Nutrient Digestibility in Labeo rohita Fingerlings Fed Citric Acid Acidified Phytase Sprayed Diet
Nutrient Digestibility in Labeo rohita Fingerlings Fed Citric Acid Acidified Phytase Sprayed Diet
Syed Zakir Hussain Shah1*, Muhammad Afzal2, Mahroze Fatima3,
Syed Makhdoom Hussain4 and Tanveer Ahmed5
1Department of Zoology, University of Gujrat, Gujrat, Pakistan
2Fish Nutrition Laboratory, Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad
3Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Lahore
4Department of Zoology, Government College University, Faisalabad
5Aquaculture Biotechnology Laboratory, Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad
ABSTRACT
A factorial experiment under completely randomized design was conducted by using citric acid (CA) supplementation (3 levels) and phytase (PHY) supplementation (3 levels) as the main effects. CA was supplemented at 0, 1.5 and 3% and PHY was added at 0, 750 and 1000 FTU/kg diet in a 3 × 3 factorial arrangement. All the nine test diets were contained 37.6% crude protein and 4.72 kcal/g gross energy. Results showed improved (p<0.05) dry matter, crude protein, crude fat and gross energy digestibility by fingerlings when fed PHY sprayed diet. Similarly, CA addition in the diet resulted in increased (p<0.05) digestibility of these nutrients. Also, the minerals digestibility was significantly (p<0.05) affected by top spraying of phytase on the diet pellets. Fish having CA acidified diet also showed improved (p<0.05) minerals digestibility performance. Interaction plots showed synergistic effect of both the supplements for crude fat and gross energy digestibility. Again, interaction data showed synergistic effect on minerals digestibility except Fe. Inclusion of both the supplements independently and in combination in the diet resulted in less nutrient contents in fish feces. In conclusion, addition of CA and PHY in the Labeo rohita diet may improve the nutrient digestibility which may lead to improved growth performance and less pollution in the water bodies.
Article Information
Received 23 September 2015
Revised 02 May 2019
Accepted 12 June 2019
Available online 11 January 2021
Authors’ Contribution
SZHS and MF conducted the experiment and wrote the manuscript. MA supervised the experiment manuscript write up. SMH and TA helped in the data collection and analysis.
Key words
Citric acid, Dietary acidification, Nutrient digestibility, Phytate, Rohu
DOI: https://dx.doi.org/10.17582/journal.pjz/20150923011725
* Corresponding author: [email protected]
0030-9923/2021/0002-0459 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
INTRODUCTION
Fishmeal is being used as a major protein source in aquafeeds (Hardy, 1995) as it has high protein content, balanced amino acid profile, utmost digestibility and presence of relatively less anti-nutritional factors. However, due to increasing demand, unstable supply and high price of fishmeal, other alternative protein sources have long been of interest (Lunger et al., 2007). Attempts are being made to use plant proteins in place of fishmeal in aquafeeds (Cain and Garling, 1995). However, these low cost plant proteins have many problems including the presence of anti-nutritional factors like phytate (myo-inositol-1, 2, 3, 4, 5, 6-hexaphosphates). Up to 80% of total phosphorus contents in plant ingredients are present in phytate form which are unavailable to agastric and mono-gastric fish species (NRC, 1993). Therefore, undigested phosphorus is excreted into water which results in eutrophication (Liebert and Portz, 2005). Besides, phytate makes insoluble chelate complexes with indispensable minerals (Papatryphon et al., 1999). Also, it reduces the availability of protein, fat and vitamins by making complexes with them (Liu et al., 1998; Sugiura et al., 2001).
Agastric fish species including L. rohita lack enzyme system to break the phytate. Supplementation of exogenous phytase (PHY) in plant based fish feed has resulted in increased bioavailability of nutrients resulting in improved growth performance. Phytase is an enzyme chemically known as myo-inositol-hexaphosphate phosphohydrolase which catalyzes the hydrolysis of phytate into inositol and orthophosphoric acid (Lei et al., 2013) resulting in release of bound nutrients. Previous studies showed improved nutrients digestibility in L. rohita fingerlings fed on phytase supplemented diets (Hussain et al., 2011a, b, c; Baruah et al., 2007b).
