Phytochemical Screening and In Vitro Antioxidant Activity of Extracts of Ipomoea purpurea Leaves from Iran
Research Article
Phytochemical Screening and In Vitro Antioxidant Activity of Extracts of Ipomoea purpurea Leaves from Iran
Fatemeh Beheshti1, Maliheh Safavi2, Mohammad Reza Akbari Eidgahi1,3, Parviz Kokhaei4, Mehdi Vazirian5 and Ali Akbar Shabani1,3*
1Research Center of Biotechnology, Semnan University of Medical Sciences, Semnan, Iran; 2Department of Biotechnology, Iranian Research Organization for Science and Technology, 13353-5111, Tehran, Iran; 3Department of Biotechnology, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran; 4Cancer Research Center, Semnan University of Medical Sciences, Semnan, Iran; 5Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
Abstract | Nowadays, the use of medicinal herbs and derivatives to improve the development of multifunctional therapeutic agents is growing. The purpose of this study is to determine the antioxidant capacity of Ipomoea purpurea leaves that grows in Iran as well as the chemical composition of the leaves. Thus, using in vitro established procedures, the antioxidant capacity of organic and aqueous extracts of I. purpurea leaves was assessed. Further, the ethanol and chloroform extracts of I. purpurea leaves were analyzed using gas chromatography-mass spectrometry (GC-MS). Concentration-dependent scavenging of 2, 2-diphenyl-2-picryl hydrazil (DPPH) was seen across all three extract types (ethanol, water, and chloroform) with the IC50 values of 42.30 ± 2.35, 54.11 ± 3.62, and 85.71 ± 4.83 μg/mL, respectively which were higher compared to the positive control (ascorbic acid = 8.07 ± 0.30 μg/mL) and (butylated hydroxyanisole or BHA = 13.9 ± 0.54 μg/mL). The ferrous reducing antioxidant power (FRAP) of the extracts was in the following order: ethanol > aqueous > chloroform. As the ethanol extract exhibited higher levels of total phenols, (29.78 ± 0.11 mg gallic acid equivalent /100 g dry leaves weight), flavonoid (19.9 ± 3.98 mg catechin equivalent /100 g dry leaves weight), and steroids content (6.85 ± 0.07 mg steroids equivalent /100 g dry leaves weight) compared to chloroform extract. In addition, GC-MS research showed that the extracts of I. purpurea leaves contain a wide diversity of chemicals. Findings from this research revealed that I. purpurea leaves contain chemicals with excellent antioxidant and biological capabilities, which might be attributed to the existence of bioactive molecules and provide additional data for future research.
Received | September 26, 2023; Accepted | Novemer 22, 2023; Published | December 01, 2023
*Correspondence | Ali Akbar Shabani, Research Center of Biotechnology, Semnan University of Medical Sciences, Semnan, Iran; Email: [email protected]
Citation | Beheshti, F., M. Safavi, M.R.A Eidgahi, P. Kokhaei, M. Vazirian and A.A. Shabani. 2023. Phytochemical screening and in vitro antioxidant activity of extracts of ipomoea purpurea leaves from Iran. Biologia (Lahore), 69(2): 32-39.
DOI | https://dx.doi.org/10.17582/journal.Biologia/2023/69.2.32.39
Keywords | Ipomoea purpurea, Antioxidant activity, Phytochemical screening, GC-MS analysis
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The pathogenesis and progression of numerous severe and fatal diseases, including breast cancer, have been interfered with by systemic oxidative stress (Nechuta et al., 2014). Oxidative stress can be a primary factor in toxicity and disease and has been associated with a wide range of pathologies (Forman and Zhang, 2021). It is caused by an imbalance between high quantities of reactive harmful chemicals and the antioxidative defense systems (Kiran et al., 2023). It can damage the different levels of biological organization and also can cause systemic inflammation (Bourgonje et al., 2021). Oxidative stress occurs when the amount of reactive oxygen species (ROS) overshadows the antioxidant capacity (Gawlik-Dziki et al., 2013). ROS has an effective influence at lower concentrations, but a large amount of this substance can damage the DNA structure (Ji and Yeo, 2021). Antioxidants are effective systems that can reduce and repair the damage of free radicals (Kiran et al., 2023). Antioxidants’ ability to scavenge free radicals and block their production is particularly useful in the prevention of cancer and other illnesses including atherosclerosis, Parkinson’s, diabetes, and heart disease. Antioxidant molecules not only reduce oxidative stress but also reduce inflammation and cancer disease. For several decades, researchers have investigated how oxidative stress can play a role in the onset and development of cancer (Ozben, 2007; Sugata et al., 2015).
