In Vitro Effectiveness of Silver Nanoparticles against Root-Knot Nematode (Meloidogyne incognita)
In Vitro Effectiveness of Silver Nanoparticles against Root-Knot Nematode (Meloidogyne incognita)
Khansa Nazir1,*, Tariq Mukhtar1 and Humayun Javed2
1Department of Plant Pathology, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi
2Department of Entomology, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi
ABSTRACT
As the use of nematicides is becoming prohibitive in many countries due to their detrimental consequences, the management of plant parasitic nematodes using nanoparticles can be one of the important alternatives. In the present study, the nematicidal activity of silver nanoparticles (AgNP) was investigated against the most destructive root-knot nematode (Meloidogyne incognita). The maximum mortality of juveniles was recorded at a concentration of 100 mg/ml followed by 75 mg/ml of AgNP. The minimum mortality was recorded with 25 mg/ml of AgNP. With the increase in concentration, there was a corresponding increase in the mortality of juveniles showing a direct relationship between mortality and concentration of nanoparticles. The effect of time on mortality was also found significant. With the increase in time, there was a corresponding increase in mortality and the relationship was found to be directly proportional. Similarly, the maximum hatching inhibition of M. incognita eggs was recorded at a concentration of 100 mg/ml of nanoparticles followed by 75 mg/ml of AgNP. The minimum inhibition was recorded with 25 mg/ml of AgNP. It was found that with the an increase in concentration, there was a corresponding increase in hatching inhibition showing direct relationship between hatching inhibition and concentrations of the nanoparticles. Similarly, the maximum hatching inhibitions were recorded after 6th day followed by 5th day. The hatching inhibition was found directly proportional to time duration as it increased with an increase in number of days. It is concluded from the present study that AgNP possess nematicidal activity against root-knot nematodes and can act as an alternative to high-risk synthetic nematicides or inconsistent biological control agents without causing any phytotoxicity.
Article Information
Received 24 December 2018
Revised 01 March 2019
Accepted 15 March 2019
Available online 13 August 2019
Authors’ Contribution
KN and TM designed the study, conducted the surveys, executed experimental work, analyzed the data and prepared the manuscript. HJ provided technical assistance. TM supervised the work.
Key words
Root-knot nematodes, Silver nanoparticles, Hatchability, Mortality, Toxicity.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.6.2077.2083
* Corresponding author: [email protected]
0030-9923/2019/0006-2077 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Many pests including plant pathogenic nematodes attack vegetables and a number of crops and are responsible for causing severe growth retardations (Ashfaq et al., 2017; Fateh et al., 2017; Iftikhar et al., 2018; Javed et al., 2017a, b; Kassi et al., 2018, 2019a, b; Nabeel et al., 2018; Aslam et el., 2019a). Among plant parasitic nematodes, root-knot nematode (Meloidogyne incognita), is one of the most important nematodes associated with low production of many vegetables in Pakistan (Kayani et al., 2017; Kayani and Mukhtar, 2018; Tariq-Khan et al., 2017). Root-knot nematodes are ranked at the top among the five major plant pathogens and the first among the ten most important genera of plant parasitic nematodes in the world (Mukhtar et al., 2017a; 2018). They have wide geographic distribution, large host range and high destructive potential. They have been reported to be implicated with other plant pathogens like Ralstonia solanacearum and result in disease complexes and aggravation of wilt diseases (Aslam et al., 2017a, b, 2019b). On the other hand, biological control agents can cause significant reductions in the soil populations of these nematodes (Khan et al., 2017; Mukhtar et al., 2017b; Rahoo et al., 2017, 2018a, b, 2019).
In Pakistan, M. incognita has been found one of the most dominant root-knot species and rampant in the vegetable-producing areas of Pakistan and considerably reduces growth and yield (Kayani et al., 2018). The worldwide distribution of this species is 47% and in Pakistan its overall occurrence is 52%. Overall yield losses of 50 to 80% have been reported to be caused by root-knot nematodes in vegetables and 24 to 38% yield losses due to root-knot nematodes have been estimated on tomato. Root-knot nematodes have become a serious threat to the profitable cultivation of vegetables in the country. The yield losses by root-knot nematodes are mainly caused due to buildup of inoculum of the nematode and repeated cultivation of same cultivars in the same land every year (Hussain et al., 2016, 2019).
