Natural Incidence of Aspergillus Mycoflora and Mycotoxins in Fresh and Ensiled Maize Fodder
Natural Incidence of Aspergillus Mycoflora and Mycotoxins in Fresh and Ensiled Maize Fodder
Nighat Sultana1,2,*, Shazia Iftikhar1, Nafeesa Qudsia Hanif2 and Iffat Tahira2
1Fatima Jinnah Women University, Rawalpindi, Pakistan
2Romer Labs Pakistan, Rawalpindi, Pakistan
ABSTRACT
The present study evaluated Aspergillus (A) spp. and mycotoxins in fresh and ensiled maize fodder using a model fermentation system and raw materials from different areas of Punjab, Pakistan. A. niger, A. flavus and A. fumigatus were the most dominant followed by A. terrus and A. ochraceous in fresh and ensiled maize fodder. Total fungal counts ranged from 1 ×103 to 4 ×103cfu/ml for all species in both fresh and ensiled maize fodder. Total aflatoxins (AFB1, AFB2, AFG1 and AFG2) and ochratoxin A (OTA) analysis showed that AFB1was present with high frequency in fresh (37.5%) and ensiled (41.66%) fodder, with an average concentration of 9.49 and 8.36ng/g respectively. Aflatoxin B2 was detected in only two samples (16%) (1.2 and 1.3ng/g), aflatoxin G1 and G2 were not found. Ochratoxin A was found more frequently in fresh (54.16%) than in ensiled (20.86 %) samples with a mean of 8.06 and 4ng/g respectively. Average values for total aflatoxins (TAFs) and OTA were below the permissible limits as per regulation of European commission (EU), i.e. 20ng/g and 10ng/g, respectively.
Article Information
Received 14 October 2015
Revised 13 August 2016
Accepted 21 November 2016
Available online 31 January 2017
Authors’ Contributions
NQH conceived and designed the study. NS analyzed mycoflora and mycotoxins, and wrote the article. IT statistically analyzed the data. SI supervised the study.
Key words
Ensiling, Maize fodder, Fungi, Total aflatoxins, Ochratoxin A.
* Corresponding author: nighatbashir77@gmail.com
0030-9923/2016/0004-1161 $ 8.00/0
Copyright 2016 Zoological Society of Pakistan
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.475.480
INTRODUCTION
Maize is most widely grown crop in Pakistan, Major consumption areas are direct in human food, wet milling industry and livestock feed (GoP, 2014-2015). Fresh maize plant fodder is also being used to preserve as silage worldwide. Maize silage has a balance between energy density of grain, fiber and digestibly of green plant that is ideal for ruminant feeding and utilized as feed source over a long time (Cavena et al., 2009).
Ensiling of maize fodder entails incorporation of whole plant and its storage is based on principle of preservation. In anaerobic conditions with help of lactic acid bacteria, sugars present in green fodder is converted into lactic acid, which lowers pH to a level at which clostridia and most fungal growth are inhibited (Sparo and Mallo, 2001). However, environmental factors such as insufficient drying, moisture content, heat, insects and other conditions may cause aerobic spoilage (Dos Santos et al., 2003). Improper compression and covering, early opening, entry of uncontrolled rodents and insects etc. may result in exposure to oxygen that may affect the quality of silage and promote the growth of mycoflora. As maize silage consists of chopped whole plant, it includes not just grain but high percentage of stalks and stover which is more susceptible for fungal attack. The dominant genera reported in maize silage include Aspergillus, Pencillium, Fusarium, Mucor, Absidia, Monascus, Scopulriopsis and Trichoderma (Velazquez et al., 2008). During ensiling, most fungi can be eliminated. However, there are other species such as Aspergillus fumigatus, Penicillium roqueforti, P. paneum, F. oxysporum and Monascus ruber that are able to tolerate both high levels of organic acids and carbon dioxide in addition to low availability of oxygen (Gallo et al., 2015). Fungal spoilage in silage can not only reduced palatability of food but also leads to production of toxic secondary substances like mycotoxins which pose a serious health hazard for dairy animals (Velazquez et al., 2008).
