Genotypic Variability and Heterotic Effects in Rapeseed for Important Traits
Genotypic Variability and Heterotic Effects in Rapeseed for Important Traits
Wajid Khan* and Raziuddin
Department of Plant Breeding and Genetics, the University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Pakistan is facing huge shortage of edible oils and its imports cost nearly 3 billion US dollars each year to feed 220 million people. This situation demands serious efforts to develop new lines/ hybrids with better yields. Rapeseed is the second largest contributor in domestic edible oil industry. Keeping in view the lower yield of rapeseed in Pakistan especially in Khyber Pakhtunkhwa this study was conducted to investigate genetic variability and heterosis in eight rapeseed genotypes for maturity, morphological and yield traits. The eight parental genotypes i.e., AUP-01, AUP-05, AUP-07, AUP-08, AUP-10, AUP-13, AUP-18 and AUP-21 were crossed in full diallel fashion to generate 56 F1 crosses. These parental genotypes and their F1 hybrids were evaluated during 2015/16 in the field at the University of Agriculture, Peshawar, Pakistan. Mean squares revealed significant genetic variability among parents and their F1 crosses for days to maturity, primary branches plant-1, pods main raceme-1, 1000-seed weight and seed yield plant-1, signifying the occurrence of considerable variability in the tested material. Mean performance identified parental genotype AUP-21 being the best for early maturity (167.0 days), pods main raceme-1 (65.3), 1000-seed weight (7.1 g) and seed yield (30.6 g). F1 cross combination AUP-10 × AUP-18 had high seed yield (36.5 g) and moderate days to maturity (177.0). Desirable significant negative best parent and commercial heterotic effects were observed in 17 and 13 hybrids for maturity, respectively. Desired significant positive best parent and commercial heterotic effects were observed in 18 and 21 hybrids for primary branches plant-1, 19 and 10 hybrids for pods main raceme-1, 11 and 05 hybrids for 1000-seed weight and 18 and 01 hybrid for seed yield plant-1, respectively. Top ranking F1 hybrids were, AUP-05 × AUP-01 for maturity and AUP-10 × AUP-18 for 1000-seed weight and seed yield plant-1. These early maturing and high yielding crosses may be exploited for developing superior hybrids through exploitation of hybrid vigor and segregation. The information generated from this study could be useful for breeders to develop hybrids with better seed yield and oil quality traits through heterosis breeding.
Received | December 25, 2018; Accepted | August 15, 2019; Published | September 12, 2019
*Correspondence | Wajid Khan, Department of Plant Breeding and Genetics, the University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Khan, W. and Raziuddin. 2019. Genotypic variability and heterotic effects in rapeseed for important traits. Sarhad Journal of Agriculture, 35(3): 1000-1010.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.3.1000.1010
Keywords | Genetic variability, Heterosis, Rapeseed
Introduction
Rapeseed (Brassica napus L.) belonging to family Brassicasae contains 44-46 percent oil and 25-30 percent protein contents in its seeds. In Pakistan rapeseed and mustard are the second largest contributor in the domestic edible oil production after cotton seed (Amjid, 2014). Pakistan has made great progress in agriculture in the last two decades. It has not only achieved self-sufficiency but has also become exporter of major crops like wheat, cotton, sugarcane, mango, dates, oranges and rice. On the other hand, for the edible oil requirements of its 210 million people it highly relies on imports which drain more than two billion US dollars each year (FBS, 2016-17). The demand of edible oils in the country is increasing 2-5% every year due to increase in population and per capita consumption (Khan et al., 2013). Average rapeseed yield in Pakistan and Khyber Pakhtunkhwa is 960 kg ha-1 and 462 kg ha-1 respectively, whereas in other countries its yield is 2000 kg ha-1 (PBS, 2016-17). Low yield per hectare of rapeseed in the country and especially in Khyber Pakhtunkhwa is partly due to non-availability of high yielding cultivars and poor management practices (Nassimi et al., 2006).
The shortage of edible oil in the country demands serious efforts to developed new high yielding oilseed crops. For conducting any breeding program, breeders need some basic information which includes genetic variability and heterosis which is important for the improvement of seed yield and other important traits. It provides an opportunity to the breeders to select desirable genotypes through direct or indirect selection of trait (Jatoi et al., 2012). To induce genetic variations and develop new varieties, inter and intra specific crosses have been used in brassica (Rameeh et al., 2012). Heterosis or hybrid vigor (the improvement of a cross over its parents) has been exploited in several crop plants and is considered effective in increasing seed yield and other parameters (Hablak, 2019; Girke et al., 2012). Three main hypotheses are used to explain the genetic basis of heterosis: the dominance, over dominance and epistasis hypotheses. The dominance hypothesis assumes that deleterious recessive alleles of one of the parents are complemented in the F1 hybrid by the dominant alleles of the other parent. As per over dominance, the heterozygous combination of alleles at a locus is higher to either of the two possible homozygous combinations. Whereas, epistasis assumes that epistatic interactions between different loci are the reason for heterosis (Goodnight and Pandy, 1999). It played a great role in improving different economical important crops including Brassica (Riaz et al., 2013). Keeping in view the current situation of seed yield and demand for edible oil in the country and role of rapeseed in the domestic production a research was conducted to determine genetic variability and heterosis for maturity, morphological and yield related traits.
Materials and Methods
The research was conducted during 2014/15 at the University of Agriculture Peshawar Pakistan. The experimental material comprising eight diverse rapeseed genotypes namely AUP-01, AUP-05, AUP-07, AUP-08, AUP-10, AUP-13, AUP-18 and AUP-21. These genotypes were crossed in a complete diallel fashion to produce 56 F1 hybrids (28 direct and 28 reciprocal). A set of 56 F1 hybrids and eight parental lines were evaluated in RCBD (randomized complete block design) with 3 replications during 2015/16. Each replication comprised of 64 sub plots (8 parents and 56 crosses) and each sub plot had three rows of each five meter long whereas, rows were kept 50 cm apart and plant to plant distance was maintained 30 cm. To reduce the environmental effects to maximum possible extent all recommended cultural practices were uniformly applied to all the entries.
Data recording and statistical analysis
Data related to days to maturity was noted on plot basis while, for remaining traits data was recorded on ten randomly selected plants in each entry at appropriate time during crop season. The recorded data were subjected to analysis of variance to test the null hypothesis of no differences among various parental lines and their F1 hybrids Steel et al. (1980).
Estimation of heterosis
The best parent (heterobeltiosis) and commercial heterosis were estimated as per formula given by Falconer and Mackay (1996) as follows.

For commercial heterosis, commercial variety, NIFA-Gold was used as check cultivar. The hybrid performance was compared with that of the commercial variety (C.V.) expressed in percentage and was calculated as under:
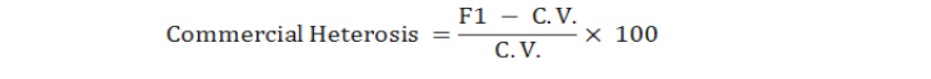
Test of significance for heterosis
The significance of best-parent and commercial heterosis was evaluated by ‘t’ test as reported by Wynne et al. (1970).