Root-knot nematodes are mainly controlled by the application of nematicides and resistant cultivars. Although nematicides can effectively manage nematodes but their usage is limited due to their short-term effects, high costs, non-availability, resistance development in nematodes, health and environmental hazards, residual toxicity and adverse effects on the beneficial micro flora and fauna in the soil besides phytotoxic effects on the crop.
To overcome the deleterious effects of nematicides, alternative strategies are being sought. Nanotechnology has the potential to revolutionize the agricultural and food industry with new tools for the molecular treatment of diseases, rapid disease detection, enhancing the ability of plants to absorb nutrients and also smart sensors and smart delivery systems which will help the agricultural industry combat viruses and other crop pathogens (Joseph and Morrison, 2006). For the last few years, nanoparticles have been encouraged to control plant pathogens including nematodes (Lara et al., 2011). Nanotechnology is mainly concerned with the production and utilization of materials whose dimensions are expressed in nanometers (1-100 nm or 1.5×109 m). The nanoparticles (NP) have a high capacity percentage in terms of volume to surface that enhances their affectivity and expected biochemical properties (Dubchak et al., 2010). Similarly, silver nanoparticles (AgNP) can be applied as sensors, catalysts, anticancer agents and antimicrobial agents as they are the advanced type of material. AgNP have shown response against different kinds of pathogens. In the present study, the nematicidal activity of silver nanoparticles was investigated against the most destructive root-knot nematode (Meloidogyne incognita).
Materials and methods
Preparation of silver nanoparticles
Silver nanoparticles (AgNP) were prepared by using onion extract as reducing agent and by the chemical decay of silver nitrate. For the extraction of onion, 25 g onion was taken and cut down into small pieces. In a pan, 300 ml distilled water was taken and boiled for 15 to 20 min under medium flame. After appearance of the bubbles, onion was added into the water until the solution color changed to light green. At the end, the solution was sieved and cooled. Silver nitrate (AgNO3) solution was made by dissolving 315 mg silver nitrate in 1 liter of distilled water and boiled for 2 to 3 min in an oven. The solution was condensed stepwise by adding 5 ml onion extract. The solution was again boiled for one minute and maintained in light for 2 to 3 min until the solution color changed. In this way 200 ppm concentration of AgNP solution was obtained and used for further applications.
Nematode culture
An indigenous population of root-knot nematode (Meloidogyne incognita) initially isolated from cucumber roots, identified on the basis of perineal pattern and maintained on the highly susceptible cultivar of tomato (money maker) was used in the assessment. The nematode was mass produced on tomato cv. Money maker (Mukhtar et al., 2017b).
Collection of juveniles and egg masses
For collection of juveniles, eggs were extracted using sodium hypochlorite (Hussey and Barker, 1973). The eggs were then processed on extraction trays for emergence of second stage juveniles (Whitehead and Hemming, 1965). The freshly hatched juveniles were collected in a beaker, standardized and concentrated. M. incognita infected tomato plants were uprooted and gently washed under running tap water. Uniform-sized egg masses were collected using forceps and the eggs per egg mass were counted (Mukhtar et al., 2013).
Effects of AgNP on juvenile mortality and egg hatching
To assess the effects of silver nanoparticles, 5 ml of solutions containing 0, 25, 50, 75 and 100 mg/ml of AgNP were added to 2.5 cm diameter plastic Petri plates. Approximately 30 juveniles of M. incognita contained in 50 µl were added to each solution. There were five replications for each concentration. The plates were incubated at room temperature in a completely randomized design. The mortality of juveniles was recorded after 12, 24, 48 and 72 h. Nematodes were considered alive if they moved or appeared as a winding shape and were considered dead if they did not move when probed with a fine needle (Mukhtar et al., 2013).
