Suppressiveness of Late Blight and Fusarium Wilt of Tomato with Trichoderma Fortified Composts
Suppressiveness of Late Blight and Fusarium Wilt of Tomato with Trichoderma Fortified Composts
Muhammad Usman Ghazanfar, Muhammad Imran Hamid, Mubashar Raza, Waqas Raza*, Misbah Iqbal Qamar
Department of Plant Pathology, College of Agriculture, University of Sargodha, 40100 Sargodha, Pakistan
Abstract | Trichoderma is widely distributed and ubiquitous in almost all type of soils and their species promote growth of the plants and commercially used as bio-fungicide against soil borne plant pathogens. The present study was conducted with the aim to determine the efficacy of Trichoderma species using dual culture and pot assays against Phytophthora infestans (late blight) and Fusarium oxysporum (wilt) of tomato on different compost including carbon rich compost, nitrogen rich compost and nutrient enriched compost. The species of Trichoderma includes T. harzianum and T. asperellum isolated from rhizosphere of tomato from different localities of district Sargodha, Punjab, Pakistan which were recognized morphologically. Three isolates of T. harzianum HM, HK, HC and one isolate of T. asperellum TH were evaluated in dual culture assays and in pots amended with different composts against P. infestans and F. oxysporum. Mycelial growth reduction and inhibition percentage of P. infestans and F. oxysporum in dual culture assay significantly higher by T. harzianum HK as compared to all other isolates. Furthermore, pot experiments conducted with carbon rich compost inoculated with Trichoderma strains with pathogens inoculums provided most effective control as compared with nitrogen rich compost and nutrient enriched compost. These results suggested the potential of Trichoderma spp. for affective control of plant pathogens by considering the growth, environmental conditions favoring the disease suppression.
Received | June 02, 2018; Accepted | May 30, 2019; Published | Jult 27, 2019
*Correspondence | Waqas Raza, Department of Plant Pathology, College of Agriculture, University of Sargodha, 40100 Sargodha, Pakistan; Email: waqasraza61@yahoo.com
Citation | Ghazanfar, M.U., M.I. Hamid, M. Raza, W. Raza, M.I. Qamar. 2019. Suppressiveness of late blight and fusarium wilt of tomato with trichoderma fortified composts. Sarhad Journal of Agriculture, 35(3): 823-833.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.3.823.833
Keywords | Trichoderma species, Morphological characterization, Phytophthora, Fusarium, Compost
Introduction
Tomato (Solanum lycopersicum Mill.) belongs to solanaceae family and extensively cultivated vegetable that grows in almost every country of the world (Peralta and Spooner, 2007; Bashir et al., 2014). It has high nutritional value and contains vitamin B, C, phosphorus and iron (Beecher, 1998) and being consumed as fresh salad, pickle ketchup (Panthee and Chen, 2010) in different regions of the world. It is affected by many foliar, seed and soil borne pathogens (Oladiran and Lwu, 1993; Panthee and Chen, 2010). Among these, Phytophthora infestans (Mont.) de Bary (Olanya et al., 2015) and Fusarium oxysporum f. sp. lycopersici (FOL) (Li et al., 2017) are most destructive diseases causing agents worldwide. Both pathogens under favorable environmental conditions cause severe financial losses up to 100% (Chaerani and Voorrips, 2006; Nowicki et al., 2012; Ayukawa et al., 2016). Both the pathogens enter through the roots resulted in yellowing of leaves (Ayukawa et al., 2016) by clogging the vascular tissues while, late blight causes damage to leaves and fruit (Soylu et al., 2006; Majeed et al., 2017). Currently, management strategies through protective copper based fungicides are being used frequently for the control these diseases (Yao et al., 2016). This result in enhanced cost of production (Yao et al., 2016), residual toxicity problems, development of fungicide resistant strains, environmental pollution (Abo-Elyousr et al., 2014). Moreover, pesticides and other environmental disturbing chemicals have been banned and forbidden in Europe (Okkerman and van der Putte, 2002). Therefore, bio-control of plant diseases through the use of biocontrol agents can be the best alternative strategy compared with fungicides (Gavrilescu and Chisti, 2005; El-Komy et al., 2015; Yao et al., 2016; Li et al., 2017).
