Molecular Cloning, E. coli Expression and Characterization of Thermostable Alanine Aminotransferase from Pyrococcus abyssi
Molecular Cloning, E. coli Expression and Characterization of Thermostable Alanine Aminotransferase from Pyrococcus abyssi
Muhammad Shahid Nadeem*, Jalaluddin Azam Khan and Firoz Anwar
Sequence of P. abyssi gene coding for ALT with restriction sites for NdeI, NcoI inside the open reading frame (ORF) and restriction site of BamHI.
SDS-PAGE of purified enzymes, M, protein marker; P, purified enzyme; E1, E2, E3, E4, and E5- extract of experimental cells with gene expression; N, negative control.
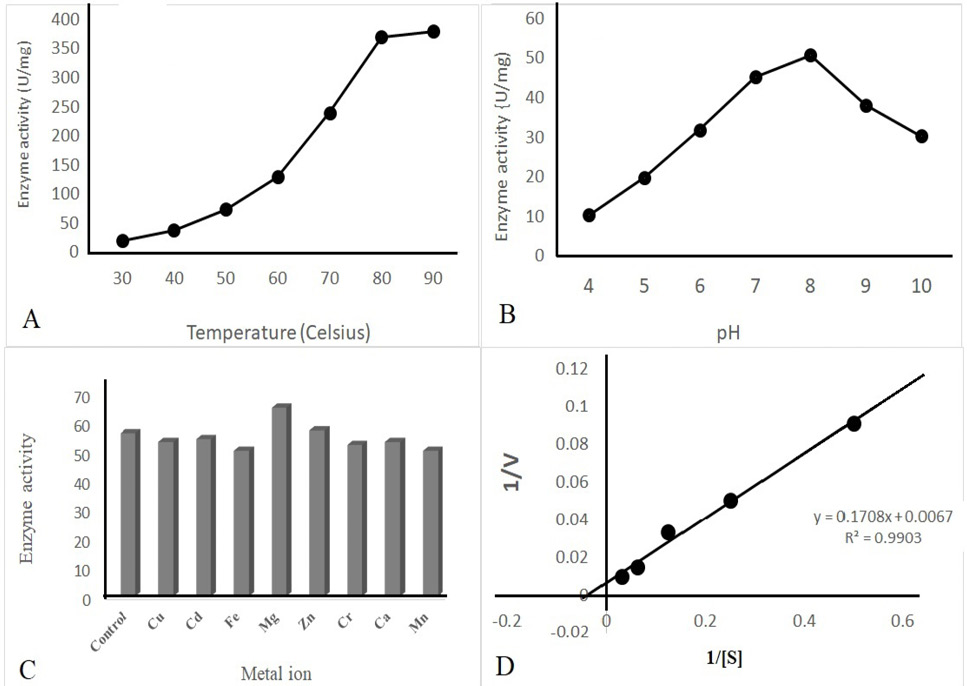
Kinetic properties of purified recombinant ALT indicating maximum activity at pH 8, above 90◦C and in the presence of Mg2+ ions. KM and Vmax values were calculated as 25µM of L-alanine and 149.25 U/mg, respectively.
3D model of ALT-PA represented as cartoon (Green) (Visualized by PyMol).
Docked complexes showing alanine aminotransferase in green pyridoxal-5-phosphate (A), glutamate (C), α ketoglutarate (E), alanine (G) and pyruvate (I) as red sticks. Zoomed view of ALT-pyridoxal-5-phosphate docked complex (B), ALT-glutamate (D), ALT- α-ketoglutarate (F) ALT-alanine (H) ALT-pyruvate docked complex (J) with interactive residues under 5Å (Visualization by PyMol).