Plants, as the main source of compounds rich in biological and medicinal properties, have an important role in the prevention and treatment of diseases and in reducing the side effects of synthetic drugs. Despite the dependence of the development of new drugs on synthetic chemicals, the importance of plants in the control and treatment of diseases is very important. Phytochemicals were among the first discovered antineoplastic drugs that have been used at the center of cancer research from the beginning (Dehelean et al., 2021). Biological control is defined as direct or indirect inhibition of a disease, or the pathogen causing the disease, by another organism or group of organisms (Collinge et al., 2022). Plant-derived secondary bioactive compounds such as phenol, alkaloids, and flavonoids are active substances that have great potential for utilization in the modern pharmaceutical industry. Alkaloids that include one or more nitrogen atoms in their molecular structure are the most common type of naturally occurring molecule generated by plants. One essential technique for the separation of various substances and identification of plant secondary bioactive metabolites is gas chromatography (GC). Plants can produce a large amount of secondary metabolites that have significant biological activities (Abo-Altemen et al., 2019; Collinge et al., 2022).
Ipomoea is a member of the family Convolvulaceae, which includes over 1650 primarily tropical species. Some important Ipomoea species have been utilized for a variety of traditional medical and dietary uses since ancient times. Some species of Ipomoea have biological and therapeutic activities such as antimicrobial and anticancer properties and for this reason, they can be used to treat plant diseases (Ojha et al., 2016; Batiga et al., 2018). Some Ipomoea species contain phytotoxic action, which suppresses the growth of invasive weeds. The flowers of I. purpurea contain antioxidant molecules such as cyanidin and pelargonidin. Also, this species along with species tricolor and violacea are enriched with ergoline derivatives such as lysergol and isoergine, so these plants are known for entheogen activities (Meira et al., 2012; Beheshti et al., 2021).
This study’s objectives were to assess I. purpurea leaves extracts’ antioxidant activity and to characterize the possible chemical components using GC-MS.
Materials and Methods
Chemicals
Analytical-grade chemicals and solvents were used throughout the study. Chloroform, hexane, ethanol, ethylacetate, Folin-Ciocalteu reagent, sodium carbonate anhydrous, and aluminum chloride hexahydrate were purchased from Merck, Germany. DPPH, ferric chloride, potassium acetate, phosphate buffer, acetate buffer, FeSO4, ascorbic acid, ferric-tripyridyltriazine (TPTZ), gallic acid, Liebermann-Burchard reagent, butylated hydroxyanisole, and catechin were acquired from Sigma-Aldrich Co., St. Louis, USA.
Sample collection and authentication
The I. purpurea was authenticated by the herbarium department of the Faculty of Pharmacy, Tehran University of Medical Sciences (6595-TEH).
