Efficacy of Dietary Polyphenols Supplementation with Soybean Meal-Based Diet on Growth, Antioxidant Status and Carcass Composition of Labeo rohita Fingerlings
Efficacy of Dietary Polyphenols Supplementation with Soybean Meal-Based Diet on Growth, Antioxidant Status and Carcass Composition of Labeo rohita Fingerlings
Mukhtiar Ali1, Syed Makhdoom Hussain1*, Muhammad Asrar1, Majid Hussain2, Muhammad Zubair ul Hassan Arsalan3, Zeeshan Yousaf1 and Aumme Adeeba Bano1
1Fish Nutrition Lab, Department of Zoology, Government College University, Faisalabad, Pakistan.
2Department of Fisheries and Aquaculture, University of Okara, Pakistan
3Department of Life Sciences, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, Pakistan
ABSTRACT
A 70-days growth trial was carried out to ascertain how dietary supplementation of polyphenols with soybean meal-based diet influences the growth, antioxidant status and carcass composition of Labeo rohita fingerlings. Seven experimental diets were formulated with graded levels, 0, 50, 100, 150, 200, 250 and 300 mg/kg of polyphenols supplementation. Each test diet was allocated randomly to triplicate tanks with 15 fingerlings in every tank. These were fed @ 5% of live wet body weight. Results showed that L. rohita fingerlings fed on test diet T4 with dietary supplementation of 150 mg/kg polyphenol showed significant (p<0.05) increase in growth performance having weight gain% (233.24%) and better feed conversion ratio (1.44). Similarly, significantly (p<0.05) improved body composition with crude protein (17.96%), crude fat (8.72%), ash (4.94%) and moisture (68.43%) were also recorded at same polyphenols supplementation level. However, in terms of antioxidant activity, increasing trend in inhibition of oxidation was recorded with increase in supplementation of polyphenols in test diets, having minimum oxidation (7.66) in fingerlings fed on T7 with 300 mg/kg level of polyphenols supplementation.
Article Information
Received 18 January 2023
Revised 20 February 2023
Accepted 13 March 2023
Available online 01 June 2023
(early access)
Published 25 October 2024
Authors’ Contribution
MA conducted the study and wrote the manuscript. SMH acquired funds, administered and supervised the project. MA and MH curated and edited the data. MZHA, ZY and AAB edited and reviewed the manuscript.
Key words
Aquaculture, Polyphenols, Growth performance, Carcass composition, Antioxidant activity
DOI: https://dx.doi.org/10.17582/journal.pjz/20230118170114
* Corresponding author: [email protected]
0030-9923/2024/0006-2823 $ 9.00/00
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Feed in aquaculture is a major factor regarding its growth and development (Gabriel et al., 2007). More commonly used aqua-feeds contain fishmeal (FM) as a source of protein owing to its balanced amino acid content, high palatability and good digestibility (Nasr et al., 2021). But FM is primarily produced from fish harvested from wild resources that are constantly diminishing and it is supposed that in the near future, current FM yield will not be able to fulfill the expanding aquaculture production. This reinforces the need to search new FM alternatives to ensure long term aquaculture sustainability (Gasco et al., 2018). Due to low cost and world-wide availability, plant protein sources are considered as the best alternatives for FM (Saleh et al., 2021). Soybean meal (SBM) is considered as an appropriate protein source and good alternative for FM in aqua-feed because of its nutritional composition that is consistent with FM, comparatively better amino acid profile, easy availability and affordable price (Krishnan and Jez, 2018).
Plant extracts having many valuable functional properties, have also gained much attention due to their positive impacts on fish health (Parrino et al., 2019; Zargar et al., 2019). Particularly, polyphenols (secondary plant metabolites) are highly perceived for their antioxidant effects that inhibit harm due to oxidative stress, abolish inflammation, enhance metabolism of lipid and decrease chances of cancer and cardiac disorders (Hussain et al., 2016; Xu et al., 2019). Until now, only 8000 plant polyphenols have been explored and only about 10% of the possible plant polyphenols are produced by using plants (Tsao, 2010). These are found in nearly all residuals of agro industrial chains. By-products of seeds, fruits, nuts, cereals, and vegetables are rich in polyphenols (Castrica et al., 2019). Supplementation of polyphenols in fish feed is necessary for enhancing the efficacy of plant based products which in return improves fish growth rate and health status. Being immunity stimulants, polyphenols are regarded as natural antibiotics that increase resistance in fish body and are used as dietary additives (Zhu, 2020).