To evaluate the effects of solutions on hatchability, 5 ml of solutions containing 0, 25, 50, 75 and 100 mg/ml of AgNP was added to 2.5 cm diameter plastic Petri plates. One uniform-sized egg mass containing approximately 200 eggs was added to each solution. There were five replications for each concentration. The plates were incubated at room temperature in a completely randomized design. The hatching of juveniles was recorded daily up to the 6th day under stereomicroscope. After each count, the egg masses were washed with 1 ml of sterilized distilled water in their respective plates and transferred to fresh solutions of the same concentration. The percent mortality and hatching inhibition in each treatment was calculated and corrected by Abbott’s formula (Abbott, 1925) as follows:
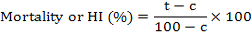
Where, HI is hatching inhibition, t is percent mortality or hatching inhibition in the extract and c is percent mortality or hatching inhibition in the control.
Statistical analysis
The data were subjected to analysis of variance using GenStat package 2009 (12th edition) version 12.1.0.3278 (www.vsni.co.uk) and the means were compared by Fisher’s Protected Least Significant Difference Test. A significance level of p≤0.05 was used in statistical analyses. The linear relationships between concentrations and time durations as independent variables (x) and mortality and hatching inhibitions as dependent variables (y) were calculated in Microsoft Excel 2007. Standard errors of means were also calculated in Microsoft Excel 2007.
Results
Effect of silver nanoparticles on mortality
The analysis of variance regarding effects of nanoparticles on mortality of M. incognita showed highly significant results. The effects of time and concentrations were also found significant. Maximum mortality of juveniles was recorded at a concentration of 100 mg/ml followed by 75 mg/ml of AgNP. The minimum mortality was recorded with 25 mg/ml of AgNP. It was also observed that with the increase in concentration there was a corresponding increase in the mortality of juveniles showing a direct relationship between mortality and concentrations of the nanoparticles as shown in Figure 1A. The effect of time on mortality was also found significant. Maximum mortality was recorded after 78 h and the minimum mortality was observed after 12 h. It was also observed that with the increase in time, there was a corresponding increase in mortality and the relationship was found directly proportional as shown in Figure 1B.
Effect of nanoparticles on hatching inhibition
The Analysis of Variance regarding effect of silver nanoparticles on hatching inhibition of M. incognita showed highly significant results. The effects of days and concentrations were also found significant. Maximum hatching inhibition of M. incognita eggs was recorded at a concentration of 100 mg/ml of nanoparticles followed by 75 mg/ml of AgNP. The minimum inhibition was recorded with 25 mg/ml of AgNP. It was found that with an increase in concentration, there was a corresponding increase in hatching inhibition showing direct relationship between hatching inhibition and concentrations of the nanoparticles as shown in the Figure 1C. Similarly, days also significantly affected hatching inhibition. Maximum hatching inhibitions were recorded after 6th day followed by 5th day. The relationship between hatching inhibition and days was found to be direct as hatching inhibition increased with an increase in number of days as shown in Figure 1D.
Discussion
In the present study, AgNP significantly caused mortality and hatching inhibition of M. incognita under lab conditions. Earlier a number of workers reported the toxicity of nanoparticles against root-knot nematodes. Taha and Abo-Shady (2016) assessed the nematicidal effects of AgNP against M. incognita juveniles in the laboratory and achieved 96.5 percent mortality after 72 h with 1500 ppm. The concentration of 200 ppm caused 52 percent mortality on the third day and 1500 ppm was found to be the most effective dose while other doses were ineffective. Ardakani et al. (2013) evaluated the efficacy of AgNP on M. incognita infecting tomato and found the doses of 800, 400 and 200 mg/ml of AgNP highly effective in causing immobility and mortality of M. incognita juveniles. Abdellatif et al. (2016) studied the influence of AgNP on the reproduction and growth of M. javanica and reported that AgNP treatment was equally effective as chemical treatment (Vydate 24% L) resulting in a reduction of egg masses per root system. AgNP were also found effective in improving growth of plants. There are reports that AgNP enhanced seed germination, seedling vigor and increased shoot length, root length, number of leaves and other plant growth criteria of different crops (Pandey et al., 2014; Yasmeen et al., 2015; Hojjat, 2016; Zuverza-Mena et al., 2016).