Plant material and extract preparation
Two hundred grams of the dry leaves powder of I. purpurea were poured into a container and added to 600 ml of 100% hexane solvent. After 24 hours, the hexane solvent was drained into the out and the extraction was repeated three times each for 24 hours. Then after making sure that the plant powder was dried, chloroform solvent was added to the plant powder and this process was repeated three times each time for 24 hours. Similar to the same process, extraction was performed on the plant powder obtained with ethyl acetate and ethanol solvents, and in some cases, if necessary, TLC was used to ensure the extraction of all effective substances. All extractions were conducted with mechanical stirring at room temperature for 24 hours (25°C) except the hot water extract (80°C), which was completed in 30 minutes. After soaking the material in organic solvents, filtering, and then extracting the residue twice more, the supernatants were combined. Freeze-drying was used to concentrate the aqueous extracts, while a rotary evaporator at 20°C (Heidolph, HeizbadHei-VAP, Germany) was used to concentrate the organic extracts. The concentrated crude extracts were preserved and used for further investigation.
Antioxidant activity of the extracts
The DPPH test was modified to assess the extracts’ capacity to scavenge free radicals (Gavamukulya et al., 2014). DPPH 0.2 mM in 95% ethanol was prepared and used immediately after preparation. Different concentrations of I. purpurea leaves extracts (20-400 μg/mL) and standard solutions including ascorbic acid and BHA (1-40 μg/mL) were prepared in ethanol as solvent. Each well of a 96-well microplate received a combination of 200 μl of ethanolic DPPH solution and 20 μl of the ethanolic extract in triplicate. The reaction mixture in the plate was vortexed thoroughly and then it was allowed to incubate for 30 min at room temperature and in the dark. Using a multi-well plate reader, the absorbance of each well was measured at a wavelength of 517 nm (Gen5, Epoch, BioTek). The following equation was used to compute the percentage of DPPH radical inhibition:
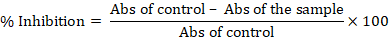
The determination of reducing ability of I. purpurea leaves extracts was done using a modified technique developed by Benzie and Strain (1999). 10 mM TPTZ solution in 20 mM FeCl3.6H2O, and 40 mM HCl and 300 mM acetate buffer were constituents of FRAP reagent. So, each time, a fresh working solution was made by mixing 2.5 mL TPTZ, 6 mL FeCl3, and 25 mL acetate buffer. In a 96-well microplate containing 200 µl of the FRAP working solution, the I. purpurea leaves extracts were allowed to react for 4 min in the dark. With the use of a microplate reader (Gen5, Epoch, BioTek), the absorbance at 595 nm was measured. The data were represented as mM of FeSO4 equivalent at 30 min and compared with that of ascorbic acid.
Phytochemical analysis
Preliminary phytochemical screening of I. purpurea leaves extracts in ethanol and chloroform was carried out to identify chemical constituents using standard procedures (Ezeldin et al., 2018).
Total phenolic, flavonoid and steroid determination
The total phenolic contents of the ethanol and chloroform extracts were measured by the Folin-Ciocalteu assay, according to the standard procedure with minor modifications (Mazandarani, 2015). The concentration of the total phenolic was measured using the equation obtained from the standard curve and presented as mg gallic acid equivalent per gram of extract (GAE mg/g dry weight).
The spectrophotometric assays based on the aluminum chloride colorimetric method were applied for the calculation of total flavonoid content of the extracts with some modifications (Sanseera et al., 2016). The standard curve was used to measure the flavonoid content, and the findings were represented as mg/g dry weight of catechin equivalent (CAE).
The spectrophotometric assays based on the Liebermann-Burchard reaction colorimetric method were used for the determination of the steroid content of the extracts with some modifications (Belani and Kaur, 2018).
GC-MS analysis
The leaves of I. purpurea were analyzed by GC-MS on Agilent Technologies GC systems with GC-7890A/MS-5975C model using HP-5MS Capillary column (30 m in length × 250 µm in diameter × 0.25 µm in the thickness of film). For GC-MS detection an electron ionization device with 70 electron volt ionization energy was applied. The carrier gas was 99.999% pure helium gas flowing at a rate of 1 mL/min. We decided on a temperature of 230°C for the injector. The baseline temperature range was designed to be between 50 and 150°C, with a rise rate of 3°C/min and a holding duration of roughly 10 min. Split mode injection of 1 µl of material at 0.5-second scan intervals and 40-450 Da scan fragments was used. With peak area normalization, the relative proportion of the extract was reported as a percentage.
