Fat Supplementation in Diet of Friesian Sahiwal Crossbred Bulls: A Novel Study on Productive and Reproductive Performance and Bio-chemicals Evaluation of Semen Quality Under Hot Summer Sub-tropical Conditions
Fat Supplementation in Diet of Friesian Sahiwal Crossbred Bulls: A Novel Study on Productive and Reproductive Performance and Bio-chemicals Evaluation of Semen Quality Under Hot Summer Sub-tropical Conditions
Naimat Ullah1, Sarzamin Khan2, Muhammad Mobashar3, Shahbaz Javaid4*, Tarique Tonio1 and Asif Mahmood5
1Livestock Management at Faculty of Sciences, Allama Iqbal Open University, Islamabad
2Department of Poultry Science, the University of Agriculture Peshawar
3Department of Animal Nutrition, the University of Agriculture Peshawar
4Animal Nutrition Program, ASI, PARC National Agricultural Research Centre, Islamabad
5Department of Animal Sciences, University of Poonch, Rawalakot, AJ&K
ABSTRACT
The current research study was planned to investigate the effect of dietary supplementation of fat on productive and reproductive performance, and semen quality of Friesian Sahiwal crossbred bulls during summer season. A total of 24 Friesian Sahiwal crossbred bulls at nearly similar age and body weight were selected and allotted erratically into 3 treatment groups, denoted as A, B and C for a period of 2 months (May and June). Each group was assigned 8 bulls. Bulls in group A were not fed with any dietary fat (control group) while bulls in group B and C were offered 100 and 200 g/kg as fat supplements, respectively. Semen quality parameters i.e. sperm motility and progressive sperm motility (%), sperm concentration (million/ml), live to dead ratio of sperm, amplitude of lateral head displacement of sperm (µm) and straightness of sperm (%) were measured at interval of 15 days. The results showed that dietary supplementation of fat didn’t influence dry matter and water intakes as well as weight gain significantly (P>0.05) among different treatments. Body conditions score was recorded significantly higher (P<0.05) in fat supplemented groups B (3.65) and C (3.78) as compared to group A (3.09). A significant increase (P<0.05) was observed in motility percentage, progressive motility percentage and semen concentration (million/ml) of group B and C. Treatment groups showed no significant effect on straightness of sperm (P>0.05) except live to dead ratio (P<0.01) at 2nd interval of 15 days in May and amplitude of lateral head displacement (µm) (P<0.05) at last interval of 15 days in June. Group B and C also showed significant increase (P<0.05) in testosterone level. However, level of glucose (mmol/lit) and blood urea nitrogen (mg/lit) and triglycerides (mg/lit) were found significantly lower. Therefore it is concluded from the present study that dietary supplementation of fat to crossbred bulls during hot summer tropical conditions can reduce the influence of heat stress on reproductive performance and thus enhances the semen quality.
Article Information
Received 04 July 2018
Revised 01 March 2019
Accepted 18 April 2019
Available online 14 August 2019
Authors’ Contribution
NU and SK conceived the idea, conducted research and wrote the manuscript. MM, TT and AM assisted in data collection and manuscript writing. SJ assisted in data analysis, manuscript reviewing.
Key words
Rumen protected fat, Body conditions score, Friesian Sahiwal crossbred, Productiv De performance, Semen quality, Serum biochemicals.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.6.2133.2140
* Corresponding author: [email protected]
0030-9923/2019/0006-2133 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
INTRODUCTION
The role of livestock in Pakistan cannot be neglected due to its agriculture based economic system. The livestock sector is fulfilling the essential needs of human population in terms of food and livelihood. Beside human diet, various rural activities are dependent on livestock especially large ruminants. Animals have special place in the transformation of agriculture foodstuffs into finished items for human consumption in daily life. Animals are important source of transformation of crop residues into edible ingredients (Bilal et al., 2006; Mobasher et al., 2018). Milk production in Pakistan has increased by more than 40% during the last decade with current value of 56,080 (‘000 tons) where the cattle contribution is 35.7% (Government of Pakistan, 2018). The livestock have share of 58.92% in agriculture and 11.11% in national GDP grew by 3.76% compared to 2.99% during corresponding period last year i.e. 2016-17.
