Response of Azadirachta indica against Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) and Amrasca biguttula Ishida (Homoptera: Cicadellidae) on Cotton Cultivars
Response of Azadirachta indica against Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) and Amrasca biguttula Ishida (Homoptera: Cicadellidae) on Cotton Cultivars
Asad Abdullah1, Muhammad Irfan Ullah1,*, Muhammad Waqar Hassan2, Samina Khalid3, Yasir Iftikhar4, Muhammad Arshad1 and Jaime Molina-Ochoa5,6
1Department of Entomology, University of Sargodha, Sargodha, Pakistan
2Department of Entomology, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Pakistan
3Department of Environmental Sciences, COMSATS Institute of Information Technology, Vehari
4Department of Plant Pathology, University of Sargodha, Sargodha, Pakistan
5Universidad de Colima-Coordinación General de Investigación Científica-Centro Universitario de Investigación y Desarrollo Agropecuario, Km. 40 autopista Colima-Manzanillo, Tecomán, Colima 28930, México
6Universidad de Colima-Facultad de Medicina Veterinaria y Zootecnia, Tecomán, Colima 28930, México
ABSTRACT
The study was conducted to evaluate the effectiveness of neem seed extract (NSE) for the management of Bemesia tabaci Gennadius, (Homoptera: Aleyrodidae) and Amrasca biguttula Ishida, (Homopter: Cicadellidae) infesting Bt and non-Bt cotton cultivars. Foliar application of neem seed, Azadirachta indica extract was applied upon reaching economic threshold levels of B. tabaci and A. biguttula. The insect pest population was recorded 24 hours before and 24h, 72h and 168h after spray. Maximum reduction of 60.20% of B. tabaci on Bt cotton was recorded at 6% NSE while at 2% concentration of NSE after 148 hrs, 39. 16% reduction was observed. While maximum reduction on non-Bt cotton at 6% recorded as 66.60% and minimum at 2% concentration recorded as 48.72% of neem seed extract against B. tabaci. In case of A. biguttula, maximum reduction in population was observed at 6% concentration of NSE (64.94%) and minimum at 2% concentration (44.50%) on Bt cotton. While maximum reduction of A. biguttula was observed at 6% concentration (69.05%) and minimum at 2% concentration (48.48%) on non-Bt cotton.
Article Information
Received 22 Decmber 2016
Revised 16 March 2017
Accepted 29 May 2017
Available online 13 October 2017
Authors’ Contribution
AA designed and conducted research, MIU helped in data anaylsis and interpretation, MWH supervised research, SK, YI and MA helped in preparation and review of manuscrip, JMO critically revised the manuscript.
Key words
Whitefly, Jassid, Cotton, Neem seed extract.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.6.1983.1987
* Corresponding author: [email protected]
0030-9923/2017/0006-1983 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Cotton (Gossypium hirsutum L.) is an economically important fiberous crop of Pakistan. Many factors like floods, heavy monsoon, infestation of sucking insect pests and cotton leaf curl virus (CLCV) disease has resulted a decline in 13.4% of cotton area during the years 2010-11 (Economic Survey of Pakistan, 2011). While during cropping season of 2009-10, an area of 3105.64 thousand hectares of cotton were sown with an average yield of 11560.1 thousand bales (Economic Survey of Pakistan, 2011). In Pakistan, there is a need to increase the per hectare yield than many other cotton growing countries. In Punjab province of Pakistan Bt (Bacillus thuringiensis) cotton was introduced in 2001 (Rao, 2006). Non Bt varieties of cotton had resulted with less quality seed, high pest attack (bollworms), lower yield, high pesticide applications usage and less resistance to drought conditions (Qaim and Zilberman, 2003). Bt cotton is frequently sown due to its advantageous features of increased yield of 7-12% than non-Bt (Bryant et al., 1999). Transgenic cotton confers a substantial benefit compared with non-transgenic due to higher yield and less use of the chemicals against the insect pest populations (Qaim and Janvry, 2004). This has resulted in overall decrease in expenses with maximum production with minimum inputs for transgenic cotton as compared to non-transgenic cotton and increased net revenue (Huang et al., 2002).