Another approach being applied in animal nutrition to break the phytate is the use of organic acids. Among these organic acids, citric acid (CA) is considered as the most promising acidifier. Zyla et al. (1995) reported that CA dephosphorylate the phytate in vitro. Beside this, these organic acids also help to reduce the gastric pH, increase the activity of digestive enzymes, improve the nutrient digestibility and reduce the bacterial load (Hossain et al., 2007). Previous studies indicated that CA (3%) improve the P utilization in L. rohita (Baruah et al., 2005) while enhanced apparent absorption of P and Mg in Oncorhynchus mykiss was also observed in CA (5%) supplemented group (Sugiura et al., 2001).
An important aspect of PHY is that it is a pH dependent enzyme and performs better at lower pH (2.5 and 5.0-5.5) (Simons et al., 1990). However, L. rohita is an agastric fish species having poor acid secretion in its gut, resulting in higher pH (above 6). Therefore, phytase performance can be enhanced by adding organic acids in the diet which may lower the dietary pH leading to decreased pH in fish gut. Present research was conducted to investigate the influence of CA acidification in PHY treated diet in improving the nutrient digestibility performance by L. rohita fingerlings. Information based on this research may prove useful to develop less expensive and less polluting feed for Labeo rohita.
MATERIALS AND METHODS
The experiment was carried out in Fish Nutrition Laboratory, Department of Zoology and Fisheries, University of Agriculture, Faisalabad, Pakistan. The experiment lasted for 60 days.
Experimental diets
Basal diet was formulated having 37.6% crude protein and 4.72 kcal/g gross energy. Fish meal and soybean meal was added as protein source in basal diet. Fish oil was used as lipids source. Composition of basal diet is shown in Table I. All ingredients were ground, sieved (0.5 mm) and were thoroughly mixed in electric mixer for 10-20 minutes with gradual addition of fish oil. In dry mixed ingredients citric acid was added at three concentrations 0%, 1.5% and 3% and partitioned into three test diets. Inert marker (1% Chromic oxide) was added for digestibility analysis. Dough for extrusion was made with addition of 10-15% of water. Pellets (2 mm) were made by using had pelletizer. These pellets were sprayed by following Jackson et al. (1996) with three concentrations of phytase; 0, 750 and 1000 FTU/kg diet. Hence, supplementation of citric acid and phytase resulted in nine test diets, ranging from T1 having 0% CA and 0 FTU/kg PHY to T9 containing 3% CA and 1000 FTU/kg PHY. Diets with 0 FTU/kg phytase concentration was sprayed with equal amount of water to make moisture contents constant. Pellets were dried up till 8-10% moisture contents remained and stored at 4oC until initiation of feeding trial.
Feeding and experimental units
Fingerlings of Labeo rohita (average weight 9 g) were taken from Government Fish Seed Hatchery, Faisalabad, Pakistan. Before feeding trial, fingerlings were treated with 5 g NaCl/L to make them ectoparasites free and acclimatized in V shaped tanks while feeding on basal diet (Allan and Rowland, 1992) with constant supply of oxygen. A group of 9 fish was stocked in each experimental V shaped tank of 70 L capacity (UA system), peculiarly designed for fecal collection from aquatic media. Triplicate tanks were allocated to each test diet. Water quality parameters (DO 5.8-7.3 mgL-1, pH 7.4-8.6 and temp 24.9-28.7oC) were monitored constantly among all tanks. Fish were fed 2% of live wet body weight twice a day and feeding was adjusted according to weekly weight measure of fish. After three hours of feeding all uneaten diet was removed with washing of tanks. After two hours of washing fecal material was collected via feaces collection tube of V shaped tanks equipped with two valves. Fecal material was collected by subsequent opening of valve I and II and was oven dried at 60oC and stored for further analysis.