Fish is also subjected to repulsive flavor and malodorous smell during its handling and processing due to oxidation of n-3 polyunsaturated fatty acids (PUFA). So, oxidation of PUFA in fish body has a greater risk for loss of fish quality. Oxidation of lipids in meat of fish and other fish products not only give rancid taste but also produce different substances in fish meat which cause health issues in humans on its consumption (Secci and Parisi, 2016). Thus, for overcoming this problem of oxidation and low immunity, antioxidants may be included in fish feed. Antioxidants capture the reactive free radicals and are thus important for removing the potentially damaging oxidizing agents which results in better health of living organism. Polyphenolic compounds show redox properties due to which they act as strong antioxidants (Adedapo et al., 2008).
Labeo rohita is globally considered a dominant freshwater tropical Indian major carp which accounts for almost 3.70 % of global aquaculture yield in 2018 (FAO, 2018). It is column feeder fish, feeding both on plant and animal matter for getting nutrients. It is an economically important species with total yield of about two million tons, enchanting an amount of US$ 3.42 billion in 2018 (FAO, 2020). Considering the commercial value of L. rohita and promising use of polyphenols in critically improving the fish health and immunity, this study was designated to investigate the effects of polyphenols supplementation with SBM-based diets on growth, antioxidant activity and carcass composition of L. rohita fingerlings.
MATERIALS AND METHODS
Culture conditions and experimental design
Fingerlings were procured from Government Fish Seed Hatchery, Faisalabad and were acclimatized with laboratory environment for 14 days. To make fingerlings free from ecto-parasites, bathed with saline water (5% NaCl) prior to experiment (Rowland and Ingram, 1991). During acclimatization period, fingerlings were fed basal diet twice daily to apparent satiety. Water quality indicators i.e., water oxygen level, pH and temperature were measured on daily basis and were adjusted within following ranges as temperature (24.8-28, 6 ºC), pH (7.4-8.5), and water oxygen level (5.8-7.4 mg/L). To all experimental tanks, aeration was provided round the clock by using capillary system. 15 fingerlings were placed in each tank having three replicates for each test diet. These were fed according to 5% live body mass. Total duration of trial was of 70-day.
Test diet formulation
Seven diets including one control and six test diets with supplementation of polyphenols in SBM-based diet on following graded levels (0, 50, 100, 150, 200, 250, and 300 mg/kg) were formulated. For supplementation, polyphenols were procured from tomato extract at Department of Applied Chemistry and Biochemistry, Government College University, Faisalabad. While rest of feed items (Table I) were bought from private feed mill and analyzed chemically according to AOAC (2005). For pellets formation, all feed ingredients were ground, mixed thoroughly by using mixer, and then fish oil was added slowly. 10 to 15% of water was also added to obtain dough which was then passed via laboratory extruder to obtain floating pellets. All the experimental diets were then oven dried at 105℃ and kept at 4ºC until use.
Table I. Ingredients composition (%) of test diets (TD).
|
Ingredients |
TD-I (Control) |
TD-II |
TD-III |
TD-IV |
TD- V |
TD-VI |
TD-VII |
|
Polyphenols (mg/kg) |
0 |
50 |
100 |
150 |
200 |
250 |
300 |
|
Soybean meal |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
|
Fish meal |
17 |
17 |
17 |
17 |
17 |
17 |
17 |
|
Wheat flour* |
12 |
12 |
12 |
12 |
12 |
12 |
12 |
|
Sunflower meal |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Fish oil |
7 |
7 |
7 |
7 |
7 |
7 |
7 |
|
Vitamin premix |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Mineral mixture |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Ascorbic acid |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Chromic oxide |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
*Polyphenols was supplemented at the cost of wheat flour.
Feeding procedure and sample collection
Fish were fed on test diets daily two times (morning and evening) at 5% of their live body mass. After 2 h. of feeding session, for measuring feed conversion ratio (FCR), unconsumed feed was collected after water drainage by disclosing the tanks valves. Tanks were cleaned properly and filled with water. After 120 min, fecal material was collected from tanks via fecal collecting tube. Proper care was done during collection of feces to prevent nutrient leaching. Then, fecal material was dried in oven at 105 ºC and after grinding stored for chemical analysis.