Mycotoxins producing fungi invade crops in field and may grow during storage under favorable environmental conditions. The detection of fungi does not necessarily entail the presence of mycotoxins, since mycotoxins production depends on various factors such as presence of toxigenic fungi, chemical composition of the substrates, moisture content, temperature, relative humidity and time course of fungal growth (Roige et al., 2009). Occurrence of mycotoxins in nature is a global problem; however geographic distribution of mycotoxins has diverse variation with respect to their occurrence (Lawlor and Lynch, 2005). Mycotoxins are mainly produced by Aspergillus, Fusarium and Penicillium genera. Major mycotoxins are trichothecenes, fumonisins, ochratoxin A, aflatoxins and zearalenone etc. Among these, Aspergillus acquires a significant attention due to their wide occurrence and climatic condition of Pakistan favors their production. Aflatoxins and ochratoxin A are produced by Aspergillus species. They are carcinogenic, teratogenic, nephrotoxic, mutagenic and immuno-suppressive in nature (Akande et al., 2006) and exert lethal health hazards in human and animals (CAST, 2003).
In Pakistan, silage making is in practice for a few decades to achieve higher production parameters and to provide a uniform diet to animals. Pakistan is situated in tropical part of world and climatic conditions are conducive for fungal contamination and mycotoxins production. Furthermore, temperate and tropical climatic conditions of Pakistan plus inadequate feed storage practices provide ideal conditions for Aspergillus growth, reduction of nutritional values and mycotoxins production. Silage can be contaminated with more than one fungi and mycotoxins at same time. However, in present study emphasis was given on Aspergillus and their toxins due to the fact that it metabolizes and carry over into milk in form of aflatoxin M1. However, on the other hand, anaerobic environment, adequate substrate and sufficient amount of lactic acid bacteria are required for good quality silage. Epiphytic population of lactic acid bacteria are in low amount that is insufficient for fermentation process. In order to increase population of lactic acid bacteria, microbial inoculant was added before ensiling to enhance the process of fermentation (Bayatkouhsar et al., 2012). A rapid decline of the pH is very important during the start of the fermentation process, because it inhibits growth of toxigenic fungi. In view of this comprehensive background, the present study was planned to assess the effect of inoculant application in fresh maize plants collected from various areas of Punjab and ensiled over a period of 60 days. The parameters under study were frequency and colony numbers of Aspergillus spp., species differentiation of Aspergillus, aflatoxins and ochratoxin A contents in fresh and ensiled maize fodder.
MATERIALS AND METHODS
Samples collection
A total of twenty-four fresh maize fodder samples (5kg each) were collected from main maize producing areas (Chakwal, Rawalpindi, Sargodha, Sahiwal, Rahimyar Khan and Multan) of the province of Punjab. Samples were divided into two portions. Half of the portion of green fodder was wrapped for process of ensiling under anaerobic conditions. Homofermentative microbial inoculants (BIOSTABIL WRAPS) that is a mixture of different homofermentative strains, Enterococcus faecium BIO 34 (DSM 3530) and Lactobacillus plantarum IFA 96 (DSM19457) was applied before ensiling at a level of 4mg/kg. Population density of bacteria in one gram of inoculant was 1 ×106cfu/g. For ensiling purpose, samples were first packed in airtight envelops and kept in air tight buckets in order to achieve anaerobic conditions. Mini silos were then opened after the 60thday of study. Samples of fresh (n=24) and ensiled (n=24) maize fodders were preserved at -4ºC until Aspergillus spp., total aflatoxins (TAFs) and ochratoxin A (OTA) analysis. In addition, the pH of fresh and ensiled samples was also measured before start of analysis.
