Population Dynamics of the Goby Trypauchen vagina (Gobiidae) at Downstream of Hau River, Vietnam
Population Dynamics of the Goby Trypauchen vagina (Gobiidae) at Downstream of Hau River, Vietnam
Quang Minh Dinh
Department of Biology, School of Education, Can Tho University, Can Tho, Vietnam
ABSTRACT
Trypauchen vagina is a commercial fish, but little is known on its population parameters. This study was carried out at downstream of Hau River in the Mekong Delta, Vietnam to provide fundamental information on the population biology of T. vagina based on length-frequency distribution analysis of 1,527 individuals. The male to female T. vagina ratio was not significantly different within and between seasons, and the parameters of the von Bertalanffy curve of this fish were given as L∞= 24.15 cm, K = 0.53 yr-1 and t0 = –0.03 yr-1. The longevity (tmax) and the growth performance (Φ’) were 5.56 yrs and 2.50, respectively. The fishing, natural and total mortalities of this fish were 1.29 yr-1, 1.44 yr-1, and 2.73 yr-1, respectively, and its exploitation rate was 0.53. There were two recruitment peaks in May and October, and relative yield-per-recruit and biomass-per-recruit analyses gave Emax = 0.735, E0.1 = 0.656 and E0.5 = 0.384. This gobiid could be potential for future aquaculture due to high growth parameter. The goby stock was subjected to overexploitation so that the mesh size of deep gill nets should be increased and avoiding catch fish during the recruitment period for sustainable fishery management.
Article Information
Received 02 December 2015
Revised 25 March 2017
Accepted 14 June 2017
Available online 28 December 2017
Key words
Trypauchen vagina, Mortality, Growth, Longevity, Exploitation rate.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.1.105.110
* Corresponding author: dmquang@ctu.edu.vn
0030-9923/2018/0001-0105 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introduction
The growth parameters and mortality rates play a fundamental role for a fish population dynamic understanding (Amezcua et al., 2006), and the exploitation rate obtained from the yield-per-recruit analysis is a valuable tool for fishery management (Al-Husaini et al., 2002; Afzaal et al., 2016). Additionally, the growth performance retrieved from growth and asymptotic length relationship is used to compare the growth rate between gender and geographic locations of a fish species or between species (Pauly and Munro, 1984). Little is known, however, on biological population parameters of fishes, especially gobiid species in the Mekong Delta.
The burrowing fish Trypauchen vagina (Bloch and Schneider, 1801) is a goby species in the Gobiidae family, and it is an elongated-bodied fish (Salameh et al., 2010; Siokou et al., 2013) and widely distributed in the estuaries in the Indo-Pacific regions (Talwar and Jhingran, 1991; Rainboth, 1996), including the Mekong Delta (Tran et al., 2013). The information of this goby species is limited to its morphology and environmental requirements (Talwar and Jhingran, 1991; Salameh et al., 2010). Although the goby T. vagina is a potential commercial fish and has been being increasingly exploited, there has no information on its population parameters, especially in the Mekong Delta. This study aims to provide helpful information on the parameters of the population biology of this goby species, enabling us to improve its stock and fishery management.
Materials and methods
Study site
This study was carried out along the downstream of Hau River in Soc Trang Province, Vietnam (9°34’12.41”N, 106°13’38.25”E), from October 2014 to September 2015. Soc Trang comprises a long coastline covered by mangroves and a large number of mudflats with the semi-diurnal tide. The dry (January–May) and wet (June–December) are two main seasons in this regions. The average annual temperature of ∼27 °C and ∼400 mm monthly precipitation in the wet season, representing the typical natural environment in Mekong Delta (Soc Trang Statistical Office, 2012).
Fish collection and analysis
The deep gill nets with 1.5 cm mesh aperture in the cod end were set at the highest tide along the mudflat and mangrove forest and retrieved after 2–3 h during ebb tide to collect monthly fish specimens in the study region. The fish was sexed based on the external morphology of genital papilla shape (female: oval shape and male: triangle sharp), before being stored in 5% formalin and transported to the laboratory. Fish specimens, in the laboratory, were measured total length (0.1 cm) and weighed (0.01 g). The water temperature and salinity at the study site were measured monthly using a thermometer and a refractometer, respectively, to test if the environmental factors influence the sex ratio.
