Ophiotaenia tessellata sp. n. (Eucestoda: Proteocephalinae) from Natrix tessellata (Laurenti, 1768) (Serpentes: Colubridae) in Egypt
Ophiotaenia tessellata sp. n. (Eucestoda: Proteocephalinae) from Natrix tessellata (Laurenti, 1768) (Serpentes: Colubridae) in Egypt
Irene S. Gamil1* and Dalia Fouad1,2
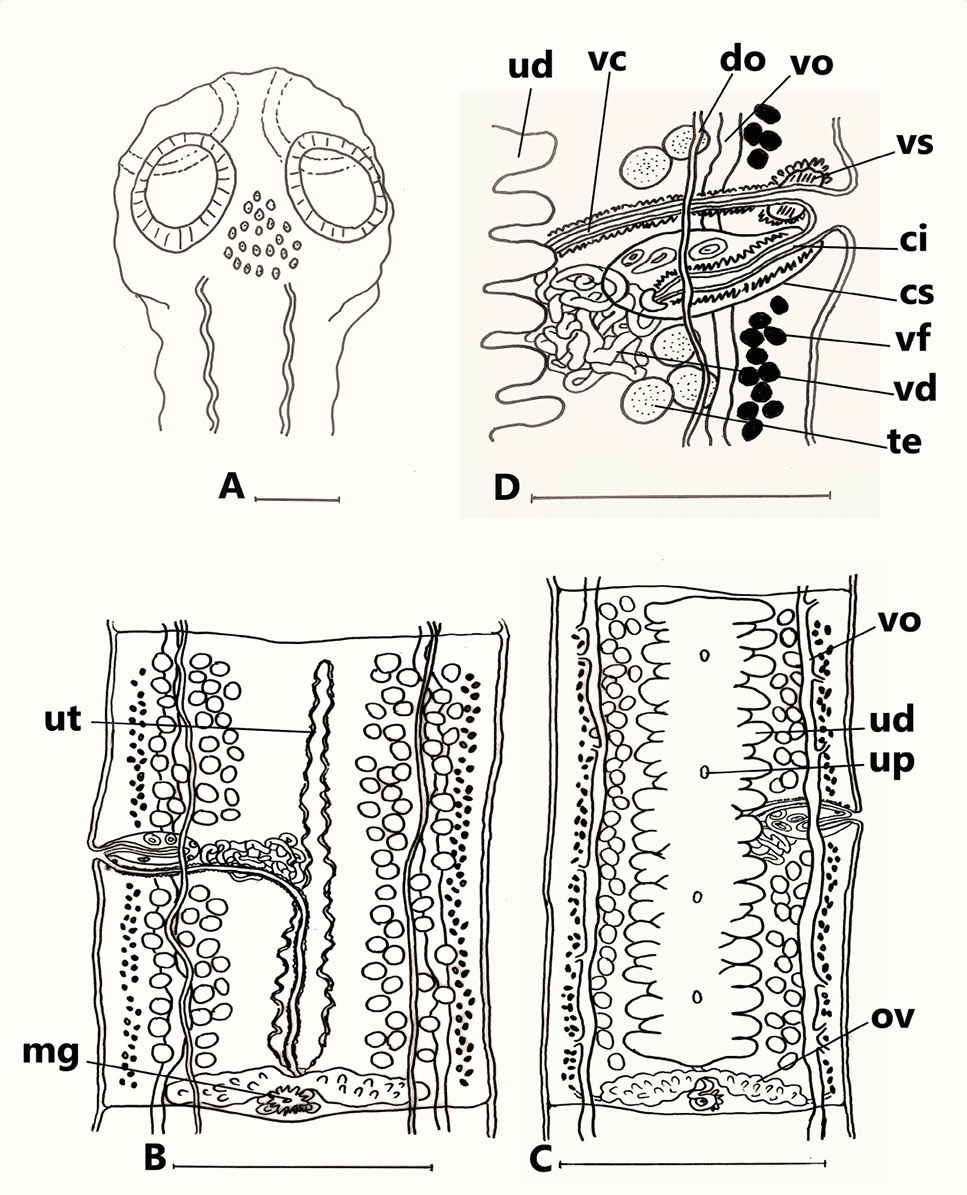
Ophiotaenia tessellata sp. n. from Natrix tessellata, Egypt. (A) Mature proglottis, transverse section at ovarian level; (B) cross-section of gravid proglottis, at level of anterior part; (C) eggs drawn after examination in distilled water. Abbreviations: do, dorsal osmoregulatory canal; dp, digitiform projections; em, embryophore; lm, internal longitudinal musculature; ln, longitudinal lateral nerves; mg, Mehlis’ gland; oe, outer envelope; om, oncospheral membrane; on, oncosphere; ov, ovary; te, testes; ut, uterus; vd, vitelline duct; vf, vitelline follicles; vo, ventral osmoregulatory canal. Scale-bars: A= 500μm; B= 250μm; C= 50μm.
(A-F, H-K) Scanning and, (G, L) Transmission electron micrographs of Ophiotaenia tessellata sp. n. from Natrix tessellata, Egypt. (A) Scolex with 4 suckers showing wrinkles and folds; (B) an enlarged sucker showing the transverse slit-like structure (sl); (C) acicular filitriches covering the proliferative zone; (D) a tumulus on the proliferative zone; (E) Burst of the tumulus on the proliferative zone forming large pore. Note also the excretory pore (ex); (F) acicular filitriches covering immature proglottis; (G) acicular filitriches and few gladiate spinitriches covering mature proglottis; (H) excretory pores scattered on mature proglottis; (I) a tumulus on mature proglottis; (J) acicular filitriches covering gravid proglottis; (K) one uterine pore; (L) an egg in a stage of development showing the oncosphere (on), oncospheral membrane (om) and bi-layered embryophore (em). Scale-bars: A= 50μm; B, K= 10μm; C, J= 1μm; D-F, L= 2μm; G= 500nm; H= 20μm; I= 5μm.
Molecular Phylogenetic analysis by Maximum Likelihood method based on 18S rRNA gene sequence of Ophiotaenia tessellata sp. n. Molecular Phylogenetic analysis by Maximum Likelihood method. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model. The tree with the highest log likelihood (-1795.64) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 23 nucleotide sequences. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 453 positions in the final dataset. Evolutionary analyses were conducted in MEGA7.