Mycological analysis
Media preparation
Aspergillus spp. was isolated by using oxytetracyline glucose agar media (OGA).For media preparation, 1.25g of yeast extract, 5g of glucose and 5g of agar were dissolved in 250ml of water and autoclaved for 20 min. Antibiotic like Terramycin capsules (250mg) was added into a little hot (~40-50°C) autoclaved media and slightly shakes the flask in order to inhibit bubble formation. Then the media was poured on autoclaved petriplates and kept at 30ºC for 24 h (Mossel et al., 1962).
Sample preparation
For each sample, 5g of sample was homogenized with 45ml of trypticase salt solution and shake for 15 min. After shaking, 10 fold dilutions were made and 1ml of each dilution sprayed in a Petri dish (90 mm diameter) under laminar flow hood containing OGA media. The tentative identity of isolates was determined by macro- and microscopic examination (Mansfield and Kuldau, 2007; Raper and Fennell, 1965). After identification of fungi, cultures were preserved on potato dextrose media (PDA). Colony forming unit was determined by using following formula:
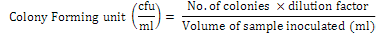
Mycotoxins analysis
For analysis of total aflatoxins (AFB1, AFB2, AFG1 and AFG2) and ochratoxin A (OTA) standard methods using Immunoaffinity chromatographic sample extract cleanup and HPLC with fluorescence detection, following international official standards were used as described by Sultana et al. (2013) and Khalil et al. (2013).
Sample preparation
Fresh and ensiled maize fodder samples (25g) were homogenized with 100ml of methanol: water (80:20; v/v) and placed in an orbital shaker for 1 h. Mixture was filtered through a Whattman No.1 filter paper. Then, 8ml of Phosphate buffer saline (pH =7.4) was added to 4ml of sample extract. The pH was adjusted to 7.0 for TAFs and 7.4 for OTA with 0.1mol/HCl or 0.1 mol/NaOH. The aliquot was then loaded on a AflaStar®Immunoaffinity column or OchraStar®Immunoaffinity column (Romer Labs, Austria). The flow rate was adjusted at 1ml/min. The column was then washed with 20ml distilled water. Aflatoxins and OTA were eluted with 3ml of methanol at flow rate of 0.5ml/min. Purified extracts were transferred into capped glass vials and evaporated by using an Evap system (Romer Labs, Inc. MO, USA) under vacuum at 60°C. Mycotoxins residue were then re-dissolved in Toluene: Acetonitrile (95:5 v/v; TAF) and toluene: acetic acid (99:1 v/v; OTA), respectively.
Analysis by high performance liquid chromatography (HPLC)
After re-dissolving, TAFs and OTA were analyzed using a Shizmadu LC20 AT system equipped with UV detector and photochemical post column derivatization cell. Flow rate of isocratic mobile phases for both toxins i.e. TAFs {Acetonitrile: methanol: water (20:20:60; v/v/v)} and OTA {acetonitrile/water/acetic acid (51:47:2, v/v/v)} was 0.8 ml/min. Temperature of column was maintained at 40ºC. Total aflatoxins and OTA were detected at wavelengths of 365nm and 333nm with injection volume of 20 µl, respectively.
RESULTS AND DISCUSSION
The present study was conducted to evaluate the growth and mycotoxins production of different Aspergillus spp. in a model system consisting of fresh (n=24) and ensiled maize fodder (n=24). The raw materials for this study were collected from the main maize growing areas (Chakwal, Rawalpindi, Sargodha, Sahiwal, Multan and Rahimyar khan) of Punjab, Pakistan. Moreover, inoculant was applied before ensiling and a drop in pH (4.0) was observed in ensiled samples at the third day of the experimental fermentation. The prime importance of inoculant is to support epiphytic bacteria with additional population for quick acidification process and instant decline in pH for nutrient preservation (Bayatkouhsar et al., 2012). Present finding revealed quick decline of pH values, which is an indicator of good quality silage having a pH value of 4.2 or lowers. For pH maintenance, various factors are being involved including water soluble carbohydrate of fresh fodder, buffering capacity, dry matter content and types of epiphytic bacteria on fresh fodder (Kadivar and Stapleton, 2003).