Although Bt cotton is resistant against bollworms but sucking insect pests are reported to attack and damage the Bt cotton (Sharma and Pampapathy, 2006). Cotton is threatened by many insect pests which are associated with this crop from vegetative growth to the harvesting (Abudulai et al., 2007). Cotton whitefly, Bemisia tabaci Genn. harm the crop by frequently sucking the cell sap which results in an estimated 50% reduction in cotton bolls. It also secretes honey dew that serve as a potential substrate for sooty mold to grow (Ahmad et al., 2002). The sucking insect pests like jassid are also more destructive to cotton. Due to the damage of jassid, 19% dropping of fruits has been reported in cotton with decreased yield (Ali, l992). Besides this, a complex of sucking insect pests viz., Amrasca biguttula biguttula (Ish.), Thrips tabaci (Lin.), Aphis gossypii (Gl.) and Bemisia tabaci (Gen.), are recognized to have engaged foremost damaging pest ranking (Mamoon-ur-Rashid et al., 2016).
Synthetic insecticides are broadly used for the management of cotton insect pests. Extensive use of synthetic pesticides has resulted in environmental complications (Abdel Bagi et al., 2006). Resistance of pest against pesticides has also increased expenses of crop which enlightened the negative impacts on the production (Assad et al., 2006). There is an increasing trend of shifting from synthetic pesticides to non-synthetic pesticides. Botanicals are valued for their insecticidal purpose that has proved beneficial against insect pest populations (Prakash and Rao, 1996). Now a days, botanicals are also widely used against cotton insect pests due to their more safety to the environment and plants. Among botanical, neem based insecticides are produced and used for the management of many insect pests (Joseph et al., 2010). Neem based pesticides contain azadirachtin as active ingredient that is used to produce neem based biopesticides (Subbalakshmi et al., 2012). Due to the germicidal and anti-bacterial properties neem oil and seed extracts are well known plant protectants for the plants against different kinds of pests (Vethanayagam and Rajendran, 2010). Neem-based pesticides do not leave any residues on the plants which is one of the most important advantage for the plant health (Subbalakshmi et al., 2012).
Materials and Methods
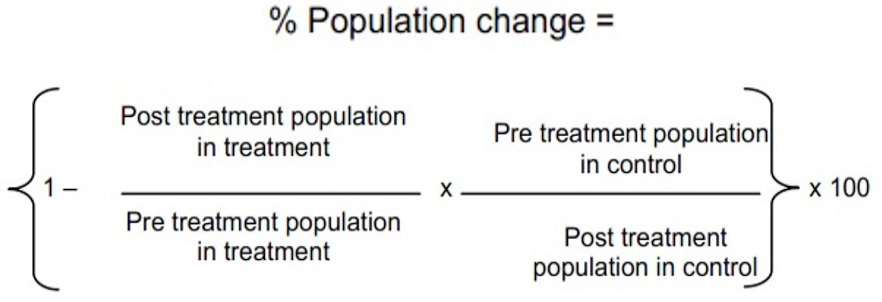
The experiment was conducted at the farm area the Islamia University of Bahawalpur, Pakistan to test efficacy of three concentrations of neem seed extract against Bemesia tabaci and Amrasa biguttula in Bt (IR-370) and no-Bt (BH-167) cotton cultivars. There were 8 treatments including control, 3 rows of each treatment and three replications with randomized complete block design (RCBD) under split plot arrangement. The plot size was kept as 2.4 x 4.6 m2, plant to plant distance was maintained at 30 cm and row to row distance was 75cm. The neem seeds were obtained from the nursery of University College of Agriculture and Environmental Sciences at the Islamia University of Bahawalpur. The covering of neem seed was removed and seed was gently ground in an electric grinder. Neem seed powder of 400g was soaked in 4 liters of water in a jar. The jar mouth was covered with the string and was left for 3 days. The jar solution was