Trichoderma species are more frequently used as antagonist against different foliar, seed borne and soil-borne pathogens (Harman et al., 2004; Nawrocka and Małolepsza, 2013). Most of the published literature reported the biocontrol efficacy of Trichoderma against various soils as well as foliar pathogens (Martínez-Medina et al., 2013; Kushwaha et al., 2014). Many isolates have proved effective against tomato wilt (Srivastava et al., 2010; Chowdappa et al., 2013; Marzano et al., 2013) and late blight pathogen (Yao et al., 2016). Trichoderma spp. has different mode of action to combat with the plant pathogens by producing lytic enzymes which alter the various morphological and biochemical changes in pathogens and induce systemic resistance in the host against adverse conditions (Harman, 2006; Ranasingh et al., 2006; Charoenporn et al., 2010; Segarra et al., 2010). A successful ecological feature of this genus Trichoderma is mycoparasitism and efficient defensive approach induced in plant (Rosado et al., 2007).
Non-chemical approaches such as organic amendment and suppressive compost having long term benefits against the management of plant diseases are available but need to be commercialized (Lazarovits, 2001; Abbasi et al., 2004; Abbasi and Lazarovits, 2005; Abbasi and Lazarovits, 2006). The efficacy of Trichoderma amended compost in reducing the plant pathogens is well documented as this results in altering the physio-chemical properties of compost (Saxena et al., 2015). Organic matter amendment improves the soil productivity by nutrient and water retention (Weil and Magdoff, 2004). Hence, organic matter amendment can enhance the natural biocontrol efficacy against soil-borne plant pathogens (Davis et al., 2001; Mader et al., 2002).
The objective of this study was to check the efficacy of composts such as carbon, nitrogen and nutrient enriched compost inoculated with Trichoderma species against P. infestans and F. oxysporum.
Materials and Methods
The research work was conducted in laboratory of Plant Pathology, College of Agriculture, University of Sargodha, Punjab, Pakistan during 2013-14 to assess the efficacy of different composts inoculated with different spp. of Trichoderma against P. infestans and F. oxysporum.
Isolation of Trichoderma and pathogens
Infected samples were collected from various localities of district Sargodha (32◦5′1″N 72◦40′16″E). Isolation of P. infestans performed on Rye agar A medium containing antibiotics (ampicillin 0.25g/lit, rifampicin 0.01g/lit, pimaricin 0.4ml/lit, PCNB 5ml/lit) and isolation of F. oxysporum was performed on antibiotic emended potato dextrose agar (potato starch 4gm, glucose 20 gm, agar 15gm, distilled water 1L, penicillin 0.25mg/lit) from the infected samples showing typical symptoms of blight and wilt while, the Trichoderma spp. from different soil samples of tomato rhizosphere of the same location by soil dilution method on Potato dextrose medium and King’s B consisting of ingredients K2HPO4-4.0 g/L; MgSO4-0.4 g/L; protease peptone-20.0 g/L; glycerol-8.0 ml/L; agar-20.0 g/L (The pH was attuned to 7.0 ± 0.2 before autoclave), later on the plates were placed in incubator (Rodriguez-Kabana, 1967).
Dual culture antagonism assay
The isolates of Trichoderma (T. harzianum HM, HK, HC and T. asperellum TH) were evaluated in vitro against P. infestans and F. oxysporum by using dual culture assay in two different ways on 90 mm PDA petri plates by inoculating the 5mm agar plug of antagonist and pathogen from seven day old culture.
Dual Culture technique 1: Each plug from seven days pure colony of antagonistic and pathogenic fungi was placed at equivalent distance from periphery.
Dual Culture technique 2: One plug from seven days pure colony was placed at the centre of Petri plate and 3 plugs of antagonistic fungi placed in a triangle form by placing at equal distance of pathogen. Inoculated PDA plates were incubated in an incubator at 25±2ºC and percentage inhibition was calculated at 14dpi by formula;
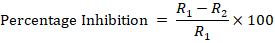
Where;
R1 is the linear growth of pathogen in check plate, R2 is the linear growth of pathogen in Trichoderma inoculated plate. Complete randomized design was followed against both techniques and the experiment were repeated twice.