‘t’ for best parent heterosis
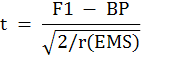
‘t’ for commercial heterosis
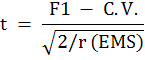
Where;
F1 = F1 cross mean; BP = value of best parent; C.V. = value of commercial variety; r = no of replication; EMS = error mean square.
Results and Discussion
Days to maturity
In Pakistan, rapeseed is generally harvested from early April to early May. Delayed maturity and harvesting exposes crop to forthcoming heat stress which resulting in heavy losses both in quantity and quality. Therefore, early maturity has prime importance in rapeseed breeding programs. Mean squares showed significant (P≤0.01) differences for days to maturity among this set of rapeseed genotypes (Table 1). Among parents, maturity ranged from 167 to 182 days with an average of 175 days where parental genotype AUP-21 matured earlier while AUP-01 and AUP-07 took maximum days to mature. Among F1 hybrids, maturity ranged from 162 to 184 days with an average of 174 days (Table 2). Cross AUP-05 × AUP-01 matured earliest while AUP-18 × AUP-10 took maximum days to mature. The current study revealed that rapeseed genotypes (parents and crosses) matured in wide span of time signifying the presence of sufficient genetic variability for maturity. Therefore, selection will be effective criterion to selecting early maturing genotype for further improvement. Our findings are supported by the results of Gul et al. (2018a, b), Naheed et al. (2017) and Ishaq and Raziuddin (2016) who found significant genetic variability for maturity in rapeseed genotypes. Similar results were recorded by Chaurasiya et al. (2018), Bibi et al. (2016), Synrem et al. (2015) and Arifullah et al. (2012) for days to maturity in Indian mustard.
In rapeseed, delayed maturity exposes crop to heat stress which damages seed and oil quality resultantly produces lower yields. Therefore, early maturing genotypes with negative heterotic effects are preferred. Current results revealed significant negative heterosis over best-parent for 17 crosses where values ranged from -4.66 to -11.14. Significant and higher negative best parent heterosis was recorded for cross AUP-05 × AUP-01. Concerning commercial heterosis, 13 out of 56 crosses revealed significant negative effects where values ranged from -4.79 to -8.73. The desirable maximum negative commercial heterosis was recorded for cross AUP-05 × AUP-01 (Table 4). Hybrids attaining significant negative heterosis for maturity may have potential for producing high-yielding single-cross hybrids of rapeseed. These results are in line with the findings of Gul et al. (2018a) and Rameeh (2017) who found desirable best parent heterosis for days to maturity in rapeseed. Meena et al. (2014) and Turi et al. (2006) also found similar results for maturity in Indian mustards L. Synrem et al. (2015) identified some F1 crosses with significant commercial heterosis in Indian mustard.
Table 1: Mean squares of morphological, yield and yield related traits in 8 × 8 F1 diallel crosses of rapeseed.
| Mean Squares | ||||
| Trait | Genotype (df=63) | Replication (df=2) | Error (df=126) | CV (%) |
| Days to maturity | 73.62** | 54.396 | 38.105 | 3.545 |
|
Primary branches plant-1 |
7.26** | 0.646 | 0.297 | 9.969 |
|
Pods main raceme-1 |
379.01** | 6.750 | 23.279 | 8.876 |
| 1000-seed weight | 1.82** | 0.538 | 0.325 | 9.545 |
|
Seed yield plant-1 |
36.13** | 4.501 | 3.796 | 7.173 |
** Significant at P≤0.01; df= degree of freedom.
Primary branches plant-1
Highly significant (P≤0.01) differences were observed for primary branches plant-1 in this set of rapeseed genotypes (Table 1). Among parents, branches plant-1 varied from 3.0 to 6.7 with an average of 4.8 branches where AUP-18 produced maximum while AUP-05 produced minimum branches. Among F1 crosses, primary branches plant-1 ranged from 3.3 to 9.0 with an average of 5.6 branches where, AUP-21 × AUP-08 and AUP-10 × AUP-07 produced minimum and maximum branches, respectively (Table 2). Branches plant-1 indirectly plays a positive role in biological and seed yield therefore, it is considered as an important parameter in rapeseed breeding programs. Current results revealed considerable variability among tested rapeseed genotypes for branches plant-1 indicating the potential for further improvement through selection. These results are in agreement to the results of Gul et al. (2018 a, b), Ishaq and Raziuddin (2016) and Ali et al. (2015) who also found highly significant variability for branches plant-1 in rapeseed genotypes. Similarly, significant variability was also reported for primary
Table 2: Mean performance of the parents, their F1 hybrids and commercial variety for days to maturity, primary branches plant-1 and pods main raceme in 8 × 8 diallel crosses of rapeseed.
| Parent/cross | Days to maturity |
Primary branches Plant-1 |
Pods main raceme-1 |
Crosses | Days to maturity |
Primary branches Plant-1 |
Pods main raceme-1 |