stained after 3 days to get clear extract. A 100 gm of surf was added as surfactant, stirred it well and then was sprayed in the field when the population of pest were reached at economic threshold level (ETL) (Fiaz et al., 2012; Schmutterer, 1988, 2002). The ETL for whitefly is five adults or nymphs per leaf and for the jassid it was a single adult or nymph per leaf. The application was carried out with hand operated knapsack sprayer having capacity of 20 liters fitted with hollow cone nozzle. The control treatments were treated with water only. Knapsack sprayer was calibrated prior to application of neem solution. Nine random plants were selected per plot for the observation of pest population early in the morning. Upper, middle and lower portions of plants were observed for the pest activity (both nymphs and adults) through every survey. To evaluate the efficacy of different concentrations, population of whitefly and jassid was observed 1, 3 and 7 days after application of neem seed extract concentrations. Statistical analysis was carried out using (ANOVA) and means were separated using LSD test. Percent change in population was determined using modified Abbot’s formula (Flemings and Ratnakaran, 1985):
Results and Discussion
For non-transgenic cotton, the results showed maximum reduction in whitefly populations (29.02±0.3%) was at 6% concentration after 24 h of application. While after 72 and 168 h at same concentration, the reduction was 43.25±0.38% and 66.60±0.79%, respectively. For Bt- cultivar, 6% concentration application has resulted in a decrease in whitefly population of 16.07±0.17%, 25.49±0.59% and 60.20±0.42% after 24, 72 and 168 h, respectively (Table I).
Table I.- Percent population reduction (SE±) of whitefly and jassid at different time intervals and at various concentrations of neem seed extract (NSE) on Bt and Non-Bt cotton cultivars.
| Treatments | Cultivar | 24HAT | 72HAT | 168HAT |
|
Whitefly |
||||
| 6% Water | Bt (IR-370) | 16.07±0.17c | 25.7±0.59d | 60.2±0.42b |
| 6% Water | N-Bt (BH-167) | 29.02±0.28a | 43.25±0.38a | 66.6±0.79a |
| 4% NSE* | Bt (IR-370) | 10.75±0.50d | 28.49±0.44c | 51.87±0.49cd |
| 4% NSE | N-Bt (BH-167) | 18.7±0.37b | 26.97±0.39cd | 55.62±0.57c |
| 2% NSE | Bt (IR-370) | 7.72±0.32e | 19.58±0.36e | 39.16±0.21e |
| 2% NSE | N-Bt (BH-167) | 11.43±0.15d | 33.35±0.44b | 48.72±0.48d |
| 0% NSE | Bt (IR-370) | 2.7±0.34f | 2.75±0.37f | 4.09±0.11f |
| 0% NSE | N-Bt (BH-167) | 3.88±0.09f | 1.94±0.06f |
5.82±0.11f |
| Cultivars x Concentrations | P < 0.05 | 0.0000 | 0.0000 | 0.0300 |
| CV | - | 2.1034 | 2.0785 |
3.6012 |
|
Jassid |
||||
| 6% Water | Bt (IR-370) | 27.5±0.28c | 45.32±0.80b | 64.94±0.62b |
| 6% Water | N-Bt (BH-167) | 34.49±0.57b | 50.25±0.74a | 69.05±0.67a |
| 4% NSE* | Bt (IR-370) | 28.63±0.33c | 39.55±0.75c | 60.82±0.48c |
| 4% NSE | N-Bt (BH-167) | 41.74±0.59a | 53.24±0.73a | 65.67±0.47b |
| 2% NSE | Bt (IR-370) | 15.03±0.22d | 22.26±0.52d | 44.5±0.58e |
| 2% NSE | N-Bt (BH-167) | 11.63±0.52d | 24.24±0.30d | 48.48±0.42d |
| 0% NSE | Bt (IR-370) | 6.39±0.15e | 9.59±0.18e | 12.89±10.25f |
| 0% NSE | N-Bt (BH-167) | 3.19±0.10e | 9.59±0.18e | 6.39±0.15g |
| Cultivars x Concentrations | P < 0.05 | 0.0000 | 0.0029 | 0.0001 |
| CV | 1.9541 | 4.5605 | 2.7832 | |
HAT, hours after treatment; *NSE, neem seed extract.
