Single Step Detergent Assisted Extraction and Solubilization of the Recombinant Matrix Protein of Newcastle Disease Virus (NDV) and Development of ELISA for Detection of Anti-NDV Antibodies in Chickens
Single Step Detergent Assisted Extraction and Solubilization of the Recombinant Matrix Protein of Newcastle Disease Virus (NDV) and Development of ELISA for Detection of Anti-NDV Antibodies in Chickens
Zahra Naz1,2, Fouzia Ismat1, Muhammad Saleem2, Mazhar Iqbal1, Aamir Shehzad1 and Moazur Rahman1,2*
Schematic representation of Newcastle disease virus (NDV) structure.
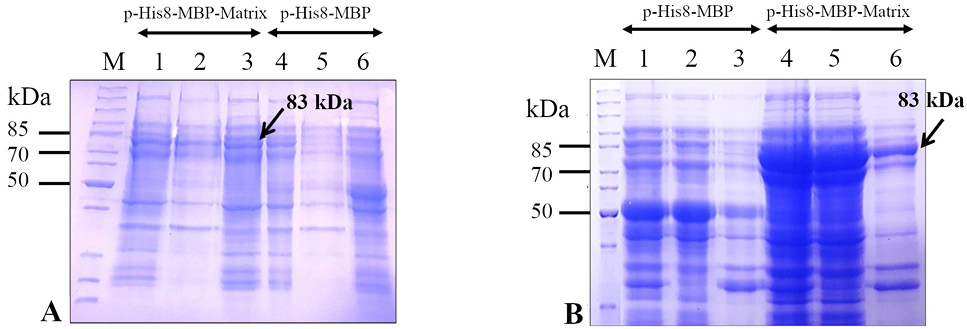
Expression analysis of the recombinant M (His8-MBP-Matrix) protein in Escherichia coli strains (BL21 (DE3) and Rosetta 2(DE3)) at 22 °C using 0.25 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). Upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), a comparison of coomassie brilliant blue stained gels containing separated protein expressed in E. coli BL21 (DE3) (panel A) and E. coli Rosetta 2(DE3) (panel B) indicates that the target protein (His8-MBP-Matrix) is better expressed in E. coli Rosetta 2(DE3) cells (panel B) than in E. coli BL21 (DE3) cells (panel A) using the expression plasmid, pHis8-MBP-Matrix. The pHis8-MBP plasmid was used as the vector-only control. Lanes 1 and 4 depict the whole cell lysate, lanes 2 and 5 represent soluble fractions, and lane 3 and 6 show insoluble fractions. Arrows indicate the band corresponding to the target (His8-MBP-Matrix) protein (~ 83 kDa). Lane M depicts the mobility of a protein molecular weight marker for estimating the size of separated proteins.
SDS-PAGE analysis of the recombinant M protein solubilized using various concentrations of lauryl-dimethylamine oxide (LDAO). The protein was solubilized using 1% (panel A), 5% (panel B), and 10% (panel C) LDAO. For SDS-PAGE analysis, samples containing different quantities (10 µg, 15 µg, and 20 µg) of the total protein were loaded. It is evident that the target protein is best solubilized using 5% LDAO. Lanes 1, 4, and 7 represent the whole cell lysate, lanes 2, 5, and 8 depict soluble fractions, and lanes 3, 6, and 9 show insoluble fractions of lysed E. coli Rosetta 2(DE3) cells containing the expressed recombinant M protein. Arrows indicate the band corresponding to the target (His8-MBP-Matrix) protein (~ 83 kDa). Lane M depicts the mobility of a protein molecular weight marker for estimating the size of separated proteins.
Analysis of the recombinant M protein purified by affinity chromatography using nickel-nitrilotriacetate (Ni-NTA) columns. Samples collected at different stages of affinity chromatography were analyzed through SDS-PAGE. Lane 1 represents the whole cell lysate, lane 2 depicts the soluble fraction, lane 3 denotes the insoluble fraction, lane 4 represents the flow-through from the column, lane 5 shows the fraction collected during stringent washing of the column, lanes 6 to 13 represent elution fractions. Arrows indicate the band corresponding to the target (His8-MBP-Matrix) protein (~ 83 kDa). Lane M depicts the mobility of a protein molecular weight marker for estimating the size of separated proteins.
Analysis of the NDV-specific antibody titer in chicken antisera through ELISA. The anti-NDV antibody titer and standard deviations (SD) among serum samples collected from chickens of different poultry farms are shown along with controls. Serum samples from NDV-infected chickens displayed a significanlty higher anti-NDV antibody titer than negative controls, confirming that the recombinant M protein can be used for detection of anti-NDV antibodies and/or NDV infection in chickens.