Chemical analysis
At the end of feeding trial samples of feed ingredients, test diets and fecal material were homogenized with the help of pestle and mortar and analyzed following AOAC (1995). Dry matter contents were determined by drying in oven at 105oC until constant weight. Crude protein (CP) was calculated by N×6.25 while N was determined by micro Kjeldahl apparatus while crude fat by ether extraction method through Soxtec HT2 1045 system. Gross energy was measured with the help of adiabatic oxygen bomb calorimeter (Parr Instrument Co., Moline, USA). For estimation of minerals, test diets and feces samples were digested in boiling nitric acid and perchloric acid mixture (2:1). After recommended dilutions, minerals were estimated on atomic absorption spectrophotometer (Hitachi Polarized Zeeman AAS, Z-8200, Japan) except P, Na and K. Na and K were estimated on flame photometer (Jenway PFP-7, UK) while P was estimated calorimetrically (UV/VIS spectrophotometer Hitachi U-2001) at 720 nm absorbance. Chromic oxide contents were measured by using UV-VIS 2001 spectrophotometer at 350 nm absorbance after oxidation with molybdate reagent (Divakaran et al., 2002).
Table I. Composition and proximate analysis of test diets.
|
CA level (%) PHY level (FTU/kg) Test diets |
0 |
1.5 |
3 |
||||||
|
0 |
750 |
1000 |
0 |
750 |
1000 |
0 |
750 |
1000 |
|
|
T1 (Control) |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
|
|
Fish meal (%) |
36 |
36 |
36 |
36 |
36 |
36 |
36 |
36 |
36 |
|
Soybean meal (%) |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
|
Rice polish (%) |
14 |
14 |
14 |
14 |
14 |
14 |
14 |
14 |
14 |
|
Wheat flour (%) |
10 |
10 |
10 |
8.5 |
8.5 |
8.5 |
7 |
7 |
7 |
|
Fish oil (%) |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
|
Vitamin premix (%) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Minerals mixture (%) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Ascorbic acid (%) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Chromic oxide (%) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Total |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
|
Proximate analysis |
|||||||||
|
Dry matter (%) |
97.43 |
97.5 |
97.67 |
97.4 |
97.43 |
97.4 |
97.53 |
97.47 |
97.53 |
|
Crude protein (%) |
37.5 |
37.8 |
37.7 |
37.63 |
37.53 |
37.93 |
37.73 |
37.43 |
37.5 |
|
Crude fat (%) |
8.7 |
8.5 |
8.87 |
8.9 |
8.87 |
8.67 |
8.7 |
8.9 |
8.63 |
|
Gross energy kcal/g |
4.72 |
4.66 |
4.74 |
4.72 |
4.73 |
4.72 |
4.69 |
4.68 |
4.68 |
Calculations and statistical analysis
Apparent nutrient digestibility coefficients (ADC%) of test and reference diets were calculated following NRC (1993).
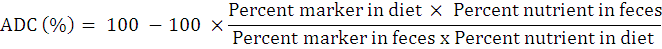
Resulting data was subjected to two way analysis of variance (Steel et al., 1996). Significant differences among means were calculated by Tukey`s Honestly Significant Difference test at 5% significance level (Snedecor and Conhran, 1991). The CoStat computer package (Version 6.303, PMB 320, Monterey, CA, 93940 USA) was used for statistical analysis while interaction plots were made by using Minitab15.
RESULTS
Effect of CA and PHY supplementations on nutrients content in feces is shown in Table II. Both the supplements result in less excretion of dry matter, crude protein, crude fat and gross energy in feces in comparison with the control group (T1), however, minimum excretion was observed in T9 diet accompanied with 3% CA and 1000 FTU/kg phytase level. Similarly, addition of both the additives (CA and PHY) resulted in reduced amount of minerals in fish feces (Table II). Among all the tried levels of phytase, minimum excretion of minerals was recorded at 1000 FTU/kg level. Similarly, CA supplementation also resulted in less discharge of minerals in feces. Also, both the additives act synergistically (p<0.05) to decrease the mineral excretion in fish feces. However, their synergistic effect was not significant for Fe excretion in feces.
Nutrients digestibility results are presented in Table III. Top spraying of phytase on extruded pellets showed improved absorption (digestibility) of dry matter, nutrients (crude protein and crude fat) and gross energy as compare to control group. However, different phytase supplementation levels showed statistically non-significant differences (p<0.05) among themselves for dry matter, crude protein and gross energy digestibility while crude fat was maximum digested by L. rohita at phytase level of 1000 FTU/kg. Similarly, dry matter, nutrients and gross energy digestibility was also enhanced by CA addition in the diet.