Results of present findings further demonstrated that A. niger was the most dominant specie followed by A. fumigatus, A. flavus, A. terrus and A. ochraceous in fresh and ensiled maize fodder, respectively (Table I). Results were further evaluated for total fungal colonies (cfu/ml). Highest fungal count was observed for A. terrus (5 ×103cfu/ml) and A. niger (2.7 ×103) in ensiled and fresh maize fodder respectively. Among areas high mean fungal densities were observed for A. flavus in Rawalpindi (4 ×103cfu/ml) and A. terrus (4.25 ×103cfu/ml) in Rahimyarkhan (Table II).
Results of recent study indicated that samples of fresh as well as ensiled maize fodder were found positive for Aspergillus spp. There is a presumption that ensiled maize fodder is less affected by spoilage microorganism due to low pH and acidic conditions. Fungi can grow under wide-ranged environmental conditions like 10-40ºC, 4-8 of pH and a water activity of 0.7. High temperature and low water activity are required for growth of Aspergillus spp. Aspergillus spp. usually found dominant in warm environment (Reyneri, 2006). Pereyra et al. (2008) also reported that Aspergillus spp. can survive under microaerophiilic conditions, which were well adapted in acidic environment.
Total fungal densities in all samples of fresh and ensiled maize fodder ranging from 1.5x103-5x103 cfu/ml that was in agreeble limit as per recommendation of quality standard of 1 ×104 cfu/ml defined by Good Managemental Practices (GMP, 2005).
|
Locations (sub-areas) |
Aspergillus niger |
Aspergillus fumigatus (%) |
Aspergillus flavus (%) |
Aspergillus terreus (%) |
Aspergillus ochraceous (%) |
|||||
| Fresh | Ensiled | Fresh | Ensiled | Fresh | Ensiled | Fresh | Ensiled | Fresh | Ensiled | |
| Chakwal |
2.5 ×103 |
1.5 ×103 |
3 ×103 |
1.5 ×103 |
3.5 ×103 |
1 ×103 |
1.5 ×103 |
1 ×103 |
2.5 ×103 |
2.5 ×103 |
| Rawalpindi |
1 ×103 |
1.5 ×103 |
3.3 ×103 |
2 ×103 |
4 ×103 |
1.5 ×103 |
ND |
1 ×103 |
1 ×103 |
1.5 ×103 |
| Sargodha |
1 ×103 |
1 ×103 |
4 ×103 |
4 ×103 |
1.5 ×103 |
2 ×103 |
4 ×103 |
1.5 ×103 |
2 ×103 |
3 ×103 |
| Sahiwal |
3 ×103 |
1 ×103 |
2 ×103 |
1 ×103 |
1 ×103 |
1.5 ×103 |
1 ×103 |
1 ×103 |
3 ×103 |
1.5 ×103 |
| Rahimyar Khan |
1.5 ×103 |
21 ×103 |
2 ×103 |
2 ×103 |
2 ×103 |
1.5 ×103 |
1 ×103 |
ND |
2 ×103 |
3.5 ×103 |
| Multan |
ND |
1 ×103 |
2 ×103 |
1.5 ×103 |
2 ×103 |
1 ×103 |
ND |
2 ×103 |
5 ×103 |
1.5 ×103 |
| Mean |
1.8 ×103 |
5 ×103 |
2.7 ×103 |
2 ×103 |
2 ×103 |
1.5 ×103 |
2 ×103 |
1.5 ×103 |
2.5 ×103 |
2 ×103 |
ND, not detected.