Data analysis
The difference in male and female T. vagina ratio was tested by χ2 performed by SPSS v.21. The population parameters of this species were calculated using FiSAT II software based on monthly total length measurement data (Gayanilo et al., 2005). The initial asymptotic length (L∞) was calculated as a/b and Z/K was defined as –(1+b)/b from the linear regression Ḹ-L’ = a+bL’, where, L’ is the cut off length; Ḹ is the mean length of all fish (≥ L’, ..............); a is the regression intercept, and b is the regression slope (Powell, 1979; Pauly, 1986; Wetherall, 1986). The preliminary asymptotic length was used to optimize the asymptotic length (L∞) and the growth parameter (K) using the ELEFAN I procedure (Pauly and David, 1981; Pauly, 1982, 1987).
The total mortality rate (Z) was calculated from the length-converted capture curve (Beverton and Holt, 1957; Ricker, 1975). The natural mortality rate (M) was obtained the equation LogM = -0.0066 – 0.279LogL∞ + 0.6543LogK + 0.463LogT, where, L∞ and K were achieved from the ELEFAN I and T was the mean of annual water temperature (oC) in the study region (Pauly, 1980). The fishing mortality (F) was estimated from the equation F = Z – M, and the exploitation rate (E) was calculated as E = F/Z (Ricker, 1975). The length-converted catch curve was used to compute the probability of capture for each size class, while the seasonal recruitment pattern was obtained from the length-frequency data set and the fish length entry firstly into the population for catching (Lc) was computed by plotting the cumulative probability of capture against the class mid-length (Pauly, 1987). The back-projected data was maximum likelihood using NORMSEP procedure (Hasselblad, 1966).
The goby stock and yield were estimated from the yield-per-recruit model of Beverton and Holt (1957) (Sparre and Venema, 1992). The yield-per-recruit (Y’/R) was calculated using the equation:
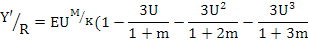
where, U=1–(Lc/L∞) is the fraction of growth to be completed after entering the exploitation phase, and the biomass-per-recruit relation (B’/R) was computed from the equation B’/R = Y’/R/F (Beverton and Holt, 1966).
The maximum yield exploitation rate (Emax), the exploitation rate with the minimal increase of 10% of (E0.1), and the exploitation rate with the reduction of stock to 50% (E0.5) were estimated from the knife-edge selection (Beverton and Holt, 1966). A combination analysis of E and isopleth ratio (Lc/L∞) was used to determine the goby fishing status based on the method of Pauly and Soriano (1986).
The index of overall growth performance (Φ’) was calculated from the equation Φ’ = LogK + 2LogL∞, where, K and L∞ are two parameters of the von Bertalanffy curve, which was used to compare the von Bertalanffy growth parameters of T. vagina and other goby fishes dwelling in the same habitat (Pauly and Munro, 1984). The longevity (tmax) of T. vagina was calculated as tmax = 3/K, where, K was the growth parameter, and t0 was the age when the egg was fertilized (Taylor, 1958; Pauly, 1980).
Table I.- The sex ratio of the goby T. vagina.
| Sampling time |
Fish groups |
Sum |
F / M |
χ2 |
P |
||
|
X |
F |
M |
|||||
| 10/2014 |
|
34 |
26 |
60 |
1:0.76 |
1.07 |
0.30 |
| 11/2014 |
|
88 |
73 |
161 |
1:0.83 |
1.40 |
0.24 |
| 12/2014 |
23 |
22 |
28 |
73 |
1:1.27 |
0.72 |
0.40 |
|
1/2015 |
6 |
90 |
78 |
174 |
1:0.87 |
0.86 |
0.36 |
| 2/2015 |
26 |
39 |
46 |
111 |
1:1.18 |
0.58 |
0.45 |
| 3/2015 |
5 |
39 |
36 |
80 |
1:0.95 |
0.12 |
0.73 |
| 4/2015 |
|
55 |
44 |
99 |
1:0.80 |
1.22 |
0.27 |
| 5/2015 |
|
53 |
48 |
101 |
1:0.91 |
0.25 |
0.62 |
| 6/2015 |
|
27 |
35 |
62 |
1:1.30 |
1.03 |
0.31 |
| 7/2015 |
7 |
107 |
99 |
213 |
1:0.93 |
0.31 |
0.58 |
| 8/2015 |
24 |
107 |
101 |
232 |
1:0.94 |
0.17 |
0.68 |
| 9/2015 |
|
90 |
71 |
161 |
1:0.79 |
2.24 |
0.13 |
| Dry |
37 |
276 |
252 |
565 |
1:0.91 |
1.09 |
0.30 |
| Wet |
54 |
475 |
433 |
962 |
1:0.91 |
1.94 |
0.63 |
| Sum |
91 |
751 |
685 |
1,527 |
1:0.91 |
3.03 |
0.08 |
X, unsexed; F, female; M, male.