Main effect data of PHY addition indicated that top spraying of phytase to plant based diet improved the minerals (P, Ca, Mg, Na, K, Fe, Cu and Zn) digestibility significantly (p<0.05) except Mn. However, both the phytase levels showed same effect (p>0.05) for most of the minerals except Fe and Mn which showed highest digestibility at 750 FTU/kg and 1000 FTU/kg phytase levels respectively.
Dietary addition of CA also improved (p<0.05) mineral absorption as compare to control group. Ca, Na, Mn and Cu showed higher digestibility at 1.5% CA level while other minerals (Mg, K and Fe) showed utmost digestibility at 3% CA level.
Table II. Concentration of nutrients in feces of fish fed CA and PHY containing diets.
|
CA level (%) PHY level (FTU /kg) Test diets |
0 |
1.5 |
3 |
Analysis of variance |
|||||||||
|
0 |
750 |
1000 |
0 |
750 |
1000 |
0 |
750 |
1000 |
|||||
|
T1 (Con-trol) |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
PSE* |
CA |
PHY |
CA× PHY |
|
|
Dry matter (%) |
77.06a |
66.20b |
65.00b |
65.26b |
56.96d |
55.83d |
62.33c |
53.60e |
50.03e |
0.911 |
S |
S |
NS |
|
Crude protein (%) |
26.90a |
21.53c |
21.90bc |
22.16bc |
18.90d |
18.63d |
23.03b |
18.63d |
17.96d |
0.693 |
S |
S |
NS |
|
Crude fat (%) |
6.43a |
5.73b |
5.20c |
5.03c |
4.53e |
4.76d |
5.13c |
4.40e |
3.93f |
0.093 |
S |
S |
S |
|
Gross energy (%) |
3.65a |
3.21c |
3.29b |
3.24b |
2.63e |
2.49f |
3.32b |
2.75d |
2.52f |
0.035 |
S |
S |
S |
|
P (%) |
0.521a |
0.452b |
0.449c |
0.434d |
0.357f |
0.336g |
0.417e |
0.330g |
0.275h |
0.007 |
S |
S |
S |
|
Ca (%) |
0.547a |
0.455b |
0.450b |
0.446c |
0.367d |
0.362d |
0.451b |
0.337e |
0.332e |
0.004 |
S |
S |
S |
|
Mg (%) |
0.423a |
0.345c |
0.344c |
0.351b |
0.227f |
0.225f |
0.348c |
0.247d |
0.233e |
0.004 |
S |
S |
S |
|
Na (mg/g) |
0.558a |
0.449c |
0.452c |
0.450c |
0.355d |
0.351d |
0.476b |
0.343e |
0.354d |
0.004 |
S |
S |
S |
|
K (%) |
0.954a |
0.757c |
0.774b |
0.678d |
0.461h |
0.481f |
0.662e |
0.470g |
0.457i |
0.004 |
S |
S |
S |
|
Mn (μg/g) |
77.66b |
83.66a |
75.66bc |
69.33cd |
67.66d |
64.66d |
75.66bc |
64.00d |
72.66c |
0.000 |
S |
NS |
S |
|
Fe (μg/g) |
0.314a |
0.2163b |
0.2153b |
0.2163b |
0.1163cd |
0.1173c |
0.2146b |
0.116cd |
0.1146d |
0.001 |
S |
S |
NS |
|
Cu (μg/g) |
41.33a |
38.00bc |
36.00c |
38.14bc |
26.01de |
27.33d |
39.03b |
25.66de |
24.33e |
0.000 |
S |
S |
S |
Data are means of three replicates. Means within rows having different superscripts are significantly different at P < 0.05. *PSE, pooled; SE, √MSE/n (where MSE, mean-squared error).