Growth measurement
L. rohita fingerlings with average weight (7.09 g) were stocked in each tank at the start of experiment. During experimental period, fingerlings were bulk weighted after every two weeks for assessment of growth. Weight gain % (WG%), and FCR were measured by following formulae:

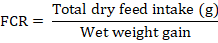
Evaluation of antioxidant activity
By utilizing 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay stated by Brand-Williams (1995), antioxidant status of samples was checked. For this purpose, 2 g of samples were homogenized in 20 ml of 80% aqueous methyl alcohol and were placed 20-25℃ for 15 min. The samples were centrifuged (5000 rpm) for 10 min at 4ºC and passed via 0.45 nm syringe filter (Whatman Inc., Clifton, NJ) before analysis. Then in 1.5 ml cuvette, 100 μl of filtered extract and 900 μl DPPH methanol solution (100 μM) was added to get final volume of 1 ml. With interval of one minutke, mixture absorbance was noted at 517 nm for 10 min by using spectrophotometer. As per inhibition the extract’s antioxidant capacity against the DPPH radicals was calculated.

Carcass composition analysis
For carcass composition analysis, 5 fingerlings were chosen at random from each tank and were air dried. Samples were homogenized by using motor and pestle prior to proximate analysis by using standard protocols (AOAC, 2005). Samples were dried in oven (105°C) for 12 h to measure moisture amount. For measurement of crude protein (CP) (N × 6.25), Kjeldahl apparatus was used while crude fat (CF) was measured by using Soxtec HT2 1045 system. Ash was analyzed by its ignition for 12 h. at 650 °C in electric furnace (Eyela-TMF 3100). For estimation of total gross energy, oxygen bomb calorimeter was utilized.
Statistical analysis
One-way analysis of variance (ANOVA) was used to evaluate the growth and proximate composition of data (Steel et al., 1996). For comparison of differences in means, Tukey’s Honesty Significant Difference Test was used and found significant at p<0.05 (Snedecor and Cochran, 1989). All was done by utilizing the Co-Stat computer software (Version 6.303, Monterey, PMB 320, 93940 USA, CA).
RESULTS
According to results of growth performance (Table II), an increasing trend in WG and WG% was recorded in fingerlings fed on polyphenols supplemented diet up to T4 (polyphenols supplementation of 150 mg/kg), with maximum and significant (p<0.05) WG% (233.24%) and WG (16.56g) with FCR (1.44), while from T5 to T7 a decreased trend in growth rate and WG was recorded. The least WG (11.43g) and WG% (161.29%) with FCR (1.94) was recorded at control diet. Antioxidant status in fingerlings fed SBM-based diet with polyphenols supplementation at various levels (0, 50, 100, 150, 200, 250 and 300 mg/kg of diet) is determined (Table III). In terms of oxidation, decreasing trend was noted with rising level of polyphenols that indicate increase in antioxidant activity with rise of dietary polyphenols level. Experimental diet T7 with 300 mg/kg polyphenols was found to be best in terms
Table II. Growth performance of L. rohita fingerlings fed on soybean meal based diets supplemented with polyphenols.
|
Growth parameters |
TD-I (Control) |
TD-II |
TD-III |
TD-IV |
TD-V |
TD-VI |
TD-VII |
|
IW (g) |
7.09±0.02a |
7.09±0.02 a |
7.08±0.02 a |
7.10± 0.01 a |
7.10± 0.02a |
7.09± 0.02a |
7.08± 0.02 a |
|
FW (g) |
18.52±0.08g |
20.84±0.07d |
21.53±0.13c |
23.66± 0.10a |
22.77± 0.12b |
20.01± 0.12e |
19.24± 0.05f |
|
WG (g) |
11.43±0.06g |
13.75±0.05d |
14.45±0.11c |
16.56± 0.09a |
15.67± 0.10b |
12.92± 0.10e |
12.16± 0.04f |
|
WG (%) |
161.29±0.59g |
193.80±0.38d |
204.14±0.93c |
233.24± 0.94a |
220.60± 0.95b |
182.23± 0.90e |
171.62± 0.19f |
|
WG (Fish-1 day-1)g |
0.16±0.01g |
0.20± 0.00d |
0.21±0.00c |
0.24± 0.02a |
0.22± 0.01b |
0.18± 0.00e |
0.17± 0.00f |
|
FI |
0.36±0.01c |
0.39±0.01b |
0.40±0.01b |
0.43± 0.03a |
0.42± 0.02a |
0.39± 0.01b |
0.36± 0.01c |
|
FCR |
1.94±0.07a |
1.97±0.04a |
1.56±0.06b |
1.44± 0.02c |
1.55± 0.02b |
1.62± 0.07b |
1.83± 0.20a |
Means within rows having different superscripts are significantly different at (p<0.05). Data are means of three replicates. IW, initial weight, FW, final weight; FI, feed intake; FCR, feed conversion ratio; WG, weight gain.