Table II.- Total fungal density (cfu/ml) of Aspergillus spp. In fresh and ensiled maize fodder from various areas of Punjab.
|
Samp ling Areas |
Aspergillus terreus (cfu/ml) |
Aspergillus niger (cfu/ml) |
Aspergillus flavus (cfu/ml) |
Aspergillus ochraceous (cfu/ml) |
Aspergillus fumigatus (cfu/ml) |
|||||
| Fresh | Ensiled | Fresh | Ensiled | Fresh | Ensiled | Fresh | Ensiled | Fresh | Ensiled | |
| Chakwal |
2.5 ×103 |
1.5 ×103 |
3 ×103 |
1.5 ×103 |
3.5 ×103 |
1 ×103 |
1.5 ×103 |
1 ×103 |
2.5 ×103 |
2.5 ×103 |
| Rawal pindi |
1 ×103 |
1.5 ×103 |
3.3 ×103 |
2 ×103 |
4 ×103 |
1.5 ×103 |
ND |
1 ×103 |
1 ×103 |
1.5 ×103 |
| Sargodha |
1 ×103 |
1 ×103 |
4 ×103 |
4 ×103 |
1.5 ×103 |
2 ×103 |
4 ×103 |
1.5 ×103 |
2 ×103 |
3 ×103 |
| Sahiwal |
3 ×103 |
1 ×103 |
2 ×103 |
1 ×103 |
1 ×103 |
1.5 ×103 |
1 ×103 |
1 ×103 |
3 ×103 |
1.5 ×103 |
| Rahim yar Khan |
1.5 ×103 |
21 ×103 |
2 ×103 |
2 ×103 |
2 ×103 |
1.5 ×103 |
1 ×103 |
ND |
2 ×103 |
3.5 ×103 |
| Multan |
ND |
1 ×103 |
2 ×103 |
1.5 ×103 |
2 ×103 |
1 ×103 |
ND |
2 ×103 |
5 ×103 |
1.5 ×103 |
| Mean |
1.8 ×103 |
5 ×103 |
2.7 ×103 |
2 ×103 |
2 ×103 |
1.5 ×103 |
2 ×103 |
1.5 ×103 |
2.5 ×103 |
2 ×103 |
ND, not detected.
Table III.- Natural prevalence of total aflatoxins and ochratoxin A in fresh and ensiled maize fodder from various areas of Punjab.
|
Area
|
Aflatoxin B1 |
Aflatoxin B2 |
Ochratoxin A |
|||||||
|
Mean (ng/g) |
Posi tive (%) |
Range |
Mean (ng/g) |
Pos itive (%) |
Range |
Mean (ng/g) |
Positive (%) |
Range |
||
|
Fresh fodder |
Chakwal |
2.95 |
25.0 |
<0.1-11.5 |
0 |
0 |
0 |
6.54 |
75.0 |
<0.1-11.28 |
| Rawalpindi |
1.33 |
25.0 |
<0.1-5.29 |
0 |
0 |
0 |
4.78 |
75.0 |
<0.1-7.17 |
|
| Sargodha |
4.37 |
100.0 |
1.54-13.4 |
0 |
0 |
0 |
6.15 |
25.0 |
<0.1-24.6 |
|
| Sahiwal |
7.85 |
50.0 |
0.1-28.2 |
0 |
0 |
0 |
- |
25.0 |
<0.1-0.41 |
|
| Rahimyar Khan |
<0.1 |
- |
- |
1.2 |
49.00 |
<0.5-3 |
6.74 |
100.0 |
<0.1-8.61 |
|
| Multan |
5.43 |
25.0 |
0.1-21.44 |
1.2 |
47.00 |
<0.5-4 |
-- |
25.0 |
<0.1-4.1 |
|
| Cumulative contamination |
9.49 |
37.5 |
0.1-28.2 |
1.2 |
16.06 |
0.5-4 |
8.06 |
54.16 |
<0.1-24.6 |
|
|
Ensiled fodder |
Chakwal |
2.57 |
25.00 |
<0.1-10 |
<0.5 |
0 |
0 |
<0.1 |
0 |
<0.1 |
| Rawalpindi |
6 |
25.00 |
<0.1-12 |
0 |
0 |
0 |
3.77 |
50.00 |
<0.1-4.92 |
|
| Sargodha |
2.43 |
50.00 |
0.1-7 |
0 |
0 |
0 |
7.79 |
50.00 |
<0.1-8.2 |
|
| Sahiwal |
6.59 |
75.00 |
1.2-24 |
0 |
0 |
0 |
<0.1 |
- |
- |
|
| Rahimyar Khan |
5.58 |
50.00 |
<0.1-20 |
1.5 |
450 |
<0.5-4 |
3.95 |
25.00 |
<0.1-15.5 |
|
| Multan |
1.3 |
25.00 |
<0.1-5 |
1.12 |
400 |
<0.5-3 |
- |
- |
- |
|
| Cumulative contamination |
8.36 |
41.66 |
<0.1-24 |
1.31 |
16.06 |
<0.5-4 |
4.00 |
20.86 |
<0.1-15.5 |
|