Among various other factors affecting animal yield, high temperature strain is a big dilemma for the animals raising people of Pakistan. Pakistan is lying in the sub-tropics (FAO, 2006). Due to its geographical location, it is a warm country of South Asia region (Sattar and Mirza, 2009). High environmental temperature and relative humidity persists for a long span in Pakistan. The outside temperature reaches 50oC in the peak hours of the day in hot summer season. Similarly, in the warm humid months, the humidity level in the air remains higher than 85%, that leads to the discomfort of animals as this much temperature and humidity is beyond the thermo-neutral zone for large ruminants. Fiaz et al. (2010) reported that Pakistan has a lengthy hot spell which starts from May till September with the average environmental temperature ranging from 30 to 45°C. Similarly, severe humid hot conditions prevail from mid-July to mid-September.
Nature has gifted our country with excellent cattle and buffalo breeds. We also have substantial number of cross-bred animals. However, milk yield of the Native breeds is strikingly low compared to world’s famous breeds. About 73% of cattle population is un-descript animals that have long calving interval (450 days) and late sexual maturity between 3-4 years. The other factors that contribute towards low productivity of cattle are low growth rate and light weight at the onset of production (Jabbar et al., 2000).
Bull sperm quality may drastically reduce due to heat stress. Recently researchers have paid more attention due to warm global climate along intensive animal production. Sub-tropical warm climatic environment is not viable for exotic breeds of countries with temperate environment and this change in environment has negatively affected the performance in terms of production and reproduction of Holstein Friesian bulls (Cook et al., 2007).
As also mentioned earlier, Pakistan is a sub-tropical country and the hot summer conditions prevail for longer duration. Use of Friesian Sahiwal crossbred bulls for breeding has been increased in recent years. Heat stress in summer season might be responsible for lower fertility in bulls. The study could be helpful to measure the reproductive performance of Friesian Sahiwal crossbred bulls in summer season and thus could improve the breeding efficiency of Friesian Sahiwal crossbred bull during hot climatic conditions in which the quantity of semen production is badly affected by heat stress.
No comprehensive research work was conducted in Pakistan related to outcome of heat stress on Friesian Sahiwal crossbred bulls performance under various feeding strategies during summer season. Therefore, the current study was designed to study the productive and reproductive performance as well as serum bio-chemicals evaluation of Friesian Sahiwal crossbred bulls during summer hot spell under dietary fat supplementation.
MATERIALS AND METHODS
Experimental site, animals and design
Research work was conducted at Military Dairy Farm, Renala Khurd, District Okara, Punjab. The experimental station is located in central irrigated area of Punjab (30°53’14”North, 73°33’18” East). Mature Friesian Sahiwal crossbred bulls (n=24) with similar age and body weight were selected from the herds maintained at Military Dairy Farm and were kept in 3 groups to study the result of high atmospheric temperature during the months of May and June under roof shed. The age of the bulls were determined from the history sheet of the bulls. The Friesian Sahiwal crossbred bulls were randomly assigned to 3 different treatment groups denoted as A, B and C with 8 animals in each group. Group A was kept as control group offered no fat supplement while group B and C were offered fat supplementation i.e. Low fat supplemented group (BM; 100 g RP-10) and high fat supplemented group (CH; 200 g RP-10). The RP-10 is rumen protected fat developed by IFFCO Company, Malaysia. Animals were offered concentrate ration (wheat bran, 40%; maize oil cake, 5%; maize grain, 20%; wheat grain, 10%; rice paddy, 8%; corn gluten, 530%; canola meal, 5%; soya bean meal, l5%; molasses, 1%; premix, 1%.) at the rate of 0.5% and green fodder at the rate of 1% body weight while wheat straw at the rate of 0.5% body weight was offered on DM basis. Experimental trial lasted for 60 days including 53 days sample collection and data recording and 7 days for adjustment period.
Housing and health conditions
For identification, the bulls were ear marked. Space requirement was calculated for each bull and given as described by Banerjee (2011). All bulls were kept under roof shades. Fresh drinking water was provided ad-libitum to animals of each treatment group on individual basis. The adjustment period of 7 days was given to all bulls before start of experiment. Vaccination schedule was: foot and mouth disease (FMD) in February-March; black quarter (BQ) in April; hemorrhagic septicemia (HS) in May-June; anthrax in August; foot and mouth disease (FMD) in September-October; hemorrhagic septicemia (HS) in November-December. (GOP, 2014). Black quarter (BQ) and hemorrhagic septicemia (HS) immunization was carried out according to the recommendations by Livestock and Dairy Development (L&DD) department (GOP, 2010) and Livestock Breeding Act (GOP, 2014) for large ruminants. Each animal was given medication for any anomaly throughout the research study. Environmental temperature (oC) and relative humidity (%) were measured at 12:30 pm to 2:30 pm by using Hygrometer (Temperature Hygrometer; HTC-1). These were 41oC and 65%, respectively. Wing velocity (Km/hr) was measured at 12:30 pm to 2:30 pm by using digital anemometer (Intel Smart Sensor; AR-816). Temperature humidity index (ITH) was calculated using following formula as described by Mader et al. (2006).