Table III. Effect of CA and PHY supplementation on apparent digestibility coefficient (ADC %) of nutrients in L. rohita fingerlings.
|
CA level (%) PHY level (FTU/kg) Test diets |
0 |
1.5 |
3 |
Analysis of variance |
|||||||||
|
0 |
750 |
1000 |
0 |
750 |
1000 |
0 |
750 |
1000 |
|||||
|
T1 (Control) |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
PSE* |
CA |
PHY |
CA×PHY |
|
|
Dry matter |
30.76f |
40.45e |
41.68e |
40.94e |
48.67c |
49.74c |
43.942e |
51.75b |
54.94a |
1.079 |
S |
S |
NS |
|
Crude protein |
36.85d |
50.03b |
49.08b |
48.08b |
55.79a |
56.93a |
46.45c |
56.33a |
57.90a |
1.921 |
S |
S |
NS |
|
Crude fat |
36.12f |
40.36e |
48.55d |
50.10c |
55.06b |
51.79c |
48.26d |
56.59b |
59.96a |
1.378 |
S |
S |
S |
|
Gross energy |
31.75f |
39.47d |
39.05d |
39.52d |
51.10b |
53.77a |
37.80e |
48.48c |
52.05b |
0.922 |
S |
S |
S |
|
P |
36.87g |
44.42f |
44.66f |
46.61e |
56.10c |
58.82b |
48.80d |
59.37b |
66.12a |
0.923 |
S |
S |
S |
|
Ca |
44.54g |
53.74f |
54.29ef |
54.49e |
62.60d |
63.21c |
54.46e |
65.21b |
66.09a |
0.349 |
S |
S |
S |
|
Mg |
36.32f |
47.14d |
47.32d |
46.13e |
65.43a |
65.53a |
46.67de |
62.39c |
64.32b |
0.579 |
S |
S |
S |
|
Na |
33.15e |
47.07c |
46.81c |
46.92c |
58.11b |
58.67ab |
44.04d |
59.50a |
58.24b |
0.561 |
S |
S |
S |
|
K |
33.11h |
47.02f |
45.85g |
52.39e |
67.73a |
66.36c |
53.83d |
67.13b |
67.99a |
0.307 |
S |
S |
S |
|
Mn |
45.51d |
40.68e |
46.93c |
52.01b |
52.91ab |
54.76a |
46.18c |
55.17a |
48.55c |
1.461 |
S |
NS |
S |
|
Fe |
33.53d |
54.13c |
54.46c |
54.32c |
75.43ab |
75.23ab |
54.48c |
75.15b |
75.69a |
0.233 |
S |
S |
NS |
|
Cu |
33.97d |
39.67cd |
42.21c |
39.61cd |
59.48ab |
55.46b |
37.78d |
59.30ab |
61.75a |
2.427 |
S |
S |
S |
Data are means of three replicates. Means within rows having different superscripts are significantly different at P < 0.05. *PSE, pooled; SE, √MSE/n (where MSE, mean-squared error).
DISCUSSION
Effects of different phytase inclusion methods in the fish diet have been studied including pre and post-treatments. Top spraying (post-treatment) of phytase on feed pellets can retain the phytase activity in the diet (Liu et al., 2013). Improved nutrients digestibility has been reported in carnivorous fish species by top spraying the phytase on dried pellets (Vielma et al., 2004; Wang et al., 2009; Tudkaew et al., 2008). However, there is scarcity of literature concerning the effect of phytase applications through top spraying on nutrients digestibility in the diets of non-carnivorous; agastric fish species.
Present study demonstrated that phytase top spraying to plant based L. rohita diet significantly enhanced the apparent digestibility of dry matter, crude protein, crude fat, gross energy and minerals. Likewise, improved apparent nutrients and minerals digestibility was also observed in our previous study (Hussain et al., 2011a, b, c) in L. rohita fingerlings fed on plant based diets sprayed with graded levels of phytase. Baruah et al. (2007a, b) also observed improved nutrients and minerals absorption in response to phytase supplementation (through spraying) to L. rohita diet. Recently, Liu et al. (2013), while working on another agastric carp species, grass carp (Ctenopharyngodon idellus), reported enhanced ADC% of crude protein, crude lipid, dry matter and minerals in phytase sprayed group in comparison of fish fed on control diet. This increased nutrient digestibility will ultimately lead to improved growth performance of fish (Hussain et al., 2011b).