Prevalence was considerabaly high but fungal biomass was far below than agreeble limits of good managemental practices. Fungi can grow in field, during sowing and harvesting but proper preventive measures like proper compression and exclusion of air may reduce their fungal biomass and inhibit growth due to acidic conditions. Another possible explanation was that in ensiled fodder, homofermentative bacterial inoculant was applied that consisting of Lactobacillus plantrum. Several studies have shown that some strains of Lactobacillus plantrum have antifungal activity and considered as natural biological antagonists. They produce phenyllactic acid, which may inhibit growth of Aspergillus niger and Aspergillus flavus (Dalie et al., 2010).
Concerning mycotoxin contamination, nine samples of fresh maize (37.5%) contained AFB1,with an average of 9.49ng/g (range of <0.1-28.2ng/g). This was quite similar with the frequency (41.66%) found in the ensiled batches of maize fodder, which contained AFB1 at a mean level of 8.36ng/g (0.1-24ng/g). AFB2 was detected in only two samples, each one of fresh and ensiled maize fodder (1.2 and 1.3ng/g). AFG1 and AFG2 was not found in any sample. Ochratoxin A contamination was found in 13 (54.16%) samples of fresh maize fodder with a mean of 8.03ng/g (0.1-24.16ng/g). In the ensiled batches, 20.86% were positive for OTA, with an average of 3.98ng/g (0.1-15.50ng/g) (Table III). Mean levels for AFB1 and OTA were below the Europeon Union regulatory limits of 20ng/g (aflatoxins) and 10ng/g (OTA) (Europeon Commission, 2005). However, some samples slightly exceeded these maximum values.Overall the values found for aflatoxins and for OTA were not alarmingly high, although the frequency of toxigenic Aspergillus species was quite high in these maize samples, both as fresh produce and after experimental model ensilage.
In the present study, samples collection were carried out during month of july. Maize is usually grown during hot weather and harvested in humid summer in Punjab. These conditions promote attack of fungi with maximum production of TAFs and OTA. Climate in most areas of Punjab remains hot, where high temperature (30 to 48 °C) and relative humidity ranging from 25% to 50%, prevail in summer (Chaudhary et al., 2009). Environmental conditions of Punjab with its warm temperature are favorable for proliferation of toxigenic fungi (Saleemullah et al., 2006; Iqbal et al., 2011). Stress factors such as shortage of water, insect infestation, and other pests attack can also enhance mycotoxins production (Sanchis and Magan, 2004; Milani, 2013). explained the stabilty of TAFs in maize silage and showed that acidic conditions can not completely inhibit toxins production. Moreover, a study conducted by Pereyra et al. (2008) shows that occurrence of aflatoxins was higher in silage than to fresh fodder that was similar to present findings in which prevalence was higher in silage (41.66%) than fresh fodder (37.5 %).