Results
A total of 1,527 individuals were caught comprising 751 females, 685 males and 91 unsexed fish in the study region. The male to female T. vagina ratio was close to 1:1 during the study period and the number of males were not significantly different from that of females within and between seasons (P > 0.05, Table I). There was no significant different in water temperature between the dry (29.07±1.32 oC) and wet seasons (28.41±0.90 oC, t-test, P > 0.05); but the water was more salter in the dry (8.86±3.75‰) compared to the wet season (2.68±2.28%, t-test, P < 0.001).
The population parameters including asymptotic length, growth rate, longevity, mortality, recruitment, exploitation indices and yield-per-recruit were estimated based on the length-frequency analysis of 1,527 individuals (6–23 cm in TL). There were six fish size groups, i.e., six growth curves represented by six dark lines (Fig. 1) in the population of T. vagina in this study area, and the bigger fish grew more slowly than small fish due to the slight slope in the larger fish compared to small fish. The growth increment data, which was obtained from NORMSEP procedure, analysis showed that the von Bertalanffy growth curve of T. vagina was Lt = 24.15 (1 – e-0.56(t+0.03)) (Fig. 2).
The length-converted catch curve analysis revealed that the total (Z), fishing (F) and natural (M) mortalities of the goby T. vagina were 2.73, 1.29 and 1.44, respectively (Fig. 3A). The exploitation rate of this fish was 0.53, and there was a variation in fishery recruitment over time of this goby with two recruitment peaks occurred in May and October (Fig. 3B). The fish length at first capture (Lc or L50) estimated from the capture probability analysis was 13.75 cm (Fig. 3C).
The yield-per-recruit and biomass-per-recruit of this goby T. vagina were analyzed showed that the optimum yield (E0.1), the yield at the stock reduction of 50% (E0.5) and the maximum sustainable yield (Emax) were 0.384, 0.656 and 0.735, respectively (Fig. 4). The yield isopleths (Lc/L∞) of this fish was 0.57, and its growth performance and longevity were 2.50 and 5.56, respectively.
Discussion
The sex ratio of some fish was strongly regulated by temperature variation, e.g., Pomatoschistus minutus in Thun Lake, Sweeden (Wedekind et al., 2013). However, the male to female of T. vagina ratio in this study was close to 1:1 that is also found in some gobies living in the same habitat of T. vagina such as Pseudapcryptes elongatus (Tran, 2008), Boleophthalmus boddarti (Dinh, 2015) and Parapocryptes serperaster (Dinh et al., 2015), suggesting that variation in temperature (26.5–30 oC) did not significantly influence the sex ratio of this goby.
The fish population structure analysis requires at least 1500 fish of specimens collected over six months with distinct peaks in length-frequency distribution (Pauly, 1987). In the present study, the fish samples (e.g., 1527 individuals collected during 12 months) adopted this sampling criterion for analysis of population parameters. Moreau et al. (1986) found that the growth performance index (Φ’) is the best growth index compared to another growth index ω = K×L∞ since Φ’ exhibits the least degree of variation when comparing growth parameters between different tilapia populations. The growth parameter Φ’ is usually similar within the related taxa and have narrow normal distributions (Moreau et al., 1986; Tran et al., 2007). When study on the population parameters of P. serperaster, Dinh et al. (2015) showed that the difference in growth performance (Φ’) between some gobiid fishes result from the variation of growth parameter (K) and asymptotic length (L∞). Likewise, K and L∞ of T. vagina were smaller than those of Periophthalmus barbarrus (Etim et al., 2002), P. elongatus (Tran et al., 2007), P. schlosseri (Mazlan and Rohaya, 2008) and P. serperaster (Dinh et al., 2015) (Table II), resulting in Φ’ of T. vagina was lower than that of other gobiid fish P. elongatus (Tran et al., 2007), P. schlosseri (Mazlan and Rohaya, 2008) and P. serperaster (Dinh et al., 2015), but higher than that of Periophthalmus barbarrus (Etim et al., 2002) (Table II).