Inclusion of organic acids in the diet of agastric fish species is an increasing practice now days, as such fishes have no acid secretion mechanism in the gut. Results of present study showed that citric acid supplementation to L. rohita feed enhanced the crude protein, crude fat, gross energy and minerals digestibility. Increased nutrient absorption will not only improved the nutrient profile of fish, but would be helpful in reduction of nutrient loss in feces, which will decrease the need of nutrient supplementation in feed and aquatic pollution caused by nutrient loss in feces. The positive effects of diet acidification by CA supplementation on nutrients absorption were also reported by Baruah et al. (2007a, b) in L. rohita fingerlings fed on plant based diet. Rainbow trout also showed enhanced minerals absorption in response to CA addition to plant based diet (Sugiura et al., 2001). Citric acid increases the bioavailability of nutrients to fish in several different ways. It physically affects the chemical bonds between phytate and different nutrients resulting in release of these bounded nutrients (Atapattu and Nelligaswatta, 2005). Also, being a strong chelator of Ca and P, it removes these minerals from the phytate making it less stable and more susceptible to endogenous phytases (Khajepour and Hosseini, 2010). Furthermore, it helps in increasing the pepsin activity and in decreasing the intestinal microbial load by lowering the gastric pH (De Wet, 2005).
In the present study, CA and PHY synergistically enhanced the crude fat and gross energy digestibility. However, both the additives showed statistically non-significant interaction for the digestibility of dry matter and crude protein digestibility. So far, few studies have been conducted to investigate the nutrient digestibility in response to combined effect of CA and PHY. Similar to our study, Baruah et al. (2007b) also reported a non-significant interaction between CA and PHY on dry matter and crude protein digestibility in L. rohita fingerlings. In this study, a positive interaction between CA and PHY was observed on minerals absorption. CA may have provided optimum conditions for PHY to work by lowering the intestinal pH which lead to their synergitic effect on minerals digestibility. Furthermore, diet acidification lowers the rate of gastric emptying (Mayer, 1994) which also provide more time to the phytases for action. In agreement to our findings, improved minerals absorption was also reported by Baruah et al. (2007a) and Sugiura et al. (2001) in L. rohita and rainbow trout respectively fed on CA acidified phytase treated plant based diets.
CONCLUSION
Both the supplements (CA and PHY) act synergistically to improve the nutrient digestibility in L. rohita fingerlings which may lead to improved growth performance. Furthermore, more nutrient digestibility mean less excretion of these nutrients in the water bodies which may result in less water pollution. The information gathered in this study would contribute to develop the more nutritionally digestible feed for fish, however, more investigations on how increased nutrient digestibility improve the growth and nutrient retention and excretion in fish are needed. In addition, the effect of CA and PHY on digestive enzyme activities also require further evaluation
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Allan, G.L. and Rowland, S.J., 1992. Development of an experimental diet for silver perch (Bidyanus bidyanus). Austasia. Aquacult., 6: 39-40.
AOAC, 1995. Official methods of analysis. 16th Edition. AOAC, Inc., Arlington, Virginia, USA.
Atapattu, N.S.B.M. and Nelligaswatta, C.J., 2005. Effects of citric acid on the performance and the utilization of phosphorous and crude protein in broiler chickens fed on rice by-products based diets. Int. J. Poult. Sci., 4: 990-993. https://doi.org/10.3923/ijps.2005.990.993
Baruah, K., Pal, A.K., Sahu, N.P., Debnath, D. and Yengkokpam., S., 2007a. Interactions of dietary microbial phytase, citric acid and crude protein level on mineral utilization by rohu, Labeo rohita (Hamilton), juveniles. J. World Aquacult. Soc., 38: 238-249. https://doi.org/10.1111/j.1749-7345.2007.00092.x
Baruah, K., Pal, A.K., Sahu, N.P., Jain, K.K., Mukherjee, S.C., and Debnath, D., 2005. Dietary protein level, microbial phytase, citric acid and their interactions on bone mineralization of Labeo rohita (Hamilton) juveniles. Aquacult. Res., 36: 803-812. https://doi.org/10.1111/j.1365-2109.2005.01290.x
Baruah, K., Sahu, N.P., Pal, A.K., Jain, K.K., Debnath, D. and Mukherjee, S.C., 2007b. Dietary microbial phytase and citric acid synergistically enhances nutrient digestibility and growth performance of Labeo rohita (Hamilton) juveniles at sub-optimal protein level. Aquacult. Res., 38: 109-120. https://doi.org/10.1111/j.1365-2109.2006.01624.x
Cain, K.D. and Garling, D.L., 1995. Pretreatment of soybean meal with phytase for salmonid diets to reduce phosphorus concentrations in hatchery effluents. J. Progr. Fish-Cultur., 57: 114-119. https://doi.org/10.1577/1548-8640(1995)057<0114:POSMWP>2.3.CO;2
De-Wet, L., 2005. Organic acids as performance enhancers. Aqua. Feeds Formul. Beyond, 2: 12-14.