For details of test diets (TDs), see Table I.
of antioxidant activity having lowest oxidation value (7.66) as compared to other test diets. In terms of L. rohita carcass composition (Table IV), increasing trend was recorded in fingerlings fed from T1 to T4, and fish fed on T4 (150 mg/kg polyphenols supplementation) has significantly (p<0.05) higher CP (17.96%), CF (8.72%), ash (4.94%) and moisture (68.43%), while fish fed T5 to T7 showed the decreasing trend in proximate body composition. Among all test diets, fingerlings fed on control diet (T1) showed the minimum proximate body composition with CP (15.03%), CF (5.71%), ash (4.04%) and moisture (67.38%).
Table III. Antioxidant activity of polyphenols supplemented soybean meal-based diet in L. rohita.
|
Experimental diets |
Polyphenols levels (mg/kg) |
Oxidation (%) |
|
TD-I (Control) |
0 |
100.00±0.00 |
|
TD-II |
50 |
96.88±0.36 |
|
TD-III |
100 |
78.34±0.84 |
|
TD-IV |
150 |
46.28±0.62 |
|
TD-V |
200 |
33.15±0.34 |
|
TD-VI |
250 |
18.23±0.38 |
|
TD-VII |
300 |
7.66±0.44 |
DISCUSSION
Polyphenols may be recognized as credible alternatives to antibiotics and other chemicals in fish farming as they may defend fishes against oxidative stress and many other diseases (Dawood et al., 2018; Van et al., 2019). A fish fed on polyphenols supplemented feed is like having a body armed with antioxidants and is more likely to combat with diseases, exhibit better carcass composition and increase in growth performance.
According to results of present study, L. rohita fingerlings exhibited significant improvement in carcass, growth performance and WG with lowest FCR value at 150 mg/kg polyphenols supplementation level in SBM- based diet. These results are in line with study of Shin et al. (2010) who noted that olive flounder showed better growth performance and lower FCR, when fed on diet supplemented with quercetin (polyphenols) at 150 mg/kg. Better growth performance was also reported in Pangasianodon gigas when fed on diet in which 5% of FM replaced with spirulina (rich in polyphenols) (Tongsiri et al., 2010). Xu et al. (2019) recorded significant improvement in growth, antioxidative activity and meat quality of Ctenopharyngodon idella fed on diet rich in polyphenols with quercetin supplementation. Significant decrease in FCR and higher growth performance in Nile tilapia was reported when fed on diet supplemented with grape seed proanthocyanidins (Zhai et al., 2014). Babaheydari et al. (2014) observed that with increase in supplementation of Stachys lavandulifolia up to 40 mg/kg, there was an increase in growth performance of tilapia. Better growth performance was reported in Cyprinus carpio when fed on diet supplemented with marshmallow extracts (Fallahpour et al., 2014). Megalobrama amblycephala showed improved immunity, growth performance and lipid metabolism when fed on high fat diet supplemented with polyphenols (Jia et al., 2019). Munglue (2016) reported higher growth performance and maximum WG in tilapia when fed on 1% Nelumbo nucifera peduncle extract. Oreochromis niloticus showed better growth performance at 0.5, 1 and 1.5% supplementation of roselle calyx, another rich source of polyphenols (El-Mesasllamy et al., 2016).
Fallahpour et al. (2014) reported that inclusion of marshmallow extract (0.25%) in feed of fish attributed to better absorption of nutrients, resulting in higher WG.