| AUP-01 | 182 | 5.0 | 61.0 | AUP-08 × AUP-18 | 171 | 7.3 | 44.7 |
| AUP-05 | 171 | 3.0 | 53.7 | AUP-08 × AUP-21 | 168 | 3.3 | 44.0 |
| AUP-07 | 182 | 5.7 | 40.3 | AUP-10 × AUP-01 | 180 | 7.3 | 47.7 |
| AUP-08 | 174 | 4.0 | 37.7 | AUP-10 × AUP-05 | 177 | 6.0 | 47.3 |
| AUP-10 | 175 | 3.3 | 45.7 | AUP-10 × AUP-07 | 168 | 9.0 | 43.3 |
| AUP-13 | 171 | 4.3 | 35.3 | AUP-10 × AUP-08 | 176 | 7.7 | 52.3 |
| AUP-18 | 179 | 6.7 | 58.7 | AUP-10 × AUP-13 | 174 | 8.0 | 66.0 |
| AUP-21 | 167 | 6.3 | 65.3 | AUP-10 × AUP-18 | 177 | 7.7 | 53.3 |
| AUP-01 × AUP-05 | 171 | 5.0 | 55.0 | AUP-10 × AUP-21 | 168 | 6.7 | 30.7 |
| AUP-01 × AUP-07 | 181 | 6.3 | 45.7 | AUP-13 × AUP-01 | 169 | 4.7 | 46.3 |
| AUP-01 × AUP-08 | 177 | 3.7 | 41.7 | AUP-13 × AUP-05 | 176 | 7.7 | 44.7 |
| AUP-01 × AUP-10 | 180 | 6.0 | 52.0 | AUP-13 × AUP-07 | 174 | 8.3 | 60.3 |
| AUP-01 × AUP-13 | 173 | 4.3 | 72.0 | AUP-13 × AUP-08 | 175 | 4.7 | 53.7 |
| AUP-01 × AUP-18 | 171 | 5.7 | 75.7 | AUP-13 × AUP-10 | 171 | 4.0 | 52.7 |
| AUP-01 × AUP-21 | 174 | 3.7 | 44.7 | AUP-13 × AUP-18 | 173 | 5.0 | 64.3 |
| AUP-05 × AUP-01 | 162 | 7.7 | 52.3 | AUP-13 × AUP-21 | 177 | 5.0 | 47.0 |
| AUP-05 × AUP-07 | 181 | 6.3 | 72.0 | AUP-18 × AUP-01 | 167 | 3.7 | 44.0 |
| AUP-05 × AUP-08 | 171 | 6.0 | 66.7 | AUP-18 × AUP-05 | 179 | 7.0 | 66.0 |
| AUP-05 × AUP-10 | 166 | 5.7 | 76.0 | AUP-18 × AUP-07 | 172 | 5.0 | 68.7 |
| AUP-05 × AUP-13 | 178 | 6.7 | 74.7 | AUP-18 × AUP-08 | 169 | 6.3 | 44.3 |
| AUP-05 × AUP-18 | 177 | 5.0 | 54.0 | AUP-18 × AUP-10 | 184 | 8.3 | 64.7 |
| AUP-05 × AUP-21 | 177 | 4.3 | 49.3 | AUP-18 × AUP-13 | 167 | 6.3 | 71.0 |
| AUP-07 × AUP-01 | 166 | 5.3 | 59.0 | AUP-18 × AUP-21 | 175 | 6.7 | 71.0 |
| AUP-07 × AUP-05 | 173 | 6.7 | 49.7 | AUP-21 × AUP-01 | 166 | 3.7 | 56.7 |
| AUP-07 × AUP-08 | 176 | 3.3 | 50.0 | AUP-21 × AUP-05 | 175 | 5.3 | 43.3 |
| AUP-07 × AUP-10 | 178 | 3.3 | 46.3 | AUP-21 × AUP-07 | 178 | 5.0 | 64.0 |
| AUP-07 × AUP-13 | 172 | 3.3 | 49.0 | AUP-21 × AUP-08 | 167 | 3.3 | 46.0 |
| AUP-07 × AUP-18 | 176 | 6.0 | 52.7 | AUP-21 × AUP-10 | 179 | 5.0 | 47.7 |
| AUP-07 × AUP-21 | 176 | 3.7 | 68.3 | AUP-21 × AUP-13 | 176 | 4.0 | 58.0 |
| AUP-08 × AUP-01 | 175 | 7.7 | 78.3 | AUP-21 × AUP-18 | 181 | 3.7 | 56.3 |
| AUP-08 × AUP-05 | 176 | 5.7 | 45.0 | Commercial variety | 177 | 5.5 | 61.2 |
| AUP-08 × AUP-07 | 167 | 4.0 | 41.7 | Parental mean | 175.1 | 4.8 | 49.7 |
| AUP-08 × AUP-10 | 180 | 4.0 | 64.3 |
F1 mean |
174.0 | 5.6 | 55.0 |
| AUP-08 × AUP-13 | 181 | 6.3 | 45.3 | LSD(0.05) | 171 | 0.9 | 7.8 |
branches plant-1 in Indian mustard (Chaurasiya et al., 2018; Dawar et al., 2018; Bibi et al., 2016) in turnip rape (Iqbal et al., 2014; Nasim et al., 2014) and Ethiopian mustard (Nausheen et al., 2015; Ali et al., 2013).
For increased seed yield, robust plants having greater number of primary branches are desired in rapeseed thus, positive heterotic effects are preferred. Analysis of best parent heterosis presented desired significant positive effects for 18 crosses where effect ranged from 14.43 to 91.70%. Significant and higher heterotic effect over best parent was recorded for cross AUP-10 × AUP-08. Significant positive commercial heterosis was recorded for 21 crosses where values ranged from 15.15 to 63.64%. Among these crosses, AUP-10 × AUP-07 had the highest heterotic effects (Table 4). Earlier, Gupta and Lal (2011) and Nassimi et al. (2006) also
Table 3: Mean performance of the parents, their F1 hybrids and commercial variety for 1000-seed weight (g) and seed yield plant-1 (g) in 8 × 8 diallel crosses of rapeseed.
| Parent/cross | 1000-seed weight |
Seed yield plant-1 |
Crosses | 1000-seed weight |
Seed yield plant-1 |
| AUP-01 | 6.5 | 27.8 | AUP-08 × AUP-18 | 6.0 | 32.0 |
| AUP-05 | 6.1 | 24.1 | AUP-08 × AUP-21 | 5.8 | 22.4 |
| AUP-07 | 4.9 | 23.3 | AUP-10 × AUP-01 | 6.2 | 26.9 |
| AUP-08 | 4.5 | 22.3 | AUP-10 × AUP-05 | 5.7 | 26.1 |
| AUP-10 | 5.1 | 25.9 | AUP-10 × AUP-07 | 5.5 | 27.1 |
| AUP-13 | 4.9 | 20.6 | AUP-10 × AUP-08 | 5.4 | 24.4 |
| AUP-18 | 6.1 | 25.2 | AUP-10 × AUP-13 | 6.3 | 29.0 |
| AUP-21 | 7.1 | 30.6 | AUP-10 × AUP-18 | 8.5 | 36.5 |
| AUP-01 × AUP-05 | 6.9 | 28.8 | AUP-10 × AUP-21 | 5.4 | 28.7 |
| AUP-01 × AUP-07 | 5.6 | 34.2 | AUP-13 × AUP-01 | 5.5 | 23.4 |
| AUP-01 × AUP-08 | 5.1 | 22.1 | AUP-13 × AUP-05 | 5.3 | 27.4 |
| AUP-01 × AUP-10 | 6.4 | 23.7 | AUP-13 × AUP-07 | 4.8 | 21.9 |
| AUP-01 × AUP-13 | 6.1 | 29.0 | AUP-13 × AUP-08 | 5.2 | 25.3 |
| AUP-01 × AUP-18 | 5.1 | 31.7 | AUP-13 × AUP-10 | 5.3 | 24.8 |
| AUP-01 × AUP-21 | 5.4 | 29.3 | AUP-13 × AUP-18 | 5.5 | 28.8 |
| AUP-05 × AUP-01 | 6.8 | 25.8 | AUP-13 × AUP-21 | 6.4 | 23.6 |
| AUP-05 × AUP-07 | 7.3 | 32.4 | AUP-18 × AUP-01 | 5.8 | 25.0 |
| AUP-05 × AUP-08 | 6.1 | 26.4 | AUP-18 × AUP-05 | 7.3 | 35.0 |
| AUP-05 × AUP-10 | 5.4 | 28.6 | AUP-18 × AUP-07 | 6.5 | 33.3 |
| AUP-05 × AUP-13 | 7.7 | 31.9 | AUP-18 × AUP-08 | 5.9 | 25.5 |
| AUP-05 × AUP-18 | 7.0 | 29.6 | AUP-18 × AUP-10 | 6.3 | 28.8 |
| AUP-05 × AUP-21 | 5.4 | 25.5 | AUP-18 × AUP-13 | 6.0 | 31.4 |