Statistical analysis
The Graph Pad Prism 9.00 software suite was used to gather experimental data. ANOVA and the Student’s t-test were used to calculate the significance of the mean differences.
Results and Discussion
In vitro antioxidant activity
The DPPH dose-response curve of I. purpurea leaves extracts is depicted in Figure 1a. In Figure 2, IC50 values of the most effective I. purpurea leaves extracts were compared with standards (ascorbic acid and BHA). The findings demonstrated that there were significant differences (P<0.0001) between each of the extracts and the standard.
The FRAP assay method revealed a similar pattern of antioxidant activity, with the ethanol extract exhibited the highest ferric ion reduction potential (1518 ± 97.58 µmol Fe2+) followed by the aqueous and chloroform extracts with FRAP values 340 ± 42.42 µmol Fe2+and 319.5 ± 26.87 µmol Fe2+, respectively. The percentage of inhibition marginally rose as the concentration of plant extract increased, indicating a dose-response relationship (Figure 1b).
Phytochemical screening
Phytochemical screening was performed on ethanol and chloroform extracts of I. purpurea leaves to identify their chemical components and the results exhibited that they contained steroids, phenols, and flavonoids (Table 1).
Table 1: Preliminary phytochemical screening of the extracts from I. purpurea leaves.
|
Phytochemical test |
Name of the test |
Solvent extract |
|
|
Ethanol |
Chloroform |
||
|
Flavonoids |
Shinoda test |
+ |
+ |
|
Steroids |
Salkowski and Liebermann-Burchard tests |
+ |
+ |
|
Saponins |
Foam test |
- |
- |
|
Tannins |
Ferric chloride test |
+ |
- |
|
Alkaloids |
Meyer’s and Wagner’s tests |
- |
- |
(+) = present, (−) = absent.
Total phenolic, flavonoid, and steroid estimation
The data in Table 2 showed that the ethanol leaves extract had higher total phenolic, flavonoid, and steroid contents as compared to the chloroform leaves extract. Differences in the extracts were found to be statistically significant (P<0.01 and P<0.0001).
Table 2: Total phenolics, flavonoids and steroids content of the extracts of I. purpurea leaves.
|
Solvent extract |
Total phenolic content (mg GAE/g extract) |
Total flavonoid content (mg CAE/g extract) |
Steroids content (mg CE/g extract) |
|
Ethanol |
29.78 ± 0.11a |
19.9 ± 3.98c |
6.85 ± 0.07e |
|
Chloroform |
6.78 ± 0.11b |
5.81 ± 0.004d |
3.41 ± 0.12f |
Each value is expressed as mean ± standard deviation, (n=3). Values in the column followed by a different letter superscript are significantly different (P< 0.01and P< 0.0001) and values having the same letters are not statistically significant.
GC-MS analysis
Several bioactive chemicals in I. purpurea leaves extracts were found using GC-MS analysis, and these compounds are shown in Tables 3 and 4 in the order in which they eluted from an HP-5MS column. Characterization of these compounds was assessed by comparison of their fragmentation pattern and records reported in the National Institute of Standards and Technology (NIST) library. The ethanol extract of the I. purpurea was found to contain 6 components, the most abundant being nonadecane with a retention time of 2.912 min (99.74%) followed by decane (0.1%). The chloroform extract of the I. purpurea contained 14 components such as nonadecane with a retention time of 2.938 min (94.63%).