THI = (0.8 × Tdb) + [(RH/100) × (Tdb − 14.4)] + 46.4
Whereas: THI, temperature humidity index, RH, relative humidity and Tdb, dry bulb temperature.
Productive performance
To determine daily feed intake, leftover was weighed and subtracted from offered feed. Sample from leftover was taken and processed for dry matter determination according to AOAC (2000). For this, 2 g air dried and ground sample in duplicate taken in pre-weighed crucible was placed in laboratory oven at 100°C until steady weightand was calculated using the following formula.
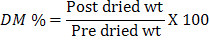
Where: DM, dry matter; wt , weight
Water intake (lit) was calculated after each 24 hrs by subtracting final value from initial value of water in watering trough. The reading was recorded on regular basis for each individual bull. Initially, the bulls were weighed at the start of experiment. Thereafter, the body weight was taken on fortnightly basis till the end of experiment and their weight gain was calculated accordingly. Before the start of experiment and subsequently at the end of experiment, a well skilled individual evaluated the body characteristics of bulls for the determination of body condition score (BCS) by following the method of Battiato et al. (2010). This method explored the feasibility of estimating the BCS of bulls from digital images by employing statistical shape analysis and regression machines. The shapes of bull body are described through a number of variations from a unique average shape. This description is used for automatic estimation of BCS through regression approach.
Serum biochemical analysis
From each individual bull, blood sample was collected on fortnightly basis and then used for determination of testosterone hormone level using testosterone enzyme immune assay test kit by Bio-Check Inc. Similarly, serum bio-chemical profile was assessed which included; glucose, triglyceride and blood urea nitrogen (BUN). The blood analysis was accomplished in the Laboratory at University of Veterinary and Animal Sciences, UVAS, Pattoki campus and University of Diagnostic Laboratory (UDL), Lahore campus. Estimation of triglycerides was done by enzymatic method using Liquiform-TG kit by Tron following method of Flegg (1973), BUN using AMP diagnostic kit (BD4002-E Vi.5 AMP, Austria) by the method of Brethelot et al. (1974) and ENZOPAK glucose kit for glucose by method of Barham and Trinder (1969).
Reproductive parameters
Fresh ejaculate sample was taken from each individual bull at 15 days interval of feeding dietary fat supplement and the collected samples were analyzed to study the parameters of sperm quality. For ejaculate collection, artificial vagina at 37°C was used to collect semen from the bulls and the collected semen samples were directly shifted to water bath pre-warmed to 37°C. Semen graduated tubes were used for recording the semen and calibrated photometer (IMV, L’Aigle, France) was used for measuring of sperm concentration. Total sperm count (volume × concentration) was calculated and recorded.
By adding commercial diluent (Triladyl, Minitube, Germany), maintained at 37°C, semen was diluted to a final concentration of 40×106 spermatozoa/ml, allowing 5 min for uniform mixing of extender and semen. Then extender mixed semen filled and packed into 0.5 ml straws (IMV, France) and for gradual cooling maintained for 2 hrs at 4°C before freezing with a computer controlled freezing system (IMV, France). The straws were organized on racks and placed into the freezing chamber. The computer controls the temperature inside the chamber and decreases it from 4°C to the lowest temperature chosen (-140°C) at the desired rate per min (15°C/ min). After the freezing process, straws were transferred to a liquid nitrogen tank (-196°C) until subsequent analysis.