| AUP-07 × AUP-01 | 5.9 | 23.9 | AUP-18 × AUP-21 | 6.3 | 30.1 |
| AUP-07 × AUP-05 | 5.9 | 24.3 | AUP-21 × AUP-01 | 5.4 | 27.7 |
| AUP-07 × AUP-08 | 5.6 | 25.3 | AUP-21 × AUP-05 | 6.3 | 28.9 |
| AUP-07 × AUP-10 | 6.3 | 25.2 | AUP-21 × AUP-07 | 5.5 | 25.5 |
| AUP-07 × AUP-13 | 6.6 | 26.9 | AUP-21 × AUP-08 | 5.2 | 25.5 |
| AUP-07 × AUP-18 | 5.6 | 26.3 | AUP-21 × AUP-10 | 5.4 | 27.0 |
| AUP-07 × AUP-21 | 6.1 | 28.9 | AUP-21 × AUP-13 | 6.7 | 30.2 |
| AUP-08 × AUP-01 | 7.8 | 33.0 | AUP-21 × AUP-18 | 5.8 | 25.1 |
| AUP-08 × AUP-05 | 5.1 | 24.6 | Commercial variety | 6.4 | 34.5 |
| AUP-08 × AUP-07 | 6.9 | 23.8 | Parental mean | 5.6 | 25.0 |
| AUP-08 × AUP-10 | 5.5 | 26.3 |
F1 mean |
6.0 | 27.5 |
| AUP-08 × AUP-13 | 6.4 | 23.8 | LSD(0.05) | 0.9 | 3.1 |
identified several crosses with significant positive mid and best parent heterosis in rapeseed for branches plant-1. Significant positive commercial heterosis for primary branches has also been recorded in Indian mustard genotypes (Synrem et al., 2015) and in Eruca sativa (Mundiyara and Jakhar, 2017). Desired mid and high parent heterosis for branches plant-1 have been reported in turnip rape (Dar et al., 2011) and Indian mustard (Turi et al., 2006). However, non-significant positive heterotic effects over mid and best parent have been reported in some rapeseed genotypes (Gupta et al., 2006).
Pods main raceme-1
In rapeseed, the final yield is highly influenced by number of pods main raceme-1 therefore, having more pods main raceme-1 result in greater seed yield. In current experiment, genotypes revealed highly significant (P≤0.01) differences for pods main raceme-1 demonstrating the presence of adequate genetic
Table 4: Best parent heterosis (BP %) and commercial heterosis (CH %) for days to maturity, primary branches plant-1and pods main receme-1 in 8 × 8 F1 diallel crosses of rapeseed.
| Days to maturity |
Primary branches plant-1 |
Pods main raceme-1 |
||||
| Hybrids | BP | CH | BP | CH | BP | CH |
| AUP-01 × AUP-05 | -6.20* | -3.66 | 0.00 | -9.09 | -9.84 | -10.13* |
| AUP-01 × AUP-07 | -0.90 | 1.78 | 11.11 | 15.15* | -25.14** | -25.38** |
| AUP-01 × AUP-08 | -2.72 | -0.09 | -26.67** | -33.33** | -31.69** | -31.92** |
| AUP-01 × AUP-10 | -1.26 | 1.41 | 20.00* | 9.09 | -14.75* | -15.03** |
| AUP-01 × AUP-13 | -5.28* | -2.72 | -13.33 | -21.21** | 18.03** | 17.65** |
| AUP-01 × AUP-18 | -6.38* | -3.85 | -15.42** | 3.03 | 24.04** | 23.64** |
| AUP-01 × AUP-21 | -4.37 | -1.78 | -41.80** | -33.33** | -31.60** | -27.02** |
| AUP-05 × AUP-01 | -11.14* | -8.73** | 53.33** | 39.39** | -14.21* | -14.49** |
| AUP-05 × AUP-07 | -0.37 | 2.16 | 11.11 | 15.15* | 34.08** | 17.65** |
| AUP-05 × AUP-08 | -1.55 | -3.66 | 50.00** | 9.09 | 24.15** | 8.93 |
| AUP-05 × AUP-10 | -4.98* | -6.48** | 71.72** | 3.03 | 41.53** | 24.18** |
| AUP-05 × AUP-13 | 3.91 | 0.28 | 55.04** | 21.21** | 39.04** | 22.00** |
| AUP-05 × AUP-18 | -1.30 | -0.47 | -25.37** | -9.09 | -8.01 | -11.76* |
| AUP-05 × AUP-21 | 3.52 | -0.09 | -31.22** | -21.21** | -24.45** | -19.39** |
| AUP-07 × AUP-01 | -9.12** | -6.67** | -6.43 | -3.03 | -3.28 | -3.59 |
| AUP-07 × AUP-05 | -4.92* | -2.35 | 16.96* | 21.21** | -7.51 | -18.85** |
| AUP-07 × AUP-08 | -3.48 | -1.03 | -41.52** | -39.39** | 24.07** | -18.30** |
| AUP-07 × AUP-10 | -2.01 | 0.47 | -41.52** | -39.39** | 1.39 | -24.29** |
| AUP-07 × AUP-13 | -5.31* | -2.91 | -41.52** | -39.39** | 21.59* | -19.93** |
| AUP-07 × AUP-18 | -3.30 | -0.85 | -10.45 | 9.09 | -10.28 | -13.94** |
| AUP-07 × AUP-21 | -3.11 | -0.66 | -41.80** | -33.33** | 4.65 | 11.66* |
| AUP-08 × AUP-01 | -4.00 | -1.41 | 53.33** | 39.39** | 28.42** | 28.00** |
| AUP-08 × AUP-05 | 1.32 | -0.85 | 41.67** | 3.03 | -16.20* | -26.47** |
| AUP-08 × AUP-07 | -8.42** | -6.10** | -29.82** | -27.27** | 3.39 | -31.92** |
| AUP-08 × AUP-10 | 2.84 | 1.22 | 0.00 | -27.27** | 40.77** | 5.12 |
| AUP-08 × AUP-13 | 4.01 | 1.78 | 47.29** | 15.15* | 21.54* | -25.93** |
| AUP-08 × AUP-18 | -4.66* | -3.85 | 9.45 | 33.33** | -23.91** | -27.02** |
| AUP-08 × AUP-21 | -3.47 | -5.54* | -47.09** | -39.39** | -32.62** | -28.10** |
| AUP-10 × AUP-01 | -1.26 | 1.41 | 46.67** | 33.33** | -21.86** | -22.11** |
| AUP-10 × AUP-05 | 1.13 | -0.47 | 81.82** | 9.09 | -11.86 | -22.66** |
| AUP-10 × AUP-07 | -7.51** | -5.16* | 57.89** | 63.64** | -5.18 | -29.19** |
| AUP-10 × AUP-08 | 0.93 | -0.66 | 91.67** | 39.39** | 14.51* | -14.49** |
| AUP-10 × AUP-13 | -0.21 | -1.78 | 86.05** | 45.45** | 44.42** | 7.84 |
| AUP-10 × AUP-18 | -1.30 | -0.47 | 14.43* | 39.39** | -9.14 | -12.85* |
| AUP-10 × AUP-21 | -3.64 | -5.16* | 5.82 | 21.21** | -53.04** | -49.89** |
| AUP-13 × AUP-01 | -7.30** | -4.79* | -6.67 | -15.15* | -24.04** | -24.29** |
| AUP-13 × AUP-05 | 2.94 | -0.66 | 78.29** | 39.39** | -16.82* | -27.02** |
| AUP-13 × AUP-07 | -4.21 | -1.78 | 46.20** | 51.52** | 49.71** | -1.42 |
| AUP-13 × AUP-08 | 0.75 | -1.41 | 8.53 | -15.15* | 42.35** | -12.31* |
| AUP-13 × AUP-10 | -1.93 | -3.47 | -6.98 | -27.27** | 15.24* | -13.94** |