Divakaran, S., Leonard, G.O. and Ian, P.F., 2002. Note on the methods for determination of chromic oxide in shrimp feeds. J. Agric. Fd. Chem., 50: 464-467. https://doi.org/10.1021/jf011112s
Engelen, A.J., Van Der Heeft, F.C., Randsdrop, P.H.G. and Smith, E.L.C., 1994. Simple and rapid determination of phytase activity. J. AOAC Int., 77: 760-764. https://doi.org/10.1093/jaoac/77.3.760
Hardy, R.W., 1995. Current issues in salmonid nutrition. In: Nutrition and utilization technology in aquaculture (eds. C.E. Lim and D.J. Sessa). AOCS Press, Champaign, IL, USA, pp. 26-35.
Hossain, M.A., Pandey, A. and Satoh, S., 2007. Effects of organic acids on growth and phosphorus utilization in red sea bream Pagrus major. Fish. Sci., 73: 1309-1317.
Hussain, S.M., Afzal, M., Rana, S.A., Javid, A. and Iqbal, M., 2011b. Effect of phytase supplementation on growth performance and nutrient digestibility of Labeo rohita fingerlings fed on corn gluten meal-based diets. Int. J. Agric. Biol., 13: 916-922.
Hussain, S.M., Afzal, M., Rana, S.A., Javid, A. and Hussain, M., 2011a. Impact of phytase supplementation on nutrient digestibility for Labeo rohita fingerlings fed on sunflower meal based diets. Pak. J. Life Soc. Sci., 9: 85-90.
Hussain, S.M., Rana, S.A., Afzal, M. and Shahid, M., 2011C. Efficacy of phytase supplementation on mineral digestibility in Labeo rohita fingerlings fed on corn gluten meal (30%) based diets. Pak. J. agric. Sci., 48: 237-241.
Khajepour, F. and Hosseini, A., 2010. Mineral status of juvenile beluga (Huso huso) fed citric acid supplemented diets. World appl. Sci. J., 11: 682-686.
Jackson, L.S., Li, M.H. and Robinson, E.H. 1996. Use of microbial phytase in channel catfish Ictalurus punctatus diets to improve utilization of phytate phosphorus 1. J. World Aquacult. Soc., 27: 309-313.
Lei, X.G., Weaver, J.D., Mullaney, E., Ullah, A.H. and Azaim, M.J., 2013. Phytase, a New Life for an “Old” Enzyme. Annu. Rev. Anim. Biosci., 1: 283-309. https://doi.org/10.1146/annurev-animal-031412-103717
Liebert, F. and Portz, L., 2005. Nutrient utilization of Nile tilapia, Oreochromis niloticus fed plant based low phosphorus diets supplemented with graded levels of different sources of microbial phytase. Aquaculture, 248: 111-119. https://doi.org/10.1016/j.aquaculture.2005.04.009
Liu, B.L., Rafing, A. and Tzeng, Y.M., 1998. The induction and characterization of phytase and beyond. Enzyme Microb. Technol., 22: 415-424. https://doi.org/10.1016/S0141-0229(97)00210-X
Liu, L.W., Luo, Y.L., Hou, H.L., Pan, J. and Zhang, W., 2013. Partial replacement of monocalcium phosphate with neutral phytase in diets for grass carp, Ctenopharyngodon idellus. J. appl. Ichthyol., 29: 520-525. https://doi.org/10.1111/jai.12021
Lunger, A.N., Mclean, E. and Craig, S.R., 2007. The effects of organic protein supplementation upon growth, feed conversion and texture quality parameters in juvenile cobia (Rachycentron canadum). Aquaculture, 264: 342-352. https://doi.org/10.1016/j.aquaculture.2006.12.012
Mayer, E.A., 1994. The physiology of gastric storage and emptying. In: Physiology of gastrointestinal tract (ed. L.R. Johnson). Raven Press, New York.