Table II.- Population parameters of various gobiid fishes.
| Species |
L∞ |
K |
tmax |
Z |
F |
M |
Lc |
E |
Φ’ |
Sources |
|
Periophthalmus barbarus |
21.60 |
0.55 |
5.45 |
4.21 |
2.86 |
1.35 |
10.2 |
0.68 |
2.41 |
|
|
Pseudapocryptes elongatus |
26.00 |
0.65 |
4.35 |
2.91 |
1.47 |
1.44 |
11.75 |
0.51 |
2.64 |
|
|
Periophthalmodon schlosseri |
29.00 |
1.40 |
2.14 |
- |
- |
- |
- |
- |
3.10 |
|
| Parapocryptes serperaster |
25.52 |
0.74 |
4.05 |
3.07 |
1.57 |
1.51 |
14.6 |
0.49 |
2.67 |
|
| Trypauchen vagina |
24.15 |
0.56 |
5.56 |
2.73 |
1.29 |
1.44 |
13.75 |
0.53 |
2.50 |
Present study |
The goby T. vagina had more potential for practice artificial spawning due to the high value of longevity that enable this fish spawn numerous time. The low value of growth parameter of T. vagina may result in its longevity was higher than that of other gobiid species in the Gobiidae family such as P. elongatus (Tran et al., 2007) and P. Serperaster (Dinh et al., 2015) in the Mekong Delta, and P. barbarrus in Nigeria (Etim et al., 2002) and P. schlosseri (Mazlan and Rohaya, 2008) in muddy flat in Malaysian water (Table II). The different geographic latitude, predation and fishing activities are also resulted in the variation of longevity among gobies (Dinh et al., 2015).
The goby in this study can adapt well to its habit compared to other co-occurring gobiid fish including P. elongatus (Tran et al., 2007) and P. serperaster (Dinh et al., 2015) as the natural mortality of T. vagina was lower than P. elongatus and P. Serperaster (Table II). The fishing mortality of T. vagina was similar to P. elongatus (Tran et al., 2007), but higher than P. serperaster (Dinh et al., 2015) (Table II), being caused by P. serperaster has been fishing increasingly due to its economic valuable (Dinh et al., 2015) or by different fishing gears. Similarly, the differences in economic value or fishing gears may lead to the different in fishing mortality between T. vagina in the present study region and P. barbarus in Nigeria (Etim et al., 2002) and the differences in length at first capture of T. vagina and other gobiid fishes (Table II). Although the L∞ of T. vagina was shorter compared to P. elongatus (Tran et al., 2007), its length at first capture was longer (Table II), suggesting the fish stock of T. vagina was more suitable exploitation than that of P. elongatus. Like P. elongatus (Tran et al., 2007) and P. serperaster (Dinh et al., 2015), T. vagina was two recruitment times from May to November, seeming recruitment is influenced by environmental factors though it is specific-species.
The goby stock was overfishing in the present study region as its exploitation rate was higher than that of E50. Moreover, the combination of yield isopleths (Lc/L∞) and exploitation rate (E) analysis showed this goby had been being fishing since its Lc/L∞ (0.57) and E (0.53) fell into developed fishery quadrant described by Pauly and Soriano (1986). Therefore, the mesh size of fishing gears should be increased and avoided fishing in the period of recruitment for sustainable fishery management.
In conclusion, the sex ratio of T. vagina was close to 1:1, and its fish stock was subjected to overexploitation in the study region. This species was high in population recruitment and could be potential for future aquaculture due to high growth constant. However, mesh size of deep gill nets should be increased for future sustainable fishery management.
Acknowledgments
I am grateful to local fishermen in Soc Trang, staff and students Can Tho University for helping me to complete this study, and Can Tho University for funding this study (T2005-86).
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Al-Husaini, M., Al-Baz, A., Al-Ayoub, S., Safar, S., Al-Wazan, Z. and Al-Jazzaf, S., 2002. Age, growth, mortality and yield-per-recruit for nagroor, Pomadasys kakaan, in Kuwait’s waters. Fish. Res., 59: 101-115. https://doi.org/10.1016/S0165-7836(01)00417-9
Amezcua, F., Soto-Avila, C. and Green-Ruiz, Y., 2006. Age, growth and mortality of the spotted rose snapper Lutjanus guttatus from the southeastern Gulf of California. Fish. Res., 77: 293-300. https://doi.org/10.1016/j.fishres.2005.10.012
Beverton, R.J.H. and Holt, S.J., 1966. Manual of methods for fish stock assessment. Part II: Tables of yield function. FAO, Roma, Italy, pp. 67.
Dinh, Q.M., 2015. Preliminary study on dietary composition, feeding activity and fullness index of Boleophthalmus boddarti in Mekong delta, Vietnam. J. Biol., 37: 252-257.
Dinh, Q.M., Qin, J.G. and Tran, D.D., 2015. Population and age structure of the goby Parapocryptes serperaster (Richardson, 1864; Gobiidae: Oxudercinae) in the Mekong Delta. Turkish J. Fish. aquat. Sci., 15: 345-357.
