as compared to the herbicides with the short term effect or with the contact herbicide where the weeds recovered after a short toxic treatment. The highest water surface coverage (21.67 %) was recorded for weedy check followed by Isoproturon (18.67 %). Moreover, the lowest water surface coverage (16.33 %) was observed for glyphosate. The same trend was found in the time afterwards i.e. in the 4th week of treatments application the maximum water surface coverage (22.67 %) were recorded for weedy check followed by Isoproturon (17.33 %) as compared to the minimum water surface coverage (14.67 %) recorded for glyphosate. Therefore, free floating aquatic weed have ability to cover the entire surface in a few days to weeks if conditions becomes favourable and they are left unchecked. But the factors limiting their growth and reproduction are always in lesser quantity and check their growth and the perfect favourable conditions are seldom achieved. These results are in line with the work of Henry-Silva et al. (2008) who reported that the difference in the concentration of various chemicals, nutrients or others can affect the growth and water surface coverage in case of aquatic weeds. The application of chemicals damage the budding and reproduction due to inhibition of these parameters which resultantly reduce the water surface coverage. While in control water surface coverage increased due to uninterrupted Lemna growth. Moreover, the glyphosate and MCPA were more effective to suppress the growth and reproduction and until the plants were killed thus also limited the water surface coverage.
Conclusions and Recommendations
Minimum fresh biomass, budding %, plant growth, root length and water surface coverage was recorded in glyposate as compared to weedy check and isoproturon, respectively. Highest plant mortality (%) were noticed for glyposate as compared to other herbicides as well as weedy check. Lowest frond diameter and growth of microscopic algae and other macrophytes associated with duckweed was recorded for MCPA as compared to control.
It is recommended to apply glyphosate and MCPA for the management of duckweed effective in large scale infestation. Bromaxonil and isoporutorun showed poor performance in duckweed control, while paraquat treated plants recovered after a short span of time and resumed normal growth.
Novelty Statement
Glyposate effectively controlled duckweed as compared to other previously used Herbicides. Moreover, glyposate is more economical and less toxic than the previously used Herbicides for duckweed control.
Author’s Contribution
Bakhtiar Gul: Supervised the research study.
Saleetha Rabial: Conducted the research and wrote the manuscript.
Haroon Khan: Helped in data analysis.
Conflict of interest
The authors have declared no conflict of interest.
References
Bejarano, J.R.C. 2005. Effect of operational variables on nitrogen transformations in Duckweed stabilization ponds. Ph.D. thesis, the UNESCO-IHE Institute for Water Education, Delft. CRC press.
Bhetalu, A.D., S.S. Patil, S.N.S.W. Ingole and S.W. Director. 2012. Studies on generation of power alcohol as a non-conventional energy source from aquatic macrophytes. A critical review. J. Eng. Res. Stud., 3(1): 9-17.
Bonomo., L., G. Pastorelli and N. Zambon. 1997. Advantages and limitations of duckweed based wastewater treatment systems. Water Sci. Technol., 35(5): 239-246. https://doi.org/10.2166/wst.1997.0207
Castro, A., I. Colares, T. Franco, M. Cutrim and R. LuvizottoSantos. 2015. Using a toxicity test with Ruppia maritima L. to assess the effects of Roundup. Mar. Pollut. Bull., 91(2): 506-510. https://doi.org/10.1016/j.marpolbul.2014.10.006
Chambers, P.A., P. Lacoul, P.K.J. Murphy and S.M. Thomaz. 2007. Global diversity of aquatic macrophytes in freshwater. In: Freshwater animal diversity assessment (pp. 9-26). Springer, Dordrecht. pp. 19-26. https://doi.org/10.1007/978-1-4020-8259-7_2
Harvey, R.M. and J.L. Fox. 1973. Nutrient removal using Lemna minor. Water Pollut. Contr. Fed., 45(9): 1928-1938.
Henry-Silva, G.G., A.F. Camargo and M.M. Pezzato. 2008. Growth of free-floating aquatic macrophytes in different concentrations of nutrients. Hydrobiologia, 610(1): 153-160. https://doi.org/10.1007/s10750-008-9430-0
Jones, T.W., W.M. Kemp, P.S. Estes and J.C. Stevenson. 1986. Atrazine uptake, photosynthetic inhibition, and short-term recovery for the submersed vascular plant, Potamogeton perfoliatus L. J. Arch. Environ. Contam. Toxicol., 15: 277-283. https://doi.org/10.1007/BF01061104
Khan, M.A., K.B. Marwat, B. Gul, F. Wahid, H. Khan and S. Hashim. 2014. Pistia stratiotes L. (Araceae): Phytochemistry, use in medicines, phytoremediation, biogas and management options. Pak. J. Bot., 46(3): 851-860.
Labrada, R. and L. Fornsari. 2002. Management of the worst aquatic weeds in Africa FAO efforts and achievements during the periods 1991-2001. FAO Rome.
Lancar, L. and K. Krake. 2002. Aquatic weed and their management. Intern. Comm. Irrig. Drain., pp. 1-72.
Lytle, J.S. and T.F. Lytle. 2001. Use of plants for toxicity assessment of estuarine ecosystems. Environ. Toxicol. Chem., 20(1): 68-83. https://doi.org/10.1002/etc.5620200107
Michael, J.L. 2002. Impact of herbicides on the forest ecosystem, aquatic ecosystems and wildlife. (The US experience) Revue Forestière Française (France), agris.fao.org., 4(6): 593-608.
Mortensen, D.A., L. Bastiaans and M. Sattin. 2000. The role of ecology in the development of weed management systems: An outlook. Weed Res. Oxford, 40(1): 49-62. https://doi.org/10.1046/j.1365-3180.2000.00174.x
Newman, J.R. 2004. Curled pond weed; Wallingford, NERC Centre for Ecology and Hydrology, Centre for Aquatic Plant Management.
Peltier, W.H. and C.I. Weber. 1985. Methods for measuring the acute toxicity of effluents to freshwater and marine organisms, 3rd Ed. EPA/600/4-85/013, U.S. Environ. Protection Agency, Cincinnati, OH.
Reddy, K.R. and W.F. DeBusk. 1984. Growth characteristics of aquatic macrophytes cultured in nutrient-enriched water: I. Water hyacinth, water lettuce, and pennywort. J. Econ. Bot., 38(2): 229-239. https://doi.org/10.1007/BF02858838
Sikorski, L., M. Baciak, MA., Bęś, A., and B. Adomas. 2019. The effects of glyphosate-based herbicide formulations on L. minor, a non-target species. Aquat. Toxicol., 209: 70-80. https://doi.org/10.1016/j.aquatox.2019.01.021
Steel, R.G.D. and J.H. Torrie. 1980. Principles and procedures of statistics. A biometrical approach. 2nd Ed., McGraw Hill, Inc., USA.
Wang, W., 1990. Literature review on duckweed toxicity testing. J. Environ. Res., 52 (1): 7-22. https://doi.org/10.1016/S0013-9351(05)80147-1
Xu., J., J.J. Cheng and A.M. Stomp. 2011. Production of high-starch duckweed and its conversion to bioethanol. Biosyst. Eng., 110 (2): 67-72. https://doi.org/10.1016/j.biosystemseng.2011.06.007
To share on other social networks, click on any share button. What are these?