A literature study reveals a scarcity of reports detailing the I. purpurea chemical composition, and most of the information is scattered (Fatima et al., 2014). Importantly, no study to our knowledge has evaluated detailed analyses by GC-MS of I. purpurea to date. The extracts with the highest antioxidant power were selected for GC analysis. The analyses showed that the extracts from I. purpurea leaves consisted mainly of aliphatic hydrocarbons such as alkanes, followed by alcohols, esters, fatty acids, and phenolic compounds. Formerly, most of the main compounds had been reported from some other plant species. Elufiyoe and Brida (2018) analyzed the antioxidant activity of Spondias purpurea L. The total phenolic and total flavonoid content of the extracts and fractions of S. purpurae were evaluated through GC-MS analysis. Compounds including hexadecanoic acid and pentadecanoic acid were among the compounds produced. The high phenolic and flavonoid contents of the stem bark extract could be responsible for its greater antioxidant activity. (Elufiyoe and Brida, 2018)
Table 3: Biologically active chemical compounds of ethanol extract from I. purpurea leaves.
|
Name of compounds (molecular formula) |
Retention time (min) |
% |
|
Nonadecane (C19H40) |
2.912 |
99.74 |
|
Tetradecane (C14H30) |
10.153 |
0.06 |
|
Dodecane (C12H26) |
14.333 |
0.04 |
|
Decane (C10H22) |
21.835 |
0.1 |
|
Bicyclo[4.2.0] octa-1,3,5- triene (C8H8) |
28.592 |
0.06 |
|
Dimethyl ether (C2H6O) |
34.618 |
0.01 |
In previous studies on the genus Ipomoea, the amount of hydrocarbons was reported lower (Prasanth et al., 2018). In other words, the profile of constituents in this species by GC-MS analysis was found different from that of the other members of the genus Ipomoea. Hence, further studies can provide the new information needed for confirmation (Adsul et al., 2009; Hue et al., 2012; Kumar et al., 2014). Probably, differences in the composition of Ipomoea extracts can be related to the extraction method and geographical location. Correspondingly with our work, a study conducted by Adsul and colleagues found hydrocarbons, fatty acids, esters, and alcohols in a hexane extract of I. carnea using GC-MS (Adsul et al., 2009). Similarly, the results of our work with the GC-MS method were somewhat consistent with the study conducted by Kumar and colleagues on the extracts of the I. pes-caprea (Kumar et al., 2014).
Table 4: Biologically active chemical compounds of chloroform extract of I. purpurea leaves.
|
Name of compounds (molecular formula) |
Retention time (min) |
% |
|
Nonadecane (C19H40) |
2.938 |
94.63 |
|
2-Hexyl-1-octanol (C14H30O) |
3.479 |
4.02 |
|
Trichloroacetic acid, hexadecyl ester (C18H33Cl3O2) |
10.032 |
0.05 |
|
Nonadecane (C19H40) |
21.844 |
0.06 |
|
Phenol,2,4-bis (1,1-dimethylethyl)- (C14H22O) |
23.766 |
0.49 |
|
Tetradecane (C14H30) |
25.534 |
0.07 |
|
2-Isopropyl-5-methyl-1-heptanol (C11H24O) |
25.815 |
0.1 |
|
2-Isopropyl-5-methyl-1-heptanol (C11H24O) |
26.099 |
0.07 |
|
Isotridecanol- (C13H28O) |
28.597 |
0.07 |
|
Benzene,1,3-bis (1, 1,-dimethylether)- (C14H22) |
32.073 |
0.17 |
|
Dodecane (C12H26) |
34.623 |
0.18 |
|
Styrene(C8H8) |
37.374 |
0.01 |
|
Carbon Tetrachloride (CCl4) |
39.245 |
0.07 |
|
Trichloromethane (CHCl3) |
40.033 |
0.01 |
Their findings identified a suitable solvent for phenolic compounds extraction which might provide a rich source of natural antioxidants. Kurniasih and colleagues evaluated and analyzed purple sweet potato (Ipomoea batatas L.) leaves ethanolic extract by GC-MS and concluded that phenolic compounds and then fatty acids comprise the most compounds in the ethanolic extract of the plant (Kurniasih and Saputri, 2019). In our research, nonadecanes were the predominant compound produced in the ethanolic and chloroform extracts of the plant. In 2020, Kavitha evaluated the ethanolic extract of the Trichosanthesis Dioica roxb and reported similar results similar to our research findings. Alcohols, esters, and fatty acids were among the compounds found in the ethanolic extract (Kavitha, 2021). Similar to our results, Bai and colleagues in 2014 investigated the chloroform extract of the Acacia nilotica L. leaves by GC method. Similar to our research, alcohols, and fatty acids were among the compounds that were registered in this research (Bai et al., 2014). Kopuswamy et al. (2013) investigated the chloroform extract of the Croton bonplandianum by GC method. In this study, GC-MS analysis showed the presence of phyto-components in the leaves of the plant and justified its medicinal usage. The results showed that alcoholic compounds followed by enthexadecane, octadecane, and nonadecane had the highest concentration among the compounds produced (Kopuswamy et al., 2013). In our research, most of the investigated compounds were nonadecans.