| AUP-13 × AUP-18 | -3.54 | -2.72 | -25.37** | -9.09 | 9.60 | 5.12 |
| AUP-13 × AUP-21 | 3.50 | -0.47 | -20.63** | -9.09 | -28.02** | -23.20** |
| AUP-18 × AUP-01 | -8.39** | -5.92** | -45.27** | -33.33** | -27.87** | -28.10** |
| AUP-18 × AUP-05 | -0.19 | 0.66 | 4.48 | 27.27** | 12.44* | 7.84 |
| AUP-18 × AUP-07 | -5.49* | -3.10 | -25.37** | -9.09 | 16.98** | 12.20* |
| AUP-18 × AUP-08 | -5.59* | -4.79* | -5.47 | 15.15* | -24.47** | -27.56** |
| AUP-18 × AUP-10 | 2.79 | 3.66 | 24.38** | 51.52** | 10.16 | 5.66 |
| AUP-18 × AUP-13 | -6.52* | -5.73* | -5.47 | 15.15* | 20.95** | 16.01** |
| AUP-18 × AUP-21 | -2.23 | -1.41 | -0.50 | 21.21** | 8.73 | 16.01** |
| AUP-21 × AUP-01 | -8.76** | -6.29** | -41.80** | -33.33** | -13.22* | -7.41 |
| AUP-21 × AUP-05 | 1.97 | -1.60 | -15.34* | -3.03 | -33.64** | -29.19** |
| AUP-21 × AUP-07 | -2.20 | 0.28 | -20.63** | -9.09 | -1.99 | 4.58 |
| AUP-21 × AUP-08 | -4.05 | -6.10** | -47.09** | -39.39** | -29.56** | -24.84** |
| AUP-21 × AUP-10 | 2.27 | 0.66 | -20.63** | -9.09 | -27.00** | -22.11** |
| AUP-21 × AUP-13 | 3.10 | -0.85 | -36.51** | -27.27** | -11.18* | -5.23 |
| AUP-21 × AUP-18 | 0.93 | 1.78 | -45.27** | -33.33** | -13.73* | -7.95 |
**, *: Significant at 1% and 5% level of probability, respectively.
Table 5: Best parent heterosis (BP %) and commercial heterosis (CH %) for 1000-seed weight and seed yield plant-1 in 8 × 8 F1 diallel crosses of rapeseed.
| Hybrids | 1000-seed weight |
Seed yield plant-1 |
||
| BP | CH | BP | CH | |
| AUP-01 × AUP-05 | 6.15 | 1.56 | 3.47 | -16.52** |
| AUP-01 × AUP-07 | -13.85* | -4.69 | 23.13** | -0.87 |
| AUP-01 × AUP-08 | -22.05** | -23.44** | -20.50** | -35.94** |
| AUP-01 × AUP-10 | -1.54 | -29.69** | -14.92** | -31.30** |
| AUP-01 × AUP-13 | -6.67 | -20.31** | 4.18 | -15.94** |
| AUP-01 × AUP-18 | -22.05** | -23.44** | 14.03** | -8.12* |
| AUP-01 × AUP-21 | -23.94** | -4.69 | -4.14 | -15.07** |
| AUP-05 × AUP-01 | 4.62 | 10.94 | -7.21 | -25.22** |
| AUP-05 × AUP-07 | 19.71** | 7.81 | 34.63** | -6.09* |
| AUP-05 × AUP-08 | -0.05 | -12.50* | 9.68 | -23.48** |
| AUP-05 × AUP-10 | -10.49 | -20.31** | 10.42* | -17.10** |
| AUP-05 × AUP-13 | 26.30** | 0.00 | 32.37** | -7.54* |
| AUP-05 × AUP-18 | 15.30* | -4.69 | 17.59** | -14.20** |
| AUP-05 × AUP-21 | -24.41** | -20.31** | -16.63** | -26.09** |
| AUP-07 × AUP-01 | -8.72 | -15.63* | -13.90** | -30.72** |
| AUP-07 × AUP-05 | -3.35 | 6.25 | 0.62 | -29.57** |
| AUP-07 × AUP-08 | 13.61 | 14.06* | 8.57 | -26.67** |
| AUP-07 × AUP-10 | 23.53** | -4.69 | -2.59 | -26.96** |
| AUP-07 × AUP-13 | 36.21** | -15.63* | 15.48* | -22.03** |
| AUP-07 × AUP-18 | -7.65 | 20.31** | 4.22 | -23.77** |
| AUP-07 × AUP-21 | -14.55* | 9.37 | -5.50 | -16.23** |
| AUP-08 × AUP-01 | 19.49** | -15.63* | 18.73** | -4.35 |
| AUP-08 × AUP-05 | -15.43* | -7.81 | 2.06 | -28.70** |
| AUP-08 × AUP-07 | 40.82** | -7.81 | 1.96 | -31.01** |
| AUP-08 × AUP-10 | 8.50 | -12.50* | 1.57 | -23.77** |
| AUP-08 × AUP-13 | 30.61** | -1.56 | 6.89 | -31.01** |
| AUP-08 × AUP-18 | -1.64 | 3.12 | 26.94** | -7.25* |
| AUP-08 × AUP-21 | -18.78** | -12.50* | -26.86** | -35.07** |
| AUP-10 × AUP-01 | -4.62 | -4.69 | -3.42 | -22.03** |
| AUP-10 × AUP-05 | -6.10 | 21.88** | 0.72 | -24.35** |
| AUP-10 × AUP-07 | 7.19 | -20.31** | 4.54 | -21.45** |
| AUP-10 × AUP-08 | 5.88 | 7.81 | -5.91 | -29.28** |
| AUP-10 × AUP-13 | 24.18** | -14.06* | 11.84* | -15.94** |
| AUP-10 × AUP-18 | 39.34** | 0.00 | 40.93** | 5.80* |
| AUP-10 × AUP-21 | -24.41** | -6.25 | -6.21 | -16.81** |
| AUP-13 × AUP-01 | -14.87* | -9.38 | -15.89** | -32.17** |
| AUP-13 × AUP-05 | -12.14 | -3.13 | 13.55* | -20.58** |
| AUP-13 × AUP-07 | -1.36 | -10.94 | -6.17 | -36.52** |
| AUP-13 × AUP-08 | 6.80 | -14.06* | 13.24* | -26.67** |
| AUP-13 × AUP-10 | 3.27 | -15.63* | -4.38 | -28.12** |
| AUP-13 × AUP-18 | -9.29 | -1.56 | 14.42* | -16.52** |
| AUP-13 × AUP-21 | -10.33 | 32.81** | -22.80** | -31.59** |
| AUP-18 × AUP-01 | -11.28 | -15.63* | -9.96* | -27.54** |
| AUP-18 × AUP-05 | 19.13** | -14.06* | 39.03** | 1.45 |
| AUP-18 × AUP-07 | 7.10 | -17.19** | 32.17** | -3.48 |
| AUP-18 × AUP-08 | -3.28 | -25.00** | 1.20 | -26.09** |
| AUP-18 × AUP-10 | 2.73 | -18.75** | 11.24* | -16.52** |
| AUP-18 × AUP-13 | -1.09 | -17.19** | 24.48** | -8.99* |
| AUP-18 × AUP-21 | -11.74* | -14.06* | -1.53 | -12.75** |
| AUP-21 × AUP-01 | -23.94** | 0.00 | -9.51* | -19.71** |
| AUP-21 × AUP-05 | -10.80* | -9.38 | -5.47 | -16.23** |
| AUP-21 × AUP-07 | -22.54** | 14.06* | -16.51** | -26.09** |
| AUP-21 × AUP-08 | -27.23** | 1.56 | -16.66** | -26.09** |
| AUP-21 × AUP-10 | -24.41** | -7.81 | -11.73* | -21.74** |
| AUP-21 × AUP-13 | -6.10 | -1.56 | -1.34 | -12.46** |
| AUP-21 × AUP-18 | -18.31** | -6.25 | -18.02** | -27.25** |