The nematicidal effect of AgNP is not specific and can be equally effective against other plant parasitic nematodes and plant pathogenic fungi in addition to root-knot nematodes. AgNP have been found associated with disrupting multiple cellular mechanisms including membrane permeability, ATP synthesis, and response to oxidative stress in both eukaryotic (Roh et al., 2009; Ahamed et al., 2010; Lim et al., 2012) and prokaryotic cells (Sondi and Salopek-Sondi, 2004; Morones et al., 2005; Lok et al., 2006; Choi and Hu, 2008). This is why, AgNP have shown a broad-spectrum antimicrobial activity and can affect plant pathogenic bacteria and fungi (Park et al., 2006; Jo et al., 2009). As such, it is possible that AgNP displays an antifungal effect on common root-associated fungal pathogens like Gaeumannomyces graminis and Rhizoctonia solani, in warm-season turfgrass. Similarly, turfgrass treated with AgNP can become more tolerant to root-knot nematode damage because of its protection from additional weakness and stress brought on by these other pathogens. Therefore, AgNP may provide an additional benefit of managing multiple turfgrass pathogens and contribute to turf quality improvement (Cromwell et al., 2014). As high frequency and application doses of AgNP are needed for effective control of root-knot nematodes, therefore, their application as fertigation and combination with compatible chemicals may enhance the applicability of AgNP. Further understanding of the mechanism in the nematicidal action of AgNP also warrants improvement of AgNP efficacy.
Conclusion
It is concluded from the present study that AgNP possess nematicidal activity against root-knot nematodes and can act as an alternative to high-risk synthetic nematicides or inconsistent biological control agents without causing any phytotoxicity.
Statement of conflict of interest
The authors declare no conflict of interest.
References
Abbott, W.S., 1925. A method of computing the effectiveness of an insecticide. J. econ. Ent., 18: 265-267. https://doi.org/10.1093/jee/18.2.265a
Abdellatif, K.F., Abdelfattah, R.H. and El-Ansary, M.S.M., 2016. Green nanoparticles engineering on root-knot nematode infecting eggplants and their effect on plant DNA modification. Iran. J. Biotech., 14: 250-259. https://doi.org/10.15171/ijb.1309
Ahamed, M., Posgai, R., Gorey, T.J., Nielsen, M., Hussain, S.M. and Rowe, J.J., 2010. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. appl. Pharmacol., 242: 263-269. https://doi.org/10.1016/j.taap.2009.10.016
Ardakani, A.S., 2013. Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology, 15: 671-677. https://doi.org/10.1163/15685411-00002710
Ashfaq, M., Saleem, A., Waqas, M. and Mukhtar, T., 2017. Natural occurrence and host range studies of cucumber mosaic virus (CMV) infecting ornamental species in the Rawalpindi-Islamabad area of Pakistan. Philipp. Agric. Scient., 100: 55-61.
Aslam, M.A., Javed, K., Javed, H., Mukhtar, T. and Bashir, M.S., 2019a. Infestation of Helicoverpa armigera Hübner (Noctuidae: Lepidoptera) on soybean cultivars in Pothwar region and relationship with physico-morphic characters. Pak. J. agric. Sci., 56: 401-405.
Aslam, M.N., Mukhtar, T., Ashfaq, M. and Hussain, M.A., 2017a. Evaluation of chili germplasm for resistance to bacterial wilt caused by Ralstonia solanacearum. Australas. Pl. Pathol., 46: 289-292. https://doi.org/10.1007/s13313-017-0491-2
Aslam, M.N., Mukhtar, T., Hussain, M.A. and Raheel, M., 2017b. Assessment of resistance to bacterial wilt incited by Ralstonia solanacearum in tomato germplasm. J. Pl. Dis. Prot., 124: 585-590. https://doi.org/10.1007/s41348-017-0100-1
Aslam, M.N., Mukhtar, T., Jamil, M. and Nafees, M., 2019b. Analysis of aubergine germplasm for resistance sources to bacterial wilt incited by Ralstonia solanacearum. Pak. J. agric. Sci., 56: 119-122.