The findings of current study are like that of Abdalla et al. (2010) who stated the 6% and 12% of the neem seed extract gave significant mortality of sucking insect pests (whitefly and jassid) as compared the control. The present experiment results are in accordance with Venkatesh et al. (1998), who showed the results that alternative application of neem seed kernel extract at 4 % reduced leaf curl virus by whitefly in chilies. Neem seed extract at various concentrations resulted in reduction of egg hatching (29%) and larval mortality (90%) (Coudriet et al., 1985), 93.7% nymphal mortality (Jayaraj et al., 1986), reduction of Bemisia tabaci (Gen.) in field experiment with neem water extract (Serra and Schmuttere, 1993) and reduction of whitefly and jassid up to 12 days (Khattak et al., 2001).
After 24, 72 and 168 h of treatment, neem seed extracts (NSE) at 4% and 6% concentration caused maximum reduction in jassid population on non Bt cotton cultivar BH-167. While on Bt cotton cultivar IR-370, the same concentrations after similar interval of time in the maximum reduction of jassid populations (Table I). The results of our study are in accordance with the findings of Jotwani and Srivastva (1981) which depicted that neem extracts proved to be effective against sucking pests. Ahmad et al. (1993) and Dhanalakshmi (2006) also found maximum reduction in jassid population after seven days and minimum after two days of treatment (Khattak et al., 2006).
Neem has been proved safer than synthetic chemicals for coccinellids and predatory spiders (Samiayyan and Chandrasekharan, 1998; Sakthivel and Qadri, 2010; Joseph et al., 2010). Kundu et al. (1998) reported neem formulation of Nimbecidine @ 5 ml/l safer to Chrysoperla carnea in cotton. Similarly, Sahayaraja and Karthickraja (2003) found 46% of hatching ability along with 98.8% of predatory potential of Rhynocoris marginatus, a predator of cotton aphid, when applied neem product; Nivaar demonstrated. Babu et al. (1998) assessed the efficacy of neem formulations for the control of jassid and brown plant hopper of rice and reported neem products were safer to predatory mirid bug, Cyrtorhinus lividipennis and predatory spiders.
Conclusion
To conclude population reduction for whitefly and jassid was maximum at 6% concentration of neem seed extract for Bt and non-Bt cotton after 168 h of spray. Similarly, population reduction after 24 h and 72 h of spray were maximum either at 4% and 6% concentrations for both varieties of cotton.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Abdalla, A.S., Mohamed, E.E. and Abdin, E.M., 2010. Insecticidal activities of neem (Azadirachta indica A. Juss) seeds under laboratory and field conditions as affected by different storage durations. Agric. Biol. J. N. Am., 1: 1001-1008. https://doi.org/10.5251/abjna.2010.1.5.1001.1008
Abdel Bagi, A.O., Ahmed, A.A.M., Elhindi, M. and Ali, A.M., 2006. Impact of pesticides and other chemicals on the environment. Workshop on Post Conflict National Plan for Environmental Management in Sudan, 18–20 July 2006. Friendships Hall, Khartoum, Sudan.
Abudulai, M., Salifu, A.B. and Abatania, L., 2007. Farmers knowledge and perceptions of cotton insect pests and their control practices in Ghana. J. Sci. Technol., 26: 39-46.
Ahmad, F., Khan, F.R., Shahid, M.I. and Ahmad, M., 1993. Field efficacy studies on different formulations and concentrations of neem (Azadirachta indica A. juss.) against major insect pest of brinjal, (Solanum melongena). Pak. Entomol., 15: 13-15.