**, *: Significant at 1% and 5% level of probability, respectively.
variability in this set of rapeseed genotypes (Table 1). Means values for pods main raceme-1 ranged from 35.3 to 65.3 for parental genotypes with an average value of 49.7 where maximum pods main raceme-1 were produced by AUP-21 while, minimum were recorded for genotype AUP-13. Among F1 crosses, data ranged from 30.7 to 78.3 with an average value of 55.0 pods main raceme-1 where, minimum and maximum values were noted for F1 crosses, AUP-10 × AUP-21 and AUP-08 × AUP-01, respectively (Table 2). These parental lines along with their F1 crosses yielding more pods main raceme-1 will be effective material for future rapeseed breeding program. Our findings are analogous to those of Gul et al. (2018 a, b), Naheed et al. (2017), Sincik et al. (2014) and Rameeh et al. (2003) who reported considerable variations for pods main raceme-1 in rapeseed genotypes. Similarly, Nasim et al. (2014) recorded significant variability for pods main raceme-1 in some turnip rape genotypes.
In rapeseed, positive heterosis effect is preferred for pods main raceme-1. Estimates of heterosis over best parent identified 19 crosses having significant positive effects where data ranged from 12.4 to 49.7%. Among these crosses, AUP-13 × AUP-07 showed highest heterosis over best parent. Among 56 crosses, 10 crosses revealed significant positive commercial heterosis ranging from 11.6 to 28.0% where highest heterosis was recorded for cross, AUP-08 × AUP-01 (Table 4). Our results are in line with the earlier findings of Ahsan et al. (2013) and Nassimi et al. (2006) who also reported significant mid and best parent heterotic effects for pods main raceme-1 in rapeseed. Significant positive commercial heterosis for pods main raceme-1 have also been reported in Indian mustard (Synrem et al., 2015; Meena et al., 2014) and turnip rape (Dar et al., 2011).
1000-Seed weight
In rapeseed, 1000-seed weight is the main yield contributing trait and influence final yield therefore; most of the rapeseed breeders are interested in heavier seeds. Highly significant (P≤0.01) differences were revealed for 1000-seed weight (Table 1) indicating the broad baseness of the tested material. Among parental lines, mean values for 1000-seed weight varied from 4.5 (AUP-08) to 7.1 g (AUP-21) with an average value of 5.6 g. Data for F1 hybrids ranged from 4.8 to 8.5 g with an average value of 6.01 g where the lowest and highest values were recorded for crosses, AUP-13 × AUP-07 and AUP-10 × AUP-18, respectively (Table 3). The best performing parental genotypes with their F1 hybrids will be helpful in the selection of high yielding genotypes in upcoming breeding progrms. Recently, Sohail et al. (2018), Kang et al. (2014), Muhammad et al. (2014), Ahmad et al. (2013) and Sadat et al. (2010) also reported highly significant genetic variability for 1000-seed weight among rapeseed genotypes. Further, Chaurasiya et al. (2018), Dawar et al. (2018) and Bibi et al. (2016) reported considerable differences for the studied trait in Indian mustard genotypes.
For 1000-seed weight, positive heterotic effect is desired. Estimates of heterosis over best parent identified 11 crosses with significant positive heterotic effects where data ranged from 15.30 to 40.82%. Among these crosses, hybrid combinations AUP-08 × AUP-07 and AUP-10 × AUP-18 (39.34%) had maximum effects. For commercial heterosis, 05 cross combinations showed significant positive heterotic effects for 1000-seed weight where data ranged from 14.06 to 32.81%. The highest commercial heterosis was expressed for cross, AUP-13 × AUP-21 (Table 5). These results were similar to earlier findings of Rameeh (2011, 2003) who reported both positive and negative best parent heterosis in rapeseed for 1000-seed weight. Similarly, Dar et al. (2011) and Singh (2005) also revealed significant positive best and mid parent heterosis for several crosses in turnip rape and Indian mustard, respectively. Further, Meena et al. (2014) revealed positive commercial heterosis while Gupta and Lal (2011) reported significant positive heterosis for mid and best parent in Indian mustard for 1000-seed weight.
Seed yield plant-1
Rapeseed breeding programs mostly focus on increased seed yield as well as oil content. Mean square values presented highly significant (P≤0.01) differences for seed yield among studied rapeseed genotypes (Table 1). Among parental genotypes, seed yield varied from 20.6 (AUP-13) to 30.6 g (AUP-21) with an average of 25.0 g. Data for F1 hybrids ranged from 21.9 to 36.5 g with an average value of 27.5 g where minimum seed yield plant-1 was attained by cross, AUP-13 × AUP-07 while AUP-10 × AUP-18 attained the maximum seed yield plant-1 (Table 3). These results showed that the studied genotypes have considerable variability which could useful to produce high yielding lines. Similarly, significant differences among rapeseed for seed yield plant-1 have been reported by Gul et al. (2018 a, b), Sohail et al. (2018), Khalil and Raziuddin (2017) and Synrem et al. (2014). Further, Chaurasiya et al. (2018), Dawar et al. (2018) and Bibi et al. (2016) also recorded highly significant variability for seed yield in Indian mustard.