In order to perform computer-assisted assessment of sperm motility, the computer assisted sperm analysis (CASA) setup (Animal Version 12.3H-CEROS, Hamilton Thorne Biosciences, Beverly, MA, USA) was pre-adjusted for bovine sperm analysis. 4 µl of diluted semen was placed on a pre-warmed glass slide and cover slip was placed on it. The slide was placed on the thermal plate of the microscope (37.5°C) for 3 min before analysis. Three randomly selected microscopic fields were scanned 6 times each. The mean of these 18 scans was used for statistical analysis. The following variables were analyzed:
The sperm motility of each bull for each of 3 treatments was evaluated on fortnightly basis using Computerized External Real Imaging Optic System (CEROS). Correspond to the feeding strategies, progressive sperm motility was determined on fortnightly basis by using CEROS. The sperm concentration was determined on fortnightly basis with the help of calibrated photometer (IMV, L’Aigle, France). Live to dead ratio of was evaluated on fortnightly basis using phase contrast microscope. A 10µL portion of semen sample was taken and mixed with eosin-nigrosin stain. Thin smear was made and air dried. 100 spermatozoa were counted for live-dead and 100 spermatozoa were counted for sperm live%. Spermatozoa considered as live in which head was not penetrated with stain and appeared as whitish whereas spermatozoa with penetrated stain in their heads considered dead. The amplitude of lateral head displacement (µm) and straightness (%) of sperm were determined on fortnightly basis with the help of CEROS.
Statistical analysis
Statistical analysis of data was performed by one-way ANOVA procedure of SAS software (SAS Institute, 2009). The significant differences in studied variables among the means of the experimental treatment groups were compared by using Duncan’s multiple range tests (Duncan, 1955). A level of P < 0.05 was used as the criterion for statistical significance.
RESULTS
In the present study, water and dry matter intake and weight gain in experimental bulls didn’t vary across different treatments, except BCS which increased significantly (P<0.05) in the animal group supplemented with 100g/kg diet as compared to no fat and high fat diet groups (Table I). This also related to dry matter intake (DMI). However, no significance was observed in DMI. Water intake decreased linearly with inclusion of fat in diet but was statistically non-significant (Table I).
Although sperm motality showed varaiable results at every interval of 15 days, but there was a significant increase (P<0.05) in sperm motility in animal group evaluated in high fat diet (200 g/kg) as compared to control group. For medium fat diet (100 g/kg), better motility was observed only on 15th June as compared to control diet (Table II). Similarly, the progressive sperm motility significantly increased in high fat diet group compared to control (Table II). The sperm concentration increased linearly with inclusion of fat in diet at almost all the time intervals studied (Table II). In this context, the live to dead sperm ratio was not signifiacantly affected by inclusion of fat in diet (Table II) except 15 June, live to dead sperm ratio was significantly greater than other intervals. Overall, sperm head displacement increased in high fat diet compared to control and medium fat diet and this was noticed only on 30th June (Table II). On other hand, head displacement was not affected by inclusion of fat at all time slots. Sperm straightness percentage was also not significantly affected by fat inclusion in diet, it remianed the same along all fat supplementation treatments (Table II). An increase in the synthesis of testosterone was observed on 31st May and 30th June in high fat diet group (200 g/kg) as compared to control group. The increase in the synthesis of testosterone is justified by the addition of fat as all the steroid hormones require cholesterol as a precursor for their production (Table II). The testosterone synthesis can have positive implication for spermatogenesis in seminiferous tubules of the bulls, thereby logically increasing sperm concentration. Sperm health can inevitably be linked to increase in sperm motility.
Table I. Effect of different levels of dietary fat supplementation on productive parameters (Mean± SEM) of Friesian Sahiwal crossed bulls during subtropical summer conditions at 15 days’ interval.
|
Treatment |
Water intake (L) |
DMI (kg) |
Weight gain |
BCS |
|
A |
35.84± 1.71 |
16.85± 0.68 |
15.62± 7.09 |
3.09± 0.09 b |
|
B |
33.24± 1.44 |
18.49± 0.71 |
25.62± 8.09 |
3.65± 0.10 a |
|
C |
31.51± 1.08 |
17.40± 0.74 |
23.72± 3.23 |
3.78± 0.12 a |
|
P –value |
0.12 |
0.27 |
0.52 |
0.0005 |
a, bMeans within same column with different superscripts are significantly different(P<0.05); A, control; B, fat supplement @100 g/Kg; C, fat supplement @ 200g/Kg); DMI, dry matter intake; BCS, body condition score.