Choi, O. and Hu, Z., 2008. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol., 42: 4583-4588. https://doi.org/10.1021/es703238h
Cromwell, W.A., Yang, J., Starr, J.L. and Jo, Y.K., 2014. Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. J. Nematol., 46: 261-266.
Dubchak, S., Ogar, A., Mietelski, J. and Turnau, K., 2010. Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Span. J. Agric. Res., 8(Suppl-I): 103-108. https://doi.org/10.5424/sjar/201008S1-1228
Fateh, F.S., Mukhtar, T., Kazmi, M.R., Abbassi, N.A. and Arif, A.M., 2017. Prevalence of citrus decline in district Sargodha. Pak. J. agric. Sci., 54: 9-13. https://doi.org/10.21162/PAKJAS/17.5643
Hojjat, S.S., 2016. The effect of silver nanoparticle on lentil seed germination under drought stress. Int. J. Farm. Allied Sci., 5: 208-212.
Hussain, M.A., Mukhtar, T. and Kayani, M.Z., 2016. Reproduction of Meloidogyne incognita on resistant and susceptible okra cultivars. Pak. J. agric. Sci., 53: 371-375. https://doi.org/10.21162/PAKJAS/16.4175
Hussain, M.A. and Mukhtar, T., 2019. Root-knot nematodes infecting okra in major vegetable growing districts of Punjab, Pakistan. Pakistan J. Zool., 50: 1137-1143. http://dx.doi.org/10.17582/journal.pjz/2019.51.3.1137.1143
Hussey, R.S. and Barker, K.R., 1973. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Pl. Dis. Rep., 57: 1025-1028.
Iftikhar, A., Aziz, M.A., Naeem, M., Ahmad, M. and Mukhtar, T., 2018. Effect of temperature on demography and predation rate of Menochilus sexmaculatus (Coleoptera: Coccinellidae) reared on Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Pakistan J. Zool., 50: 1885-1893. https://doi.org/10.17582/journal.pjz/2018.50.5.1885.1893
Javed, H., Hussain, S.S., Javed, K., Mukhtar, T. and Abbasi, N.A., 2017a. Comparative infestation of brinjal stem borer (Euzophera perticella) on six aubergine cultivars and correlation with some morphological characters. Pak. J. agric. Sci., 54: 753-758.
Javed, H., Mukhtar, T., Javed, K. and Ata ul Mohsin, 2017b. Management of eggplant shoot and fruit borer (Leucinodes orbonalis Guenee) by integrating different non-chemical approaches. Pak. J. agric. Sci., 54: 65-70. https://doi.org/10.21162/PAKJAS/17.5282
Jo, Y.K., Kim, B.H. and Jung, G., 2009. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Pl. Dis., 93: 1037-1043. https://doi.org/10.1094/PDIS-93-10-1037
Joseph, T. and Morrison, M., 2006. Nanotechnology in agriculture and food. A nanoforum report. Institute of Nanotechnology, Glasgow, UK. Available online at: http://www.nanoforum.org
Kassi, A.K., Javed, H. and Mukhtar, T., 2018. Screening of okra cultivars for resistance against Helicoverpa armigera. Pakistan J. Zool., 50: 91-95. https://doi.org/10.17582/journal.pjz/2018.50.1.91.95
Kassi, A.K., Javed, H. and Mukhtar, T., 2019a. Relationship of physico-morphic characters of okra cultivars with their resistance to Helicoverpa armigera. Pakistan J. Zool., 51: 835-841. https://doi.org/10.17582/journal.pjz/2019.51.3.835.841
Kassi, A.K., Javed, H. and Mukhtar, T., 2019b. Screening of different aubergine cultivars against infestation of brinjal fruit and shoot borer (Leucinodes orbonalis Guenee). Pakistan J. Zool., 51: 603-609. https://doi.org/10.17582/journal.pjz/2019.51.2.603.609