Ahmad, M., Arif, M.I., Ahmad, Z. and Denholm, I., 2002. Cotton whitefly (Bemisia tabacii, resistance to organophosphate and pyrethroids insecticides in Pakistan. Pest Manage. Sci., 58: 203-208. https://doi.org/10.1002/ps.440
Ali, A., 1992. Producer and consumer subsidy equivalent of agricultural policies in Pakistan. Concept, measurement and implications. Pak. J. agric. Econ., 1: 1-23.
Assad, Y.O.H., Bashir, N.H.H. and Eltoum, E.M.A., 2006. Evaluation of various insecticides on the cotton whitefly, Bemisia tabaci (Genn.); population control and development of resistance in Sudan Gerira. Resist. Pest Manage. Newsl., 15: 7-12.
Babu, G.R., Rao, G.M. and Rao, P.A., 1998. Efficacy of neem oil and neem cake for the control of green leaf hoppers, brown plant hoppers and their effect on predators of brown plant hoppers. Shashpa, 5: 91-94.
Bryant, K.J., Robertson, W.C. and Lorenz III, G.M., 1999. Economic evaluation of Bollgard cotton in Arkansas. Proc. Beltw. Cotton Conf., 1: 349-350.
Coudriet, D.L., Prabhaker, N. and Meyerdirk, D.E., 1985. Sweetpotato whitefly Bemisia tabaci (Homoptera: Aleyrodidae): Effects of neem seed extract on oviposition and immature stages. Environ. Ent., 14: 776-779. https://doi.org/10.1093/ee/14.6.776
Dhanalakshmi, D.N., 2006. Studies on storability and utilization of indigenous materials on okra pests. M.Sc. (Agric.) thesis, Univ. Agric. Sci., Dharwad, India.
Economic Survey of Pakistan, 2011. Economic Advisor’s Wing, Finance Division, Government of Pakistan, Islamabad.
Fiaz, M., Hameed, A. Hassan, M. and Wakil, W., 2012. Efficacy of plant extracts on some cotton (Gossypium hirsutum) pests: Amrasca bigutulla bigutulla ishida and Thrips tabaci Lindeman. Pakistan J. Zool., 44: 277-283.
Fleming, R. and Retnakaran, A., 1985. Evaluating single treatment data using Abbot’s formula with modification. J. econ. Ent., 78: 1179. https://doi.org/10.1093/jee/78.6.1179
Huang, D.H., Chang, Y.L., Yang, C.C., Pan, I.C. and King, B., 2002. Pipsqueak encodes a factor essential for sequence-specific targeting of a polycomb group protein complex. Mol. cell. Biol., 22: 6261-6271. https://doi.org/10.1128/MCB.22.17.6261-6271.2002
Jayaraj, S., Rangarajan, G.A.V., Murugesan, S., Santharam, G., Vijayaraghavan, S. and Thangaraj, D., 1986. Outbreak of whitefly Bemisia tabaci (Gennadius.) on cotton in Tamil Nadu and its management. All India group Discussion on whitefly in cotton, Guntur, April 29-30.
Joseph, A., Premila, R., Nisha, K.S.V.G., Rajendran, S. and Mohan, S.S., 2010. Safety of neem products to tetragnathid spiders in rice ecosystem. J. Biopest., 3: 88-89.
Jotwani, M.G. and Srivastava, K.P., 1981. Neem insecticides of the future. II-Protection against field pests. Pesticides, 15: 19-23.
Kundu, S.K., Jeyakumar, P., Meenakshi, G. and Gupta, P., 1998, Conservation of Chrysoperla carnea Steph. in cotton ecosystem for sustainable IPM programme. Indian J. Ent., 60: 297-300.