Serum glucose and blood urea nitrogen (BUN) was not affected by inclusion of fat (Table III). Serum triglycerides increased initially on 15th May in both medium (100g /kg) and high fat diet groups (200g/kg) compared to control group (Table III). However, serum triglycerides later on were not affected by the fat treatment. The normal glucose and BUN levels indicated that inclusion of fat had no adverse effects on glucose homeostasis and protein metabolism. The initial increase in triglyceride due to fat inclusion also seemed to be metabolically adjusted later on by the fat supplemented bulls. However, other lipid profiles like serum cholesterol, elevated density lipoproteins and low density lipoproteins could be of benefit in this context.
Table II. Effect of different levels of dietary fat supplementation on sperm motility and progressive motility sperm (%) sperm concentration (million/ml) live to dead sperm ratio (%), testosterone level, amplitude of lateral head displacement (µm) and sperm straightness (%) (Mean ± SEM) of Friesian Sahiwal crossed bulls during subtropical summer conditions at 15 days interval.
|
Treatment |
15th May |
31st May |
15th June |
30th June |
Overall |
|
|
Mortality |
||||||
|
A |
70.10 ± 4.00 b |
71.25 ± 2.25 b |
64.12 ± 4.90 b |
72.12 ± 1.93 b |
69.37 ± 1.80 b |
|
|
B |
78.08 ± 3.18 ab |
71.37 ± 3.40 b |
76.00 ± 3.95 a |
69.00 ± 4.03 b |
73.59 ± 2.00 b |
|
|
C |
86.04 ± 1.38 a |
85.62 ± 1.43 a |
84.37 ± 1.96 a |
85.00 ± 1.94 a |
85.28 ± 0.35 a |
|
|
P-value |
0.005 |
0.0006 |
0.004 |
0.001 |
0.0002 |
|
|
Progressive sperm motility (%) |
||||||
|
A |
23.01 ± 3.27 b |
21.12 ± 0.74 b |
22.50 ± 3.06 |
16.50 ± 1.94 c |
20.78 ± 1.48 b |
|
|
B |
24.05 ± 2.54 b |
23.12 ± 3.86 b |
23.50 ± 3.99 |
22.75 ± 1.73 b |
23.34 ± 0.26 b |
|
|
C |
34.07 ± 1.85 a |
31.62 ± 1.92 a |
31.12 ± 2.68 |
32.87 ± 2.27 a |
32.40 ± 0.64 a |
|
|
P-value |
0.013 |
0.018 |
0.144 |
0.000 |
0.000 |
|
|
Sperm concentration (million/ml) |
||||||
|
A |
356.13±34.82c |
312.75±36.60c |
393.88±14.74c |
398.13±32.91c |
365.22±19.87c |
|
|
B |
632.25±45.19b |
584.50±22.06b |
575.00±29.89b |
554.25±14.34b |
586.50±16.50b |
|
|
C |
786.25±16.58a |
772.50±27.35a |
768.88±16.56a |
822.38±17.97a |
787.50±12.21a |
|
|
P-value |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
|
Live to dead sperm ratio (%) |
||||||
|
A |
58.37 ± 1.55 |
58.37 ± 1.55 |
56.75 ± 0.95 b |
55.37 ± 1.75 |
57.21 ± 0.72 |
|
|
B |
58.50 ± 2.29 |
58.50 ± 2.29 |
58.50 ± 2.39 a |
58.50 ± 0.90 |
60.00 ± 1.50 |
|
|
C |
61.20 ± 1.73 |
61.00 ± 1.73 |
64.75 ± 1.38 b |
60.62 ± 2.17 |
60.34 ± 0.53 |
|
|
P-value |
0.549 |
0.549 |
0.010 |
0.112 |
0.107 |
|
|
Testosterone |
||||||
|
A |
12.43 ± 0.88 |
11.36 ± 0.77 b |
11.45 ± 0.97 |
11.18 ± 0.28 b |
11.60 ± 0.28 c |
|
|
B |
12.25 ± 0.65 |
12.41 ± 0.53ab |
13.25 ± 0.73 |
12.42 ± 0.45 a |
12.58 ± 0.22 b |
|
|
C |
13.26 ± 0.41 |
13.80 ± 0.63 a |
13.78 ± 0.32 |
13.06 ± 0.36 a |
13.47 ± 0.18 a |
|
|
P-value |
0.540 |
0.049 |
0.082 |
0.006 |
0.001 |
|
|