Results of several investigations showed that long-chain alkanes, 2, 4-bis (1,1-dimethyl ethyl) phenol, 2-isopropyl-5-methyl-1-heptanol, and 2-hexyl-1-octanol possessed diverse pharmacological effects (Mannaa and Kim, 2018). They may have pharmacologic effects that contribute to the healing potential of the plant (Casuga et al., 2016). Al-Owaisi et al. (2014) investigated the compounds in the extract of Moringa pepegrina (Forssk) Fiori leaves. The results showed that many bioactive compounds such as alkaloids, flavonoids, and steroids were produced by the plant and the extracts of the leaves of this plant were very rich in phenolic compounds (Al-Owaisi et al., 2014). In our research, the ethanol and chloroform extracts of the plant were more rich in nonadecanes than other compounds. There is a need for more study to back up biological investigations, although the results provided here represent the foundational work of chemical composition and biological qualities.
Conclusions and Recommendations
There is a trend to determine antioxidant materials from natural products in the new medical world. In summary, our work exhibits that the extracts from I. purpurea leaves are high in phenolic contents and express strong antioxidant power. The existence of bioactive chemicals with established therapeutic value has also been shown by GC-MS analysis.
Acknowledgments
This study was supported in part by grant 1130 from the Semnan University of Medical Sciences, Faculty of Medicine, and Iranian Research Organization for Science and Technology.
Novelty Statement
This study proved strong antioxidant power and high phenolic content in I. purpurea leaves.
Author’s Contribution
FB performed the biological experiments and wrote the manuscript. MS supervised and designed the current project and was involved in editing and correction of the manuscript. MRAE evaluated the data and edited the manuscript. PK collaborated in data observation and editing the manuscript. MV conceived and designed the biological experiments. AAS supervised and was involved in revising the manuscript.
Conflict of interest
The authors have declared no conflict of interests.
References
Abo-Altemen, R.A., A.M. Al-Shammari, M.S. Shawkat. 2019. GC-MS analysis and chemical composition identification of Cyperus rotundus L. from Iraq. Energy Proc., 157: 1462-1474. https://doi.org/10.1016/j.egypro.2018.11.311
Adsul, V., E. Khatiwora, M. Kulkarni, A. Tambe, P. Pawar, N. Deshpande. 2009. GC-MS study of fatty acids, esters, alcohols from the leaves of Ipomoea carnea. Int. J. Pharma. Technol. Res., 1(4): 1224-1226.
Al-Owaisi, M., N. Al-Hadiwi, S.A. Khan. 2014. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac. J. Trop. Biomed., 4(12): 964-970. https://doi.org/10.12980/APJTB.4.201414B295
Bai, S., L. Seasotiya, A. Malik, P. Bharti and S. Dalal. 2014. GC-MS analysis of chloroform extract of Acacia nilotica L. leaves. J. Pharmacogn. Phytochem., 2(6): 79-82.