Etim, L., King, R.P. and Udo, M.T., 2002. Breeding, growth, mortality and yield of the mudskipper Periophthalmus barbarus (Linneaus 1766) (Teleostei: Gobiidae) in the Imo River estuary, Nigeria. Fish. Res., 56: 227-238. https://doi.org/10.1016/S0165-7836(01)00327-7
Hasselblad, V., 1966. Estimation of parameters for a mixture of normal distributions. Technometrics, 8: 431-444. https://doi.org/10.1080/00401706.1966.10490375
Mazlan, A.G. and Rohaya, M., 2008. Size, growth and reproductive biology of the giant mudskipper, Periophthalmodon schlosseri (Pallas, 1770), in Malaysian waters. J. appl. Ichthyol., 24: 290-296. https://doi.org/10.1111/j.1439-0426.2007.01033.x
Pauly, D., 1980. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J. Conseil, 39: 175-192. https://doi.org/10.1093/icesjms/39.2.175
Pauly, D., 1986. On improving operation and use of the ELEFAN programs. Part II. Improving the estimation of L∞. Fishbyte, 4: 18-20.
Pauly, D., 1987. A review of the ELEFAN system for analysis of length-frequency data in fish and aquatic invertebrates. In: The international conference on the theory and application of length-based methods for stock assessment (eds. D. Pauly and G. Morgan). ICLARM, Mazzara del Vallo, Sicily, Italy.
Pauly, D. and David, N., 1981. ELEFAN I, a BASIC program for the objective extraction of growth parameters from length-frequencies data. Meeresforschung, 28: 205-211.
Pauly, D. and Munro, J.L., 1984. Once more on the comparison of growth in fish and invertebrates. Fishbyte, 2: 21.
Pauly, D. and Soriano, M.L., 1986. Some practical extensions to Beverton and Holt’s relative yieldper-recruit model. In: The first Asian fisheries forum (eds. J.L. Maclean, L.B. Dizon and L.V. Hosillo). Asian Fisheries Society, Manila.
Powell, D.G., 1979. Estimation of mortality and growth parameters from the length frequency of a catch. Rapp. Proces-Verb. Reun., 175: 167-169.
Rainboth, W.J., 1996. Fishes of the Cambodian Mekong. FAO, Roma, Italy.
Ricker, W.E., 1975. Computation and interpretation of biological statistics of fish populations. Department of the Environment, Fisheries and Marine Service, pp. 382.
Salameh, P., Sonin, O. and Golani, D., 2010. First record of the burrowing goby, Trypauchen vagina (Actinopterygii: Gobiidae: Amblyopinae), in the Mediterranean. Acta Ichthyol. Piscat., 40: 109-111. https://doi.org/10.3750/AIP2010.40.2.03
Siokou, I., Ates, A.S., Ayas, D., Souissi, J.B., Chatterjee, T., Dimiza, M., Durgham, H., Dogrammatzi, K., Erguden, D. and Gerakaris, V., 2013. New Mediterranean Marine biodiversity records. Mediterranean Mar. Sci., 14: 238-249. https://doi.org/10.12681/mms.450
Sparre, P. and Venema, S., 1992. Introduction to tropical fish stock assessment - part I: manual. FAO, Roma, Italy.
Talwar, P.K. and Jhingran, A.G., 1991. Inland fishes of India and adjacent countries. Balkema, Rotterdam, Netherlands.
Taylor, C.C., 1958. Cod growth and temperature. J. Conseil, 23: 366-370. https://doi.org/10.1093/icesjms/23.3.366
Tran, D.D., Ambak, M.A., Hassan, A. and Nguyen, T.P., 2007. Population biology of the goby Pseudapocryptes elongatus (Cuvier, 1816) in the coastal mud flat areas of the Mekong Delta, Vietnam. Asian Fish. Sci., 20: 165-179.
Tran, D.D., Shibukawa, K., Nguyen, T.P., Ha, P.H., Tran, X.L., Mai, V.H. and Utsugi, K., 2013. Fishes of Mekong Delta, Vietnam. Can Tho University Publisher, Can Tho.
Wedekind, C., Evanno, G., Székely, T., Pompini, M., Darbellay, O. and Guthruf, J., 2013. Persistent unequal sex ratio in a population of grayling (Salmonidae) and possible role of temperature increase. Conserv. Biol., 27: 229-234. https://doi.org/10.1111/j.1523-1739.2012.01909.x