Amplitude of lateral head displacement (µm) |
||||||
|
A |
6.26 ± 0.27 |
6.50 ± 0.81 |
6.62 ±0.20 |
6.02 ± 0.26 b |
6.35 ± 0.13 |
|
|
B |
6.02 ± 0.35 |
6.06 ± 0.20 |
5.89 ± 0.26 |
6.11± 0.17 b |
6.03 ± 0.14 |
|
|
C |
6.62 ± 0.12 |
6.31 ± 0.09 |
6.10 ± 0.14 |
6.71± 0.09 a |
6.44 ± 0.03 |
|
|
P-value |
0.268 |
0.209 |
0.073 |
0.047 |
0.076 |
|
|
Sperm straightness (%) |
||||||
|
A |
84.50 ± 0.82 |
83.50 ±1.08 |
83.87 ± 1.07 |
84.50 ± 0.56 |
84.09 ± 0.24 |
|
|
B |
83.12 ± 1.10 |
84.37 ± 0.84 |
84.10 ± 0.90 |
84.50 ± 0.68 |
84.24 ± 0.39 |
|
|
C |
83.87 ± 3.63 |
84.50 ± 0.98 |
85.37 ± 0.94 |
85.00 ± 0.75 |
83.18 ± 1.44 |
|
|
P-value |
0.205 |
0.735 |
0.721 |
0.833 |
0.664 |
|
a, bMeans within same column with different superscripts are significantly different(P<0.05); A, control; B, fat supplement @100 g/Kg; C, fat supplement @ 200g/Kg); DMI, dry matter intake; BCS, body condition score.
Table III. Effect of different levels (Mean ± SEM) of dietary fat supplementation on glucose (mmol/lit), blood urea nitrogen (mg/lit), and triglyceride production (mg/lit) of Friesian Sahiwal crossed bulls during subtropical summer conditions at 15 days.
|
15th May |
31st May |
15th June |
30th June |
Overall |
||
|
Glucose |
||||||
|
A |
74.52 ± 4.96 |
88.19 ± 4.66 |
81.89 ± 2.96 |
71.41 ± 4.15 |
79.00 ± 7.76 |
|
|
B |
81.42 ± 2.58 |
83.92 ± 1.53 |
80.23 ± 1.65 |
81.22 ± 3.54 |
81.67 ± 0.78 |
|
|
C |
72.80 ± 7.68 |
70.97 ± 7.10 |
72.62 ± 7.39 |
78.42 ± 2.37 |
73.70 ± 1.62 |
|
|
P-value |
0.512 |
0.059 |
0.348 |
0.140 |
0.110 |
|
|
Blood urea nitrogen |
||||||
|
A |
19.22 ± 0.79 |
22.67 ± 1.33 |
23.92 ± 1.22 |
19.02 ± 0.72 |
21.22 ± 1.24 |
|
|
B |
20.56 ± 0.73 |
20.64 ± 0.66 |
21.18 ± 0.22 |
20.59 ± 0.61 |
20.74 ± 0.14 |
|
|
C |
19.85 ± 0.84 |
20.87 ± 1.18 |
21.02 ± 1.05 |
20.80 ± 0.62 |
20.63 ± 0.26 |
|
|
P-value |
0.498 |
0.377 |
0.067 |
0.137 |
0.839 |
|
|
Triglycerides |
||||||
|
A |
100.64 ± 11.05 b |
118.65 ± 6.31 |
129.57±12.82 |
114.68 ± 9.60 |
115.89 ± 5.97 |
|
|
B |
140.44 ± 8.34 a |
131.56 ± 12.52 |
142.41 ± 7.58 |
132.78 ± 10.50 |
136.80 ± 2.71 |
|
|
C |
137.42 ± 13.32 a |
122.67 ± 7.78 |
151.14±14.36 |
125.53 ± 9.96 |
134.19 ± 6.49 |
|
|
P-value |
0.034 |
0.608 |
0.452 |
0.452 |
0.426 |
|
For abbreviations and statistical details, see Table I.
DISCUSSION
Animal production contributes major input to agriculture value added services (GOP, 2018). Conversion of agriculture products and byproducts into finished items for human consumption is the main role of Livestock. Animals are main source for conversion of crop residues into edible items (Bilal et al., 2006). The results of current study showed decrease in water intake among groups offered dietary fat supplement but was not significant. The decrease in water intake even statistically non-significant could be enhanced in summer by addition of fat, because of clinical implications of dehydration in individual bulls. Similarly BCS remained higher in group offered 200 g fat/kg. There was no significant change in dry matter intake was observed.