Greater seed yield is the main objective of majority of rapeseed breeding programs therefore; positive heterosis is desirable for seed yield plant-1. In current study, estimates of heterosis over best parent identified 18 crosses with significant positive heterotic effects for seed yield where values ranged from 10.42 to 40.93%. Maximum heterosis was expressed by hybrid, AUP-10 × AUP-18 followed by AUP-18 × AUP-05 (39.0%). For commercial heterosis, only AUP-10 × AUP-18 revealed significant positive heterotic effect (5.80%) (Table 5). Top ranking crosses could be an asset for breeding high yielding genotypes. Our results are supported by Gul et al. (2018a), Liton et al. (2017) and Verma et al. (2011) who also reported several crosses with significant positive heterosis for seed yield in rapeseed genotypes. Tanaka and Niikura (2006) reported best-parent heterotic effect for seed yield in rapeseed. Similarly, Gami and Chauhan (2014) identified several hybrids with significant positive heterobeltiosis for seed yield in Indian mustards whereas, Mundiyara and Jakhar (2017) found commercial heterosis for seed yield in Eruca sativa.
Conclusions and Recommendations
The analysis of variance revealed highly significant differences signifying the prevalence of sufficient genetic variability for the studied traits in rapeseed genotypes. Mean performance identified AUP-21 as the best parent for most of the important traits. Whereas, among F1 crosses, AUP-05×AUP-01 and AUP-10×AUP-18, performed better and showed desirable heterotic effects for maturity and seed yield plant-1. Therefore, these Parental lines and crosses could be an asset for rapeseed breeding programs and could be exploited for developing superior lines.
Author’s contribution
Wajid Khan designed, conducted research, collected, analyzed data and prepared rough draft of the manuscript. Raziuddin supervised all the research activities and made final draft and reviewed it.
Acknowledgement
The authors are thankful to financial support of Higher Education Commission Pakistan through indigenous 5000 PhD fellowship program.
Novelty Statement
This research will be helpful for breeders to develop hybrids with better seed yield and oil quality traits through heterosis breed-ing.
References
Ahmad, B., S. Mohammad, F. Azam, I. Ali, J. Ali and S. Rehman. 2013. Studies of genetic variability, heritability and phenotypic correlations of some qualitative traits in advance mutant lines of rapeseed. Amer. Eur. J. Agric. Enviro. Sci., 13(4): 531-538.
Ahsan, M.Z., F.A. Khan, S.A. Kang and K. Rasheed. 2013. Combining ability and heterosis analysis for seed yield and yield components in B. napus L. J. Biol. Agric. Health. 9(3): 31-36.
Ali, N., J. Bakht, K. Naveed, S. Ali, M. Khan and M. Salim. 2015. Heterosis for some fatty acids composition of Indian mustard. J. Anim. Plant Sci. 25(3): 587-592.
Ali, Y., H. Farhatullah, A. Rahman, S.M. Nasim, Azam and A. Khan. 2013. Heritability and correlation analysis for morphological and biochemical traits in Brassica carinata L. Sarhad J. Agric. 29(3): 359-370.
Amjad, M. 2014. Oilseed crops of Pakistan (status paper). PARC-Islamabad: 1-40. https://doi.org/10.18356/a55dee27-en
Arifullah, M., M.M. Abid, S. Mahmood and G. Shabbir. 2012. Combining ability of some yield attributes in Indian mustard. Pak. J. Agric. Res. 25(2): 104-109.
Bibi, T., S. Rauf, T. Mahmood, Z. Haider and Salahuddin. 2016. Genetic variability and heritability studies in relation to seed yield and its component traits in mustard (Brassica juncea L.). Acad. J. Agric. Res. 4(8): 478-482.
Chaurasiya, J.P., M. Singh, R.K. Yadav and L. Singh. 2018. Heterosis and combining ability analysis in Indian mustard (Brassica juncea L.). J. Pharm. Phytochem. 7(2): 604-609.
Dar, Z., A. Shafiq, M.A. Wani and M. Habib. 2011. Heterosis and combining ability analysis for seed yield and its attributes in Brassica rapa. J. Oilseed Bras. 2(1): 21-28.
Dawar, S., N. Kumar and S.P. Mishra. 2018. Genetic variability, correlation and path coefficient analysis in Indian mustard varieties grown in Chitrakoot. India. I. J. Curr. Microbiol. App. Sci. 7(3): 883-890. https://doi.org/10.20546/ijcmas.2018.703.103
Falconer D.S. and T.F.C. Mackay. 1996. Introduction to quantitative genetics, 4th ed. Long man scientific and technical, London.
FBS. 2016-17. Agricultural statistics of Pakistan, Fed. Bur. Stat. Islamabad, Pak.
Gami, R.A. and R.M. Chauhan. 2014. Genetic analysis for oil content and oil quality traits in Indian mustard (Brassica juncea L.). Int. J. Agric. Sci. 10(1): 146-150.
Girke, A., A. Schierholt and H.C. Becker. 2012. Extending the rapeseed gene pool with resynthesized Brassica napus L. II: Heterosis. Theor. Appl. Genet. 124: 1017-1026. https://doi.org/10.1007/s00122-011-1765-7
Goodnight, C.J. and S. Pandy. 1999. The genetics and exploitation of heterosis in crops. Am. Soc. Agron. Madison, USA. 59-68.
Gul, S., Raziuddin, N.U. Khan, M. Arif, R. Goher and M. Zakaria. 2018b. Inheritance studies through combining ability for morphological and yield traits in F1 populations of Brassica napus L. J. Ani. Plant Sci. 28(4): 1094-1102.
Gul, S., Raziuddin, N.U. Khan, M.S. Khan, S.U. Khan and R. Goher. 2018a. Heterotic and genetic effects in intra-specific populations of Brassica napus L. Pak. J. Bot. 50(5): 1951-1963.
Gupta, P.C. and S.K. Lal. 2011. Heterosis and combining ability analysis for yield and its components in Indian mustard. Acad. J. Plant Sci. 4(2): 45-52.
Gupta, S.K., N. Karuna and T. Dey. 2006. Heterosis and combining ability in rapeseed (Brassica napus L.). J. Res. Skuast. 5(1): 42–47.
Hablak, S. 2019. New theory of the mechanism of heterosis. Acta Sci. Pharm. Sci. 3(1): 10-16.
Iqbal, S., Farhatullah, S. Shah, M. Kanwal, L. Fayyaz and M. Afzal. 2014. Genetic variability and heritability studies in indigenous B. rapa accessions. Pak. J. Bot. 46(2): 609-612.