Total aflatoxins and OTA were prevalent in warm and humid environment and gain much importance due to their carcinogenic, teratogenic, immunosuppresive, nephrotoxic and mutagenic effects. Total aflatoxins metabolized in liver and excreted in bile. It increases the apparent protein requirement of cattle and pose as potent cancer causing agent (Chohan et al., 2016). It has been classified as class 1A carcinogen by international agency of research on cancer (IARC, 2002). Total aflatoxin also have carryover effect in milk in form of AFM1 that have adverse effects on animal as well as on human (CAST, 2003). Ochratoxin A, a well known nephrotoxin (class 2B carcinogen) has been associated with fatal human kidney disease referred to as balkan endemic nephropathy. It aslo increased the incidence of tumors of upper urinary tract in human as well as in animals (JECFA, 2001).
Present study has provided baseline in identifying problem of mycoflora and mycotoxins contamination in fresh and ensiled maize fodder that affects quality of silage. In maize silage, grains basically become the source of aflatoxins contamination in addition to compound feed. Although the levels noticed in maize silage are not significantly higher but contribution from feed may exert negative impact on animal’s health. Sometimes mycotoxins in combination may exert synergistic, additive and anatagonistic effects on animal. Proper silage making and use of inoculant (homofermentative type) maintains stability of silage and prevent it from spoilage. Mycotoxins contamination of silage seems to be unavoidable to a certain degree during processing and storage of silage. Moreover, to prevent spoilage of silage, care must be taken during feed out phase as there is chance of oxygen penetration that may lead to silage spoilage. Furthermore, good managemental practices during silage making plays an important role in reduction of fungal count and mycotoxins contamination.
Statement of conflict of interest
The primary data is available with us and may be provided when required. The authors declare that there is no conflict of interest. We are giving copyright reserved to Journal.
REFERENCES
Akande, K.E., Abubakar, M.M., Adegbola, T.A. and bogoro, S.E., 2006. Nutritional and health implications of mycotoxins in animal feed. Pakistan J. Nutri., 5: 398-403. https://doi.org/10.3923/pjn.2006.398.403
Chaudhary, Q.Z., Mahmood, A., Rasul, G. and Afzal, M., 2009. Climate change indicators of Pakistan. Pakistan Meteorological Department, Islamabad. Technical Report No. PMD-22/2009, p. 1–43.
Chohan, K.A., Awan, F., Ali, M.M., Iqbal, U. and Ijaz, M., 2016. Assessment of aflatoxin in dairy concentrate feeds, total mixed rations, silage and various feed ingredients in Pakistan. Pakistan J. Zool., 42: 697-700.
Caneva, G., Nugari, M.P. and Salvadori, O., 2009. Plant biology for cultural heritage: Biodeterioation and conservation. ISBN 978-0-89236-939-3, 70 2008.
CAST, 2003. Mycotoxins: Risks in plants, animals and humans. Task Force Report no. 139. Council for Agriculture Science and Technology (CAST), Ames, lowa, USA.
Economic Survey of Pakistan, 2014-2015. Government of Pakistan, Ministry of Finance Islamabad.
EC, 2005. Commission Regulation (EC1881/2006). http://europe.eu.int/eurlex/lex/LexUriserv/site/en/oj/2006/1/9120040528en00010052.pdf
Dos Santos, V.M., Dorner, J.W. and Carreira, F., 2003. Isolation and toxigenicity of Aspergillus fumigatus from moldy silage. Mycopathology, 156: 133-138. https://doi.org/10.1023/A:1022996911563
Dalie, D.K.D., Deschamps, A.M. and Richard, F.F., 2010. Lactic acid bacteria - Potential control of mould growth and mycotoxin: A review. Fd Contr., 21: 370-380. https://doi.org/10.1016/j.foodcont.2009.07.011
Gallo, A., Giuberti, G., Frisvad, J.C., Bertuzzi, T. and Nielsen, F., 2015. Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins, 7: 3057-3111. https://doi.org/10.3390/toxins7083057
Iqbal, S.Z., Asi, M.R. and Arino, A., 2011. Aflatoxin M1 contamination in cow and buffalo milk samples from the North West Frontier Province (NWFP) and Punjab provinces of Pakistan. Fd. Add. Contam., Part B, 4: 282–288.