High environmental stress is a big issue for livestock production of Pakistan. During high uncomfortable environmental temperature, to which the animals are exposed during heat stress and mechanisms behind this trigger to sustain an animal’s body thermal balance (Marai et al., 2010). Due to dissimilarity in energy level and slow reproductive process, the fertility is reduced during high temperature stress. Heat stress more likely has a damaging outcome on semen production and fertility (Murugaiyah, 1992; Sinclair, 2000). Elevated body temperature is injurious for spermatogenesis and also reduces testosterone levels (Murray, 1997). Diet is a main element in managing fruitfulness by its immediate or backhanded activities. Moreover, it specifically impacts the process of spermatozoa development; in addition, it affects sustenance on the convergence of the hormones and different metabolites required for semen production (Wathes et al., 2007).The testosterone synthesis can have positive implication for spermatogenesis in seminiferous tubules of the bulls, thereby logically increasing sperm concentrations (Smith et al., 2014).
The semen parameters showed similar results with marked increase in semen quality of group offered 200g/kg as feed supplement. The sperm motlity, progressive sperm motility, semen concentration, live to dead percentage and straightness remained significantly (P<0.05) higher in group offered 200 g/kg. Similarly Amplitude of lateral head displacement (ALH) remained signaficantly (P<0.05) lower in group offered 200 g/kg. There is the crucial role of fat in the fertility improvement of males as fats are consumed as source of energy. Also there are various critical components of spermatozoa membrane (Santos et al., 2008). These results show that the inclusion of fat in diet had positive effect on the semen quality.
Serum glucose and BUN was not affected by inclusion of fat. Furthermore, glucose and BUN levels indicated that inclusion of fat had no adverse effects on glucose homeostasis and protein metabolism. These findings are supported by Kheiri and Nasr (2012), who also observed no effect of dietary supplementation of fat on blood glucose and BUN; however, these results are in contrast to Park et al. (1983) and Stephan et al. (2014) who found an increase in glucose and BUN with fat supplement in diet. Serum triglycerides increased initially on 15th May in both medium and high fat diet groups compared to control. However, serum triglycerides were not affected later on by the fat treatment. The present results are in agreement with Zhang et al. (2011) and Stephan et al. (2014) who also observed slightly increase in serum triglycerides with fat supplement in diet.
CONCLUSION
It can be concluded from present study that dietary fat supplementation is helpful in improving the semen quality and provides animals relief from heat stress under hot summer subtropical conditions. Further, trials should be conducted on exotic breeds instead of cross bred cattle to determine the effect of fat supplementation on milk production under hot summer subtropical conditions.
Statement of conflict of interest
The authors declare no conflict of interest.
REFERENCES
Association of Official Analytical Chemists (AOAC), 2000. Official methods of analysis. 15th Ed., Washington, DC, USA.
Banerjee, D. and Ashutosh, H., 2011. Effect of thermal exposure on diurnal rhythms of physiological parameters and feed, water intake in Tharparkar and Karan Fries heifers. Biol. Rhythm Rev., 42: 39-51. https://doi.org/10.1080/09291011003726490
Barham, D. and Trinder, P., 1969. Methods for determination of blood glucose level by spectrophotometer. Analyst, 97: 142. https://doi.org/10.1039/an9729700142
Battiato, S., Farinella, G.M., Guarnera, G.C. and Puglisi, G., 2010. Assessment of cow’s body condition score through statistical shape analysis and regression Machines. MLR: In Proc. Workshop Applic. Patt. Analy., 66: 73-76.
Berthelot, A., 1974. Clinical chemistry. 25: 336. https://doi.org/10.1088/0031-9112/25/8/012
Bilal, Q.M., Suleman, M. and Raziq, A., 2006. Buffalo: Black gold of Pakistan. Livest. Res. Rural Develop., 9: 18-25.
Cook, N.B., Mentink, R.L., Bennett, T.B. and Burgi, K., 2007. The effect of heat stress and lameness on time budgets of lactating dairy cows. J. Dairy Sci., 90: 1674-1682. https://doi.org/10.3168/jds.2006-634
Duncan, D.B., 1955. Multiple range and multiple F tests. Biometrics, 11:1-42. https://doi.org/10.2307/3001478
FAO, 2006. Irrigation in Southern and Eastern Asia in figures. AQUASTAT Survey, pp. 1-19.