Khattak, M.K., Khan, L., Awan, M.N. and Hussain, A.S., 2001. Evolution of some insecticidal combination and neem, Azadirachta indica (A. Juss) extract against jassid and whitefly on cotton and their effect on the yield. Pak. J. biol. Sci., 4: 419-421. https://doi.org/10.3923/pjbs.2001.419.421
Khattak M.K., Rashid, M., Hussain, S.A.S. and Islam, T., 2006. Comparative effect of neem (azadirachta indica. Juss) oil, neem seed water extract and baythroid TM against whitefly, jassids and thrips on cotton. Pak. Entomol., 28: 31-37.
Mamoon-Ur-Rashid, M., Jilani, M.S., Khan, Q., Hashim, M.M., Sayal, O.U., Khan, M.P., Latif, A., Waseem, K. And Nawaz, S., 2016. Evaluation of neem (Azadirachta indica) derivatives against Jassids (Emrasca devastans) and cotton mealybug (Phenacoccus solenopsis), and side effects on the feeding potential of green lacewing (Chrysoperla carnea) on cotton aphid (Aphis gossypii). Pak. J. Zool., 48: 1763-1768.
Prakash, A. and Rao, J., 1996. Botanical pesticides in agriculture. CRC Press New Delhi, India. (http://factbotanicals.htm; http://www.cplbookshop.com).
Qaim, M. and de Janvry, A., 2004. Cheaper GM seeds could boost adoption, farm benefits and company profits: The case of Bt cotton in Argentina. Crop Biotech. Brief, 4:1-4.
Qaim, M. and Zilberman, D., 2003. Yield effects of genetically modified crops in developing countries. Science, 299: 900-902. https://doi.org/10.1126/science.1080609
Rao, I.A., 2006. Pakistan- GM cotton grown. Available at: http://www.afaa.com.au/news/n_news-1758.asp.
Sahayaraja, K. and Karthikraja, S., 2003. Effect of bio pesticides on Rhynocoris marginatus (Fab). J. Bio. Cont., 17: 43-45.
Sakthivel, N. and Qadri, S.M.H., 2010. Impact of insecticides and botanicals on population build-up of predatory coccinellids in mulberry. J. Biopest., 3(Special issue 1): 85-87.
Samiayyan, K. and Chandrasekharan, B., 1998. Influence of botanicals on the spider populations of rice. Madras Agric. J., 85: 479-480.
Schmutterer, H., 1988. Potential of azadirachtin-containing pesticides for integrated pest control in developing and industrial countries. J. Insect Physiol., 34: 713-719. https://doi.org/10.1016/0022-1910(88)90082-0
Schmutterer, H., 2002. The neem tree (Azadirachta indica A. Juss.) and other malicious plants: Sources of unique natural products for integrated pest management, medicine, industry and other purposes. Neem Foundation, Mumbai, India, pp. 893.
Serra, C.A. and Schmutterer, H., 1993. Die Bekämpfung der Tabakmottenschildlaus Bemisia tabaci Gennadius. Mit Niemextrakten in Tomatenfeldern in der Dominikanischen Republic. Mitt. Deutsch Gesell Allg. angew. Ent., 8: 795-801.
Sharma, H.C. and Pampapathy, G., 2006. Influence of transgenic cotton on the relative abundance and damage by target and non-target insect pests under different protection regimes in India. Crop Prot., 25: 800–813. https://doi.org/10.1016/j.cropro.2005.11.002
Subbalakshmi L., Muthukrishnan, P. and Jeyaraman, S., 2012. Neem products and their agricultural applications. J. Biopest., 5(Suppl.): 72-76.
Venkatesh, H.M., Muniyappa, V.J., Ravi, K.S. and Krishnaprasad, P.R., 1998. Management of chili leaf curl complex. Proc. First Nation. Symp. Pest Manag. Horti. Crops, Bangalore, pp. 48.
Vethanayagam, S.M. and Rajendran, S.M., 2010. Bioefficacy of neem insecticidal soap (NIS) on the disease incidence of bhendi, Abelmoschus esculentus (L.) Moench under field conditions. J. Biopest., 3: 246-249.
To share on other social networks, click on any share button. What are these?