JECFA, 2001. Safety evaluation of certain food additives and contaminants: aflatoxins (WHO Food Additive series 40), 49th Meeting of the joint FAO/WHO Expert committee on Food Additives (JECFA). International Programon Chemical Safety, World Health Organization, Geneva.
Kadivar, H. and Stapleton, A.E., 2003. Ultraviolet radiation alters maize phyllosphere bacterial diversity. Microb. Ecol., 45: 353-361. https://doi.org/10.1007/s00248-002-1065-5
Khalil, M.M.H., Gooma, A.M. and Sebaei, A.S., 2013. Reliable HPLC determination of aflatoxin M1 in eggs. J. Analyt. Meth. Chem., 2013: 1-5. https://doi.org/10.1155/2013/817091
Lawlor, P.G. and Lynch, P.B., 2005. Mycotoxin management. Afr. Farm. Fd. Process, 46: 12-13.
Mansfield, M.A. and Kuldau, G.A., 2007. Microbiological and molecular determination of mycobiota in fresh and ensiled maize silage. Mycologia, 99: 269-278. https://doi.org/10.3852/mycologia.99.2.269
Mossel, D.A.A., Visser, M. and Mengerink, W.H.J., 1962. A comparison of media for the enumeration of moulds and yeasts in foods and beverages. Lab. Pract., 11: 109.
Pereyra, C.M., Alonso, V.A., Rosa, C.A.R., Chiacchiera, S.M., Dalcero, A.M. and Cavaglieri, R.R., 2008. Gliotoxin natural incidence and toxigenicity of Aspergillus fumigatus isolated from maize silage and ready dairy cattle feed. World Mycotoxin J., 3: 457-462. https://doi.org/10.3920/WMJ2007.1012
Roige, M.B., Sandra, M.A., Maria, B.R., Silvia, P., Alejandro, L.S. and Maria, O.T., 2009. Mycobiota and mycotoxins in fermented feed, wheat grains and corn grains in Southeastern Buenos Aires Province, Argentina. Rev. Iberoam Micol., 26: 233-237. https://doi.org/10.1016/j.riam.2009.03.003
Reyneri, A., 2006. The role of climatic condition on mycotoxins production in cereals. Vet. Res. Commun., 30: 87-92. https://doi.org/10.1007/s11259-006-0018-8
Sultana. N., Rashid, A., Tahira, I., Hanif, H.U. and Hanif, N.Q., 2013. Distribution of various mycotoxins in compound feed, total mix ration and silage. Pakistan Vet. J., 33: 200-204.
Saleemullah, Khalil, I.A. and Shah, H.U., 2006. Aflatoxin contents of stored and artificially inoculated cereals and nuts. Fd. Chem., 98: 699–703. https://doi.org/10.1016/j.foodchem.2005.06.034
Sanchis, V. and Magan, N., 2004. Environmental conditions affecting mycotoxins. In: Mycotoxins in food. (eds. N. Magan and M. Olsen), Woodhead Publishing. Cambridge, pp. 174–89. https://doi.org/10.1533/9781855739086.2.174
Velazquez, W.P.R., Victor, H.I.E., Federico, R., Cecilia, J.P., Ernesto, L.P., Jorge, H.G. and Augustin, R.A., 2008. Occurrence of fungi and mycotoxins in maize silage, Jalisco State, Mexico. Rev. Iberoam. Micol., 25: 182-185.