Ishaq, M. and Raziuddin. 2016. Combining ability analysis for maturity and plant architecture traits in intra-specific crosses of rapeseed. Sarhad J. Agric. 32(3): 168-176. https://doi.org/10.17582/journal.sja/2016.32.3.168.176
Jatoi, S.A., A. Javaid, M. Iqbal, O.U. Sayal, M.S. Masood and S.U. Siddiqui. 2012. Genetic diversity in radish germplasm for morphological traits and seed storage proteins. Pak. J. Bot. 43(5): 2507-2512.
Kang, S.A., F. Saeed and M. Riaz. 2014. Breeding for improving the seed yield and yield contributing traits in B. napus L. by using line × tester analysis. J. Plant Breed. Genet. 1(3): 111-116.
Khalil, I.A. and Raziuddin. 2017. Combining ability for seed yield in indigenous and exotic Brassica napus L. genotypes. Sarhad J. Agric. 33(1): 177-182. https://doi.org/10.17582/journal.sja/2017.33.1.177.182
Khan, F., Raziuddin and I.A. Khalil. 2013. Correlation and factor wise contributions of various traits related to yield in rapeseed (Brassica napus L.). Am. Eur. J. Agric. Environ. Sci. 13(1): 101-104.
Liton, M.M.U., N. Zeba and M.H. Rashid. 2017. Estimation of heterosis for yield and its attributes in Brassica rapa L. Asian Res. J. Agric. 4(4): 1-13. https://doi.org/10.9734/ARJA/2017/33085
Meena, H.S., B. Ram, V.V. Singh and D. Singh. 2014. Heterobeltiosis and standard heterosis for seed yield and important traits in Brassica juncea. J. Oilseed Bras. 5(2): 134-140.
Muhammad, A., Raziuddin, M. Sajid, Q.U. Bacha, 1.A. Rahman and S.A. Khan. 2014. Combining ability and heritability in F2 populations of Brassica napus. Am. Eur. J. Agric. Environ. Sci. 14(6): 509-515.
Mundiyara, R. and M.L. Jakhar. 2017. Economic heterosis for yield and yield characters in taramira (Eruca sativa Mill.), Int. J. Pure App. Biosci. 5(2): 81-91. https://doi.org/10.18782/2320-7051.2862
Naheed, H., Raziuddin, S. Abid, Q. Sohail, G. Hassan and M. Arif. 2017. Heritability and combining ability of vegetative growth and phenological development of diallel crosses of rapeseed. Genet. 49(1): 117-126. https://doi.org/10.2298/GENSR1701117N
Nasim, A., Farhatullah, N.U. Khan, M. Afzal, S.M. Azam, Z. Nasim and N. Amin. 2014.Combining ability and heterosis for yield and yield contributing traits in Brassica rapa. Pak. J. Bot. 46(6): 2135-2142.
Nassimi, A.W., Raziuddin, A. Sardar and T.A. Turi. 2006. Study on heterosis in agronomic characters of rapeseed using diallel. J. Agron. 5: 505-508. https://doi.org/10.3923/ja.2006.505.508
Nausheen, Farhatullah, I.H. Khalil and Amanullah. 2015. Heterosis and heterobeltiotic studies of F1 hybrids in Brassica carinata. Pak. J. Bot. 47(5): 1831-1837.
PBS. 2016-17. Pakistan Bureau of Statistics. Year book. Govt. Pak. Islamabad, Pakistan.
Rameeh, V. 2011. Heritability and other genetic parameters assessment for flowering associated stress indices in oil seed rape. Int. J. Plant Breed. Genet. 5(3): 268-27. https://doi.org/10.3923/ijpbg.2011.268.276
Rameeh, V. 2012. Combining ability analysis of plant height and yield components in spring type of rapeseed varieties. Int. J. Agric. For. 2(1): 58-62. https://doi.org/10.5923/j.ijaf.20120201.10
Rameeh, V. 2017. Hybrid performance and combining ability analysis in rapeseed using Line×Tester mating design. Int. J. Res. Agric. Forest. 4(8): 22-28.
Rameeh, V., A. Rezail and G. Saeidil. 2003. Estimation of genetic parameters for yield, yield components and glucosinolate in rapeseed. J. Agric. Sci. Tech. 5: 143-151.
Riaz, A., Farhatullah, R.S. Khan and C.F. Quiros. 2013. Inheritance of fertility restorer gene for cytoplasmic male sterility in B. napus and identification of closely linked molecular markers to it. Euphytica. 194: 351-360. https://doi.org/10.1007/s10681-013-0942-y
Sadat, H.A., G.A. Nematzadeh, N.B. Jelodar and O.G. Chapi. 2010. Genetic evaluation of yield and yield components at advanced generations in rapeseed (Brassica napus L.). Afr. J. Agric. Res. 5(15): 1958-1964.
Sincik, M., E. Sozen, K.C. Falk, A.T. Goksoy and E. Acikgoz. 2014. Heterosis and combining ability in a diallel cross of turnip rape genotypes. Turk. J. Field Crops. 19(2): 219-22. https://doi.org/10.17557/tjfc.27610
Singh, M. 2005. Genetics of seed yield and its attributes in Indian mustard. Plant Arch. 5(1): 217-221.
Sohail, A., S. Shah, S.M.A. Shah, Farhatullah, S. Ali, A. Izzam and Q. Hussain. 2018. Assessment of genetic variability, heritability and selection response for morph-yield traits in brassica. Pure Appl. Biol. 7(1): 50-56. https://doi.org/10.19045/bspab.2018.70007
Steel, R.G.D. and J.H. Torrie. 1980. Principles and procedures of statistics. 2nd ed. McGraw-Hill Book Co. Inc. New York, USA.
Synrem, G.J., N.R. Rangare and D.M. Bahadur. 2014. Variability studies in intra specific crosses of Indian mustard genotypes. J. Agric. Vet. Sci. 7(9): 29-32. https://doi.org/10.9790/2380-07932932
Synrem, G.J., N.R. Rangare, M. Ibadaiahun, T.N. Bhusal and D.M. Bahadure. 2015. Estimation of heterosis for seed yield and yield attributing traits in Indian mustard. Electr. J. Plant. Breed. 6(1): 274-281.
Tanaka, N. and S. Niikura. 2006. Genetic analysis of the characteristics related to the earliness of head formation in Brassica oleracea L. Breed. Sci. 56: 147-153. https://doi.org/10.1270/jsbbs.56.147
Turi, N.A., S.S. Shah and S. Ali. 2006. Estimation of heterosis for some important traits in mustard (Brassica juncea L.). J. Agric. Biol. Sci. 1(4): 6-10.
Verma, O.P., R. Yadav, K. Kumar, R. Singh, K.N. Maurya and R. Ranjana. 2011. Combining ability and heterosis for seed yield and its components in Indian mustard (Brassica juncea). Plant Arch. 11: 863-865.
Wynne, J.C., D.A. Emery and P.W. Rice. 1970. Combining ability estimates in Arachis hypogaea L. and field performance of F1 hybrids. Crop Sci. 10: 713-715. https://doi.org/10.2135/cropsci1970.0011183X001000060036x
To share on other social networks, click on any share button. What are these?