Batiga, S., M. Valli, M.L. Zeraik, K. Fraige, G.M. Leme, N.S. Pitangui, A.M.F. Almeida, S. Michel, M.C.M. Young, V.S. Bolzani. 2018. Chemical composition and biological properties of Ipomoea procumbens. Rev. Brasil. Farmacogn., 29: 191-197. https://doi.org/10.1016/j.bjp.2018.08.010
Beheshti, F., A.A. Shabani, M.R.A. Eidgahi, P. Kokhaei, M. Vazirian and M. Safavi. 2021. Anticancer activity of Ipomoea purpurea leaves extracts in monolayer and three-dimensional cell culture. Evid. Based Complement. Altern. Med., https://doi.org/10.1155/2021/6666567
Belani, S. and C. Kaur. 2018. Qualitative and quantitative analysis of phytochemicals of barleria prionitis. Int. J. Recent Trends Sci. Technol., (Special Issue): 250-254.
Benzie, I.F., J.J. Strain. 1999. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol., 299: 15-27. https://doi.org/10.1016/S0076-6879(99)99005-5
Bourgonje, M.F., A.R. Bourgonje, A.A. Abdulle, L.M. Kieneker, S.L.B. Gemert, R.T. Gansevoort, S.J.L. Bakker, D.J. Mulder, A. Pasch, J. Saleh, S.J. Gordijn, H. Goor. 2021. Systemic oxidative stress, aging and the risk of cardiovascular events in the general female population frontiers in cardiovascular medicine, pp. 8. https://doi.org/10.3389/fcvm.2021.630543
Casuga, F.P., A.L. Castillo, M.J.A. Corpuz. 2016. GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pac. J. Trop. Biomed., 6(11): 957-961. https://doi.org/10.1016/j.apjtb.2016.08.015
Collinge, D.B., D.F. Jensen, M. Rabiey, S. Sarrocco, M.W. Shaw, R.H. Shaw. 2022. Biological control of plant diseases. What has been achieved and what is the direction? Plant Pathol., 71: 1024–1047. https://doi.org/10.1111/ppa.13555
Dehelean, C.A., Marcovici, I., Soica, C., Mioc, M., Coricovac, D., Iurciuc, S., Cretu, O.M., Pinzaru, I. 2021. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules, 26: 1109. https://doi.org/10.3390.
Elufioye, T.O. and T.I. Berida. 2018. GC-MS Analysis and antioxidant activity of Spondias purpurea L (Anacardiaceae). Pharmacogn. J., 10(5): 941-945. https://doi.org/10.5530/pj.2018.5.159
Ezeldin, M., C.Y. Ishak, M. El-Jack and S. Milad. 2018. Gas chromatography mass spectrometry analysis and phytochemical screening of sterculia setigera oil. Chem. Methodol., 2(1): 64-72.
Fatima, N., M.M. Rahman, A. Khan, M.J. Fu. 2014. A review on Ipomoea carnea: Pharmacology, toxicology and phytochemistry. J. Complement. Integr. Med., 11(2): 55-62. https://doi.org/10.1515/jcim-2013-0046
Forman, H.J. and H. Zhang. 2021. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov., 20: 689-709. https://doi.org/10.1038/s41573-021-00233-1
Gavamukulya, Y., F. Abou-Elella, F. Wamunyokoli and H.A. El-Shemy. 2014. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Biomed., 1(7): 355-363. https://doi.org/10.1016/S1995-7645(14)60258-3
Gawlik-Dziki, U., M. Swieca, M. Sułkowski, D. Dziki, B. Baraniak and J. Czyz. 2013. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts in vitro study. Food Chem. Toxicol., 57: 154-160. https://doi.org/10.1016/j.fct.2013.03.023
Hue, S.M., A.N. Boyce and C. Somasundram. 2012. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (Ipomoea batatas). Aust. J. Crop Sci., 6(3): 375-380.