Table IV. Proximate composition (%) of L. rohita carcass fed on soybean meal based diet supplemented with polyphenols.
|
Experimental diets |
Polyphenols levels (mg/kg) |
Protein % |
Fat % |
Ash % |
Moisture % |
|
TD-I (Control) |
0 |
15.03±0.03d |
5.71±0.37d |
4.04±0.03c |
67.38±0.12cd |
|
TD-II |
50 |
15.15±0.14d |
6.39±0.04bc |
4.15±0.03b |
67.94±0.23bc |
|
TD-III |
100 |
15.49±0.09c |
6.42±0.16bc |
4.20±0.03b |
67.08±0.11d |
|
TD-IV |
150 |
17.96±0.01a |
8.72±0.04a |
4.94±0.03a |
68.43±0.59b |
|
TD-V |
200 |
15.99±0.08b |
6.58±0.12b |
4.19±0.09b |
69.33±0.10a |
|
TD-VI |
250 |
15.05±0.05d |
6.02±0.12cd |
4.17±0.06b |
67.73±0.22bcd |
|
TD-VII |
300 |
15.06±0.02d |
5.92±0.04cd |
4.13±0.04b |
67.67±0.49bcd |
Means within rows having different superscripts are significantly different at (p<0.05). Data are means of three replicates.
Enhanced nutrient digestibility leads to improved carcass composition of fish in terms of fat, protein, ash and moisture. Jiang et al. (2016) reported that supplementation of curcumin (polyphenols) in feed of Carassius auratus is beneficial in enhancing the absorptive ability and digestion of nutrients leading to higher growth rates. According to Nandeesha et al. (2001), L. rohita fingerlings showed significant improvement in lipid content when fed on spirulina supplemented diet. Similarly, protein content was significantly different from control group when fish fed on diet supplemented with green tea extracts and propolis extracts (Wafaa et al., 2013).
Antioxidants capture the free radicals, thus have health promoting benefits by preventing the peroxidative damage to the body (Biglari et al., 2008). The results of this study proved that antioxidant activity of L. rohita increased with increased polyphenols level in diet. Maximum inhibition of oxidation was reported with 300 mg/kg of polyphenols level among all test diets while the fish fed on controlled diet showed the maximum oxidation. Amer (2016) reported that spirulina supplementation worked as antioxidant, hence enhances the immunity in body of Nile tilapia. Darsini et al. (2013) reported that Limonia acidissima supplementation significantly (p<0.01) enhances the number of antioxidant enzymes in common carp leading to decrease lipid oxidation. Hwang et al. (2013) studied that supplementation of green tea extracts in diet of Sebastes schlegeli improved lipid utilization along with increased lysozymal activity for better immunity. Curcumin supplementation boosted the immunity and antioxidative status of C. carpio and C. idella (Giri et al., 2019; Ming et al., 2020). Wang et al. (2020) observed that quercetin supplementation fortified the antioxidative status along with improvement in immunity of Danio rerio.
However, contradictory results have also been noticed by other researchers. Spirulina supplementation showed no significant effects when fed to olive flounder (Kim et al., 2013). Hwang et al. (2013) mentioned that fish fed on diet having 5% supplementation of green tea extracts showed significantly lowered specific growth rate, WG, and feed efficiency in comparison with fish fed on control diet. Frejnagel and Wroblewska (2010) observed that polyphenols supplementation resulted in decreased nutrients absorption in gut of monogastric animals resulting in poor growth performance. These contradictions in results can be due to differences in fish species, nutrient requirements, feeding conditions and polyphenols supplementation levels (Zhai and Liu, 2013).
CONCLUSION
Results of current study showed that polyphenols are good antioxidants and its inclusion in SBM-based diet at 150 mg/kg level has significant positive impacts on growth and proximate body composition. By increase in polyphenols supplementation level in diet, effective inhibition of oxidation occurred indicating improved antioxidant activity. Hence, SBM-based diet with polyphenols supplementation ensured cheap and effective feed additive referred for use in aqua-feed that will help in producing healthy fish, overcoming the issue of shortage and high price of FM leading to aquaculture industry development.
Funding
The funds for this project were provided by HEC, Project no. 20-4892/NRPU/RandD/HEC/14/1145 and 5649/Punjab/NRPU/RandD/HEC/2016.