Kayani, M.Z. and Mukhtar, T., 2018. Reproductivity of Meloidogyne incognita on fifteen cucumber cultivars. Pakistan J. Zool., 50: 1717-1722. https://doi.org/10.17582/journal.pjz/2018.50.5.1717.1722
Kayani, M.Z., Mukhtar, T. and Hussain, M.A., 2017. Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Prot., 92: 207-212. https://doi.org/10.1016/j.cropro.2016.09.007
Kayani, M.Z., Mukhtar, T. and Hussain, M.A., 2018. Interaction between nematode inoculum density and plant age on growth and yield of cucumber and reproduction of Meloidogyne incognita. Pakistan J. Zool., 50: 897-902. https://doi.org/10.17582/journal.pjz/2018.50.3.897.902
Khan, A.R., Javed, N., Sahi, S.T., Mukhtar, T., Khan, S.A. and Ashraf, W., 2017. Glomus mosseae (Gerd & Trappe) and neemex reduce invasion and development of Meloidogyne incognita. Pakistan J. Zool., 49: 841-847. https://doi.org/10.17582/journal.pjz/2017.49.3.841.847
Lara, H.H., Garza-Treviño, E.N., Ixtepan-Turrent, L. and Singh, D.K., 2011. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol., 9: 30. https://doi.org/10.1186/1477-3155-9-30
Lim, D., Roh, J.Y., Eom, H.-J., Hyun, J.W. and Choi, J., 2012. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem., 31: 585-592. https://doi.org/10.1002/etc.1706
Lok, C.N., Ho, C.M., Chen, R., He, Q.Y., Yu, W.Y., Sun, H., Tam, P.K., Chiu, J.F. and Che, C.M., 2006. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteom. Res., 5: 916-924. https://doi.org/10.1021/pr0504079
Morones, J.R., Elechiguerra, J.L., Camacho, A., Holt, K., Kouri, J.B., Ramirez, J.T. and Yacaman, M.J., 2005. The bactericidal effect of silver nanoparticles. Nanotechnology, 16: 2346-2353. https://doi.org/10.1088/0957-4484/16/10/059
Mukhtar, T., 2018. Management of root-knot nematode, Meloidogyne incognita, in tomato with two Trichoderma species. Pakistan J. Zool., 50: 1589-1592. https://doi.org/10.17582/journal.pjz/2018.50.4.sc15
Mukhtar, T., Arooj, M., Ashfaq, M. and Gulzar, A., 2017a. Resistance evaluation and host status of selected green gram germplasm against Meloidogyne incognita. Crop Prot., 92: 198-202. https://doi.org/10.1016/j.cropro.2016.10.004
Mukhtar, T., Hussain, M.A. and Kayani, M.Z., 2017b. Yield responses of 12 okra cultivars to southern root-knot nematode (Meloidogyne incognita). Bragantia, 76: 108-112. https://doi.org/10.1590/1678-4499.005
Mukhtar, T., Jabbar, A., Raja, M.U. and Javed, H., 2018. Re-emergence of wheat seed gall nematode (Anguina tritici) in Punjab, Pakistan. Pakistan J. Zool., 50: 1195-1198. https://doi.org/10.17582/journal.pjz/2018.50.3.sc4
Mukhtar, T., Kayani, M.Z. and Hussain, M.A., 2013. Nematicidal activities of Cannabis sativa L. and Zanthoxylum alatum Roxb. against Meloidogyne incognita. Ind. Crops Prod., 42: 447-453. https://doi.org/10.1016/j.indcrop.2012.06.027
Nabeel, M., Javed, H. and Mukhtar, T., 2018. Occurrence of Chilo partellus on maize in major maize growing areas of Punjab, Pakistan. Pakistan J. Zool., 50: 317-323. https://doi.org/10.17582/journal.pjz/2018.50.1.317.323