Ji, L.L. and D. Yeo. 2021. Oxidative stress: An evolving definition. Fac. Rev., 10(13). https://doi.org/10.12703/r/10-13
Kavitha, R., 2021. Phytochemical screening and GC-MS Analysis of bioactive compound present in ethanolic extracts of leaf and fruit of Trichosanthesis Dioica roxb. Int. J. Pharma. Sci. Res., 12(5): 2755-2764.
Kiran, T.R., O. Otlu and A.B. Karabulut. 2023. Oxidative stress and antioxidants in health and Disease. J. Lab. Med., 47(1): 1–11. https://doi.org/10.1515/labmed-2022-0108
Kopuswamy, K.M., B. Jonnalagadda and S. Arockisamy. 2013. GC-MS Analysis of chloroform extract of Croton bonplandianum. Int. J. Pharma Bio Sci., 4(4): 613–617.
Kumar, A., Paul, S., Kumari, P., Somasundaram, S. and Kathiresan, K., 2014. Antibacterial and phytochemical assessment on various extracts of Ipomoea Pes-caprae (L.) R. Br through FTIR and GC-MS spectroscopic analysis. Asian J. Pharma. Clin. Res., 7(3): 134-138.
Kurniasih, S. and D.D. Saputri. 2019. Phytochemical screening and gas cromatography-mass spectrometry (GC-MS) analysis ethanol extract of purple sweet potato (Ipomoea batatas L). J. Sci. Innov., 2(2): 28-30. https://doi.org/10.33751/jsi.v2i2.1527
Mannaa, M. and L.D. Kim. 2018. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. predominant in stored rice grains: Study II. Mycobiology, 46(1): 52-63. https://doi.org/10.1080/12298093.2018.1454015
Mazandarani, M., 2015. Autecology, total phenol and total flavonoid content, antioxidant activity and ethno-pharmacological survey of nigella sativa Linn. in traditional medicine of Golestan province, North of Iran. Crescent J. Med. Biol. Sci., 2(3): 95-99. https://doi.org/10.15296/ijwhr.2015.21
Meira, M., E.P.D. Silva, J.M. David, J.P. David. 2012. Review of the genus Ipomoea: Traditional uses, chemistry and biological activities. Rev. Brasil. Farmacogn., 22(3): 682-713. https://doi.org/10.1590/S0102-695X2012005000025
Nechuta, S., Q. Cai, Y. Zheng, G.L. Milne, H. Cai, Q. Dai, G.G. Yang, W. Zheng, W. Lu, X.O. Shu. 2014. Urinary biomarkers of oxidative stress and breast cancer survival. Cancer Causes Contr., 25(6): 701-707. https://doi.org/10.1007/s10552-014-0373-7
Ojha, G., K. Mishra and A. Mishra. 2016. Pharmacological uses and isolated chemical constituents of Ipomoea digitata: A review. J. Pharma. Biol. Sci., 11(3): 1-4.
Ozben, T., 2007. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharma. Sci., 96(9): 2181-2196. https://doi.org/10.1002/jps.20874
Prasanth, B., N. Aleykutty and J. Harindran. 2018. Phytochemical Studies on Ipomoea sepiaria Roxb. Saudi J. Life Sci., 3(4): 339-349.
Sanseera, D., B. Liawruangrath, S.G. Pyne, S. Liawruangrath. 2016. Determination of antioxidant and anticancer activities together with total phenol and flavonoid contents of Cleidion javanicum Bl. and Bridelia retusa (L.) A. Chiang Mai J. Sci., 43(3): 535-546.
Sugata, M., C.Y. Lin, Y.C. Shih. 2015. Anti-inflammatory and anticancer activities of Taiwanese purple-fleshed sweet potatoes (Ipomoea batatas L. Lam) extracts. BioMed. Res. Int., https://doi.org/10.1155/2015/768093
To share on other social networks, click on any share button. What are these?