Fiaz, M., Usmani, R.H., Abdullah, M. and Ahmad, T., 2010. Evaluation of semen quality of Holstein Friesian and Jersey bulls maintained under subtropical environment. Pak. Vet. J., 30: 75-78.
Flegg, H.M., 1973. An investigation of the determination of serum cholesterol by an enzymatic method. Annls clin. Biochem., 10: 79. https://doi.org/10.1177/000456327301000125
Government of Pakistan (GOP), 2018. Economic survey of Pakistan 2017-18, Economic Advisor Wing, Ministry of Finance, Islamabad, Pakistan.
Government of Punjab (GOP), 2010. Raising cattle and buffaloes manual. Livestock and Dairy Development Department, Lahore, Pakistan. www.livestockpunjab.gov.pk
Government of Punjab (GOP), 2014. Punjab livestock breeding act. Livestock and Dairy Development Department, Lahore, Pakistan. www.livestockpunjab.gov.pk
Jabbar, M.A., Hussain, M. and Pasha, T.N., 2000. Effect of different dietary energy levels on growth and onset of sexual maturity in Sahiwal heifers. 21st Annual Report. Livestock Production Research Institute. Bahadurnagar, Okara-Pakistan, pp. 76-77.
Kheiri, F. and Nasr, J., 2012. Growth and blood biochemical effects of dietary supplementation with ractopamine, a β-adrenergic agonist, in female broiler chickens. Rev. Med. Vet., 163: 411-414.
Mader, T.L., Davis, M.S. and Brown, B.T., 2006. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci., 84: 712-719. https://doi.org/10.2527/2006.843712x
Marai, I.F.M. and Haeeb, A.A.M., 2010. Buffalo’s biological functions as affected by heat stress: A review. Livest. Sci., 127: 89-109. https://doi.org/10.1016/j.livsci.2009.08.001
Mobashar, M., Tahir, M., Javaid, S., Anjum, M.I., Gul, I. and Sami, A., 2018. Nutritional evaluation of various stages of maturity of oat hay and its effect on milk production and composition in lactating Holstein Friesian cows. Pakistan J. Zool., 50: 2209-2216.
Murray, M.T., 1997. Male infertility: A growing concern. Am. J. nat. Med., 4: 9-16.
Murugaiyah, M., 1992. Changes in the semen characteristics of Kmbing Katjan crossbreed buck under hot and humid environmental temperatures. In Proceedings of 5th International Conference on Goat Production, New Delhi, pp. 1126-1129.
Park, C.S. and Rafalowski, W., 1983. Effect of dietary supplement on lipid metabolism of Friesian heifers. J. Dairy Sci., 66: 528-534. https://doi.org/10.3168/jds.S0022-0302(83)81821-9
SAS (Statistical Analytical System), 2009. User’s Guide: Statistics, Version 3.2. 2nd Ed., SAS Institute Inc., Cary, NC, USA.
Santos, J.E., Bilby, T.R., Thatcher, W.W., Staples, C.R. and Silvestre, F.T., 2008. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reprod. Domest. Anim., 43: 23-30. https://doi.org/10.1111/j.1439-0531.2008.01139.x
Sattar, A. and Mirza, R.H., 2009. Hematological parameters in exotic cows during gestation and lactation under subtropical conditions. Pak. Vet. J., 29: 129-132.
Sinclair, S., 2000. Male infertility: Nutritional and environmental considerations-alternative medicine review. J. clin. Therap., 5: 28-38.
Stephan, S.R., Mote, P.L. and Flegal, J.M., 2014. Dietary supplementation with Lovaza and krill oil shortens the life span of long-lived F1 mice. Age, 36: 1345-1352. https://doi.org/10.1007/s11357-014-9659-7
Smith, L.B. and Walker, W.H., 2014. The regulation of spermatogenesis by androgens. Cell Develop. Biol., 30: 2-13. https://doi.org/10.1016/j.semcdb.2014.02.012
Wathes, D.C., Abayasekara, D.R. and Aitken, R.J., 2007. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod., 77: 190-201. https://doi.org/10.1095/biolreprod.107.060558
Zhang, H., Wang, Z., Liu, G., He, J. and Su, C., 2011. Effect of dietary fat supplementation on milk components and blood parameters of early-lactating cows under heat stress. Slovak J. Anim. Sci., 44:52-58.
To share on other social networks, click on any share button. What are these?