IBR approval
The experiment was carried out in-line with the IRB guidelines of Government College University, Faisalabad.
Ethical statement
All the procedures and methods used in this study followed the ethical guidelines provided by Government College University, Faisalabad.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Adedapo, A.A., Jimoh, F.O., Afolayan, A.J. and Masika, P.J., 2008. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complement. Altern. Med., 8: 1-7. https://doi.org/10.1186/1472-6882-8-54
Amer, S.A., 2016. Effect of Spirulina platensis as feed supplement on growth performance, immune response and antioxidant status of mono-sex Nile Tilapia (Oreochromis niloticus). Benha med. J., 30: 1-10. https://doi.org/10.21608/bvmj.2016.31332
AOAC (Association of Official Analytical Chemists), 2005. Official methods of analysis. 15th Ed. Association of Official Analytical chemists, Washington, D.C. USA., 1094.
Babaheydari, S.B., Dorafshan, S., Heyrati, S.P. and Soofiani, N.M., 2014. Effect of wood betony (Stachys lavandulifolia Vahl) extract on some serum biochemical changes and acute stress response in juvenile common carp (Cyprinus carpio). Iran. J. aquat. Anim. Hlth., 1: 17-26. https://doi.org/10.18869/acadpub.ijaah.1.1.17
Biglari, F., AlKarkhi, A.F. and Easa, A.M., 2008. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Fd. Chem., 107: 1636-1641. https://doi.org/10.1016/j.foodchem.2007.10.033
Brand-Williams, W., Cuvelier, M.E. and Berset, C.L.W.T., 1995. Use of a free radical method to evaluate antioxidant activity. LWT Fd. Sci. Technol., 28: 25-30. https://doi.org/10.1016/S0023-6438(95)80008-5
Castrica, M., Rebucci, R., Giromini, C., Tretola, M., Cattaneo, D. and Baldi, A., 2019. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci., 18: 336-341. https://doi.org/10.1080/1828051X.2018.1529544
Darsini, D.T.P., Maheshu, V., Vishnupriya, M., Nishaa, S. and Sasikumar, J.M., 2013. Antioxidant potential and amino acid analysis of underutilized tropical fruit Limonia acidissima L. Free Radic. Antioxid., 3: 62-69. https://doi.org/10.1016/j.fra.2013.08.001
Dawood, M.A., Koshio, S. and Esteban, M.Á., 2018. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac., 10: 950-974. https://doi.org/10.1111/raq.12209
El-Mesasllamy, A.M., Ahmad, M.H., Souleman, A.M., El-Morsy, A.T. and Abd El-Naby, A.S., 2016. Effects of Roselle calyx (Hibiscus sabdariffa L.)-supplemented diets on growth and disease (Aeromonas hydrophila) resistance in Nile tilapia (Oreochromis niloticus L.). Egypt. Pharm. J., 15: 78-87. https://doi.org/10.4103/1687-4315.190403
Fallahpour, F., Banaee, M. and Javadzade, N., 2014. Effects of dietary marshmallow (Althaea officinalis L.) extract on growth performance and body composition of common carp (Cyprinus carpio). Int. J. Adv. Biol. Biomed. Res., 2: 2453-2460.
FAO, 2018. The state of world fisheries and aquaculture. Meeting the sustainable development goals. Rome.
FAO, 2020. The state of world fisheries and aquaculture. Sustainability in action. Rome. https://doi.org/10.4060/ca9229en
Frejnagel, S. and Wroblewska, M., 2010. Comparative effect of green tea, chokeberry and honeysuckle polyphenols on nutrients and mineral absorption and digestibility in rats. Ann. Nutr. Metab., 56: 163-169. https://doi.org/10.1159/000278747
Gabriel, U.U., Akinrotimi, O.A., Bekibele, D.O., Onunkwo, D.N. and Anyanwu, P.E., 2007. Locally produced fish feed: Potentials for aquaculture development in subsaharan Africa. Afr. J. Agric. Res., 2: 287-295.