NRC (National Research Counsil), 1993. Nutrient requirements of fish. Nat. Acad. Press, Washington DC, USA, pp. 114.
Papatryphon, E., Howell, R.A. and Soares, J.H., 1999. Growth and mineral absorption by striped bass, Morone saxatilis fed a plant feedstuff based diet supplemented with phytase. J. World Aquacult. Soc., 30: 161-173. https://doi.org/10.1111/j.1749-7345.1999.tb00863.x
Robinson, E.H., Li, M.H. and Manning, B.B., 2002. Comparison of microbial phytase and dicalcium phosphate for growth and bone mineralization of pond-raised channel cat fish, Ictalurus punctatus. J. appl. Aquacult., 12: 81-88. https://doi.org/10.1300/J028v12n03_08
Rowland, S.J. and Ingram, B.A., 1991. Diseases of Australian native fishes. NSW Fisheries, Sydney, NSW, Australia.
Simons, P.C.M., Versteegh, H.A.J., Jongbloed, A.W., Kemme, P.A., Slump, P., Bos, K.D. Wolters, W.G.E., Beudeker, R.F. and Verschoor, G.J. 1990. Improvement of phosphorus availability by microbial phytase in broilers and pigs. Br. J. Nutr., 64: 525-540. https://doi.org/10.1079/BJN19900052
Snedecor, G.W. and Cochran, W.G., 1991. Statistical methods. 8th Ed. Iowa State Univ. Press, Ames. USA, p. 503.
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1996. Principles and procedures of statistics, 3rd Ed. McGraw Hill International Book Co. Inc., New York. USA. pp. 336-352.
Sugiura, S.H., Gabaudan, J., Dong, F.M. and Hardy, R.W., 2001. Dietry microbial phytase supplementation and the utilization of phosphorus, trace minerals and protein by rainbow trout (Oncorhynchus mykiss Walbaum). Aquacult. Res., 32: 583-592. https://doi.org/10.1046/j.1365-2109.2001.00581.x
Tudkaew, J., Gabaudan, J. and Phromkunthong, W., 2008. The supplementation of phytase RONOZYME P on the growth and utilization of phosphorus by sex-reversed red tilapia (Oreochnoromis niloticus Linn.). Songklanakarin J. Sci. Technol., 30: 17-24.
Vielma, J., Ruohonen, K., Gabaudan, J. and Vogel, K., 2004. Top-spraying soybean meal-based diets with phytase improves protein and mineral digestibilities but not lysine utilization in rainbow trout, Oncorhynchus mykiss (Walbaum). Auacult. Res., 35: 955-964. https://doi.org/10.1111/j.1365-2109.2004.01106.x
Wang, F., Yang, Y.H., Han, Z.Z., Dong, H.W., Yang, C.H. and Zou, Z.Y., 2009. Effects of phytase pretreatment of soybean meal and phytase-sprayed in diets on growth, apparent digestibility coefficient and nutrition excretion of rainbow trout (Oncorhynchus mykiss Walbaum). Aquacult. Int., 17: 143-157. https://doi.org/10.1007/s10499-008-9187-5
Zyla, K., Ledoux, D.R., Garcia, A. and Veum, T.L., 1995. An in vitro procedure for studying enzymatic phosphorylation of phytate in maize-soybean feeds for turkey poults. Br. J. Nutr., 74: 3-17. https://doi.org/10.1079/BJN19950102
To share on other social networks, click on any share button. What are these?