Pandey, C., Khan, E., Mishra, A., Sardar, M. and Gupta, M., 2014. Silver nanoparticles and its effect on seed germination and physiology in Brassica juncea L. (Indian mustard) plant. Adv. Sci. Lett., 20: 1673-1676. https://doi.org/10.1166/asl.2014.5518
Park, H.-J., Kim, S.H., Kim, H.J. and Choi, S.H., 2006. A new composition of nanosized silica-silver for control of various plant diseases. Pl. Pathol. J., 22: 295-302. https://doi.org/10.5423/PPJ.2006.22.3.295
Rahoo, A.M., Mukhtar, T., Abro, S.I., Bughio, B.A. and Rahoo, R.K., 2018b. Comparing the productivity of five entomopathogenic nematodes in Galleria mellonella. Pakistan J. Zool., 50: 679-684. https://doi.org/10.17582/journal.pjz/2018.50.2.679.684
Rahoo, A.M., Mukhtar, T., Bughio, B.A. and Rahoo, R.K., 2019. Relationship between the size of Galleria mellonella larvae and the production of Steinernema feltiae and Heterorhabditis bacteriophora. Pakistan J. Zool., 51: 79-84. http://dx.doi.org/10.17582/journal.pjz/2019.51.1.79.84
Rahoo, A.M., Mukhtar, T., Gowen, S.R., Rahoo, R.K. and Abro, S.I., 2017. Reproductive potential and host searching ability of entomopathogenic nematode, Steinernema feltiae. Pakistan J. Zool., 49: 229-234. https://doi.org/10.17582/journal.pjz/2017.49.1.229.234
Rahoo, A.M., Mukhtar, T., Jakhar, A.M. and Rahoo, R.K., 2018a. Inoculum doses and exposure periods affect recovery of Steinernema feltiae and Heterorhabditis bacteriophora from Tenebrio molitor. Pakistan J. Zool., 50: 983-987. https://doi.org/10.17582/journal.pjz/2018.50.3.983.987
Roh, J.-Y., Sim, S.J., Yi, J., Park, K., Chung, K.H., Ryu, D.Y. and Choi, J., 2009. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ. Sci. Technol., 43: 3933-3940. https://doi.org/10.1021/es803477u
Sondi, I. and Salopek-Sondi, B., 2004. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interf. Sci., 275: 177-182. https://doi.org/10.1016/j.jcis.2004.02.012
Taha, E.H. and Abo-Shady, N.M., 2016. Effect of silver nanoparticles on the mortality, pathogenicity and reproductivity of entomopathogenic nematodes. Int. J. zool. Res., 12: 47-50. https://doi.org/10.3923/ijzr.2016.47.50
Tariq-Khan, M., Munir, A., Mukhtar, T., Hallmann, J. and Heuer, H., 2017. Distribution of root-knot nematode species and their virulence on vegetables in northern temperate agro-ecosystems of the Pakistani-administered territories of Azad Jammu and Kashmir. J. Pl. Dis. Prot., 124: 201-212. https://doi.org/10.1007/s41348-016-0045-9
Whitehead, A.G. and Hemming, J.R., 1965. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annls. appl. Biol., 55: 25-38. https://doi.org/10.1111/j.1744-7348.1965.tb07864.x
Yasmeen, F., Razzaq, A., Iqbal, M.N. and Jhanzab, H.M., 2015. Effect of silver, copper and iron nanoparticles on wheat germination. Int. J. Biosci., 6: 112-117. https://doi.org/10.12692/ijb/6.4.112-117
Zuverza-Mena, N., Armendariz, R., Peralta-Videa, J.R. and Gardea-Torresdey, J.L., 2016. Effects of silver nanoparticles on radish sprouts: Root growth reduction and modifications in the nutritional value. Front. Pl. Sci., 7: 90. https://doi.org/10.3389/fpls.2016.00090
To share on other social networks, click on any share button. What are these?