Gasco, L., Finke, M.D. and Huis, A.A., 2018. Can diets containing insects promote animal health? J. Insects Fd. Feed., 4: 1-4. https://doi.org/10.3920/JIFF2018.x001
Giri, S.S., Sukumaran, V. and Park, S.C., 2019. Effects of bioactive substance from turmeric on growth, skin mucosal immunity and antioxidant factors in common carp, Cyprinus carpio. Fish Shellf. Immunol., 92: 612-620. https://doi.org/10.1016/j.fsi.2019.06.053
Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M.C. and Rahu, N., 2016. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev., 2016: 7432797. https://doi.org/10.1155/2016/7432797
Hwang, J.H., Lee, S.W., Rha, S.J., Yoon, H.S., Park, E.S., Han, K.H. and Kim, S.J., 2013. Dietary green tea extract improves growth performance, body composition, and stress recovery in the juvenile black rockfish, Sebastes schlegeli. Aquac. Int., 21: 525-538. https://doi.org/10.1007/s10499-012-9586-5
Jia, E., Yan, Y., Zhou, M., Li, X., Jiang, G., Liu, W. and Zhang, D., 2019. Combined effects of dietary quercetin and resveratrol on growth performance, antioxidant capability and innate immunity of blunt snout bream (Megalobrama amblycephala). Anim. Feed Sci. Technol., 256: 114268. https://doi.org/10.1016/j.anifeedsci.2019.114268
Jiang, J., Wu, X.Y., Zhou, X.Q., Feng, L., Liu, Y., Jiang, W.D., Wu, P. and Zhao, Y., 2016. Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture, 463: 174–180. https://doi.org/10.1016/j.aquaculture.2016.05.040
Kim, S., Rahimnejad, S., Kim, K., Lee, B. and Lee, K., 2013. Effects of dietary supplementation of spirulina and quercetin on growth, innate immune responses, disease resistance against Edwardsiella tarda, and dietary antioxidant capacity in the juvenile olive flounder (Paralichthys olivaceus). Fish. Aquat. Sci., 16: 1-8. https://doi.org/10.5657/FAS.2013.0007
Krishnan, H.B. and Jez, J.M., 2018. The promise and limits for enhancing sulfur-containing amino acid content of soybean seed. Pl. Sci., 272: 14-21. https://doi.org/10.1016/j.plantsci.2018.03.030
Ming, J., Ye, J., Zhang, Y., Xu, Q., Yang, X., Shao, X., Qiang, J. and Xu, P., 2020. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-jB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol., 97: 540–553. https://doi.org/10.1016/j.fsi.2019.12.074
Munglue, P., 2016. Effect of lotus (Nelumbo nucifera Gaertn.) fruit extract on growth performances of Nile Tilapia (Oreochromis niloticus). Raj. Agric. J., 15: 59-65.
Nandeesha, M.C., Gangadhara, B., Manissery, J.K. and Venkataraman, L.V., 2001. Growth performance of two Indian major carps, catla (Catla catla) and rohu (Labeo rohita) fed diets containing different levels of Spirulina platensis. Bioresour. Technol., 80: 117-120. https://doi.org/10.1016/S0960-8524(01)00085-2
Nasr, M.A.F., Reda, R.M., Ismail, T.A. and Moustafa, A., 2021. Growth, haemato-biochemical parameters, body composition, myostatin gene expression of Clarias gariepinus fed by replacing fish meal with plant protein. Animals, 11: 889-896. https://doi.org/10.3390/ani11030889
Parrino, V., Kesbiç, O.S., Acar, Ü. and Fazio, F., 2019. Hot pepper (Capsicum sp.) oil and its effects on growth performance and blood parameters in rainbow trout (Oncorhynchus mykiss). Nat. Prod. Res., 37: 1-5. https://doi.org/10.1080/14786419.2018.1550769
Rowland, S.J. and B.A. Ingram., 1991. Diseases of Australian native freshwater fishes, with particular emphasis on the ectoparasitic and fungal diseases of Murray cod (Maccullochella peeli), golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus). Fish. Bull., vol. 4, NSW Agriculture and Fisheries, Sydney.
Saleh, N.E., Helal, M., Ali, N.G., Abbas, E. and Abdel-Tawwab, M., 2021. Effects of using vital wheat gluten in practical diets on growth, intestinal histopathology, proinflammation-related gene expression, and resistance of white seabream (Diplodus sargus) to Staphylococcus epidermidis infection. Aquaculture, 537: 736508. https://doi.org/10.1016/j.aquaculture.2021.736508
Secci, G. and Parisi, G., 2016. From farm to fork: Lipid oxidation in fish products. A review. Ital. J. Anim. Sci., 15: 124-136. https://doi.org/10.1080/1828051X.2015.1128687
Shin, H.S., Yoo, J.H., Min, T.S., Lee, K.Y. and Choi, C.Y., 2010. The effects of quercetin on physiological characteristics and oxidative stress resistance in olive flounder (Paralichthys olivaceus). Asian Australas. J. Anim. Sci., 23: 588-597. https://doi.org/10.5713/ajas.2010.90624
Snedecor, G.W. and Cochran, W.G., 1989. Statistical methods, 8th Ed. Iowa State University Press, Ames Iowa. pp. 71-82.
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1996. Principles and procedures of statistics. 3rd edition. McGraw Hill International.
Tongsiri, S., Mang-Amphan, K. and Peerapornpisal, Y., 2010. Effect of replacing fishmeal with Spirulina on growth, carcass composition and pigment of the Mekong giant catfish. Asian J. agric. Sci., 2: 106-110.
Tsao, R., 2010. Chemistry and biochemistry of dietary polyphenols. Nutrients, 2: 1231-1246. https://doi.org/10.3390/nu2121231
Van, D.H., Hoseinifar, S.H., Sringarm, K., Jaturasitha, S., Yuangsoi, B., Dawood, M.A.O., Esteban, M.A., Ringo, E. and Faggio, C., 2019. Effects of Assam tea extract on growth, skin mucus, serum immunity and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish Immunol., 93: 428–435. https://doi.org/10.1016/j.fsi.2019.07.077
Wafaa, E., Doaa, I., El-Murr, A. and Rania, M., 2013. Effects of dietary inclusion of black cumin seeds, green tea and propolis extraction growth parameters, body composition and economic efficiency of Nile tilapia (Oreochromis niloticus). World J. Fish. Mar. Sci., 6: 447-452.
Wang, J., Zhang, C., Zhang, J., Xie, J., Yang, L., Xing, Y. and Li, Z., 2020. The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebra fish (Danio rerio). Fish Physiol. Biochem., 46: 759–770. https://doi.org/10.1007/s10695-019-00750-2
Xu, X., Chen, X., Huang, Z., Chen, D., He, J., Zheng, P.F. and Yu, J., 2019. Effects of dietary apple polyphenols supplementation on hepatic fat deposition and antioxidant capacity in finishing pigs. Animals, 9: 937-942. https://doi.org/10.3390/ani9110937
Xu, Z., Li, X., Yang, H., Liang, G., Gao, B. and Leng, X., 2019. Dietary quercetin improved the growth, antioxidation, and flesh quality of grass carp (Ctenopharyngodon idella). J. World Aquac. Soc., 50: 1182-1195. https://doi.org/10.1111/jwas.12663
Zargar, A., Rahimi-Afzal, Z., Soltani, E., Taheri Mirghaed, A., Ebrahimzadeh-Mousavi, H.A., Soltani, M. and Yuosefi, P., 2019. Growth performance, immune response and disease resistance of rainbow trout (Oncorhynchus mykiss) fed Thymus vulgaris essential oils. Aquacult. Res., 50: 3097-3106. https://doi.org/10.1111/are.14243
Zhai, S.W. and Liu, S.L., 2013. Effects of dietary quercetin on growth performance, serum lipids level and body composition of tilapia (Oreochromis niloticus). Ital. J. Anim. Sci., 12: 85-87. https://doi.org/10.4081/ijas.2013.e85
Zhai, S.W., Lu, J.J. and Chen, Z.H., 2014. Effects of dietary grape seed proanthocyanidins on growth performance, some serum biochemical parameters and body composition of tilapia (Oreochromis niloticus) fingerlings. Ital. J. Anim. Sci., 13: 33-57. https://doi.org/10.4081/ijas.2014.3357
Zhu, F., 2020. A review on the application of herbal medicines in the disease control of aquatic animals. Aquaculture, 526: 422-735. https://doi.org/10.1016/j.aquaculture.2020.735422
To share on other social networks, click on any share button. What are these?