Growth room pot experiment
Pot experiments were conducted with three replicates to compare three different composts viz, carbon rich compost, nitrogen rich compost and nutrient enriched compost comprising of sulphur and zinc mixed at the ratio of 1:10 (1kg compost: 10kg soil) with soil for performance evaluation of most competent isolates of T. harzianum and T. asperellum against P.infestans and F. oxysporum causing under growth room conditions. The fifteen days old seedlings of tomato variety (Early boy) were sown in surface sterilized plastic pots treated with Trichoderma and pathogens filled with different composts according to the description of Mwangi et al. (2011).
Inoculation of the inoculum
The antagonist and both the pathogens were isolated from infected tissue using standard laboratory techniques as described earlier. After the seven days of the colony growth, the colony grown on petri plates was wet-scraped using distilled sterile water and then the inoculum was observed at the microscope and the spores counted. The total spore suspension (1×107 spores/ml) of Trichoderma isolates counted by using haemocytometer were applied with pathogen inoculums while control treatments were treated only with pathogen P. infestans and F. oxysporum separately. The plant parameters such as, plant height, number of leaves, fresh weight of shoot, dry weight of shoot, and fresh weight of root as well as dry weight of root were recorded at 15 days of inoculation on carbon rich compost, nitrogen rich compost and nutrient enriched compost respectively.
Statistical analysis
The collected data were analyzed with the help of statistical software R by using least significant difference test (LSD). The results which have P<0.05 were considered as significant.
Results
Isolation and identification of Trichoderma spp and pathogens
The four isolates of Trichoderma were selected among 15 isolates based on their morphological characteristics as T. asperellum (TH) and T. harzianum (HM, HK and HC) as described by Castle et al. (1998) for further biological assays. The pathogens, Phytophthora infestans and Fusarium oxysporum was identified under the light microscope on the basis of morphological characteristics such as length, width of spores and colonies patterns according to the description of (Abad, 2008) and Leslie et al. (2006) respectively.
Pathogen suppression in dual culture antagonism test
The experimental findings in dual culture techniques showed the significant inhibition of P. infestans and F. oxysporum by Trichoderma species (Figure 1). It was also observed that both dual culture techniques were significantly effective against both plant pathogens (Figure 2).
Pot experiment
The effects of Trichoderma species with carbon, nitrogen and nutrient enriched (Phosphorous and zinc) compost was observed on plant height, number of leaves, shoot fresh weight, shoot dry weight, root fresh weight and root dry weight after 15 days of seed potting. The results revealed that comparing different composts, carbon rich compost was found to be the best rather than nitrogen rich and nutrient enriched compost when compared with control. Carbon rich compost inoculated with T. harzianum HK was found to be most successful for root and shoot development followed by T. harzianum HM, T. asperellum and T. harzianum HC against both pathogens. Carbon rich compost when used after inoculation with T. harzianum against F. oxysporum resulted in increase of plant height (9.95cm), number of leaves (62.0), shoot fresh weight (22.2 wt.), shoot dry weight (2.54g), root fresh weight (3.53g) and dry weight (2.4g) in all tested treatments as compared to control while T. harzianum compost when used against P. infestans resulted in increase of plant height (8.64cm), number of leaves (49.66), shoot fresh weight (19.67 wt.), shoot dry weight (1.96 g), root fresh weight (2.86g) and dry weight (1.7 g) (Table 3). The soil amended with different compost materials and Trichoderma species lead to increase in area and numbers of leaves of plant according to results and evidence shown in statistical analyzed data (Table 1, 2).
Discussion
The antagonistic ability of Trichoderma spp. varies from different regions of the world and may be inconsistent i.e. it may act poorly against pathogen for which it proved efficient in other regions (Hajieghrari et al., 2008; Otadoh et al., 2011). Therefore, it is imperative to use indigenous isolates for efficient products development which are compatible with environment and other related factors of the region where they will be used (Rabeendran et al., 2006). Furthermore, by molecular characterization, the chances of duplication of microbial control agents can be minimized (Stocco et al., 2016).
F. oxysporum and P. infestans cause wilt and late blight of tomato is yield limiting factors of tomato plant. Chemical control is not successful to control these diseases due to soil borne nature of the plant pathogens. Some limitations are common of all bio-control agents in varying environmental conditions related to their inconsistency. A large number of Trichoderma spp. isolated from soil were analyzed against F. oxysporum and P. infestans (Rai et al., 2016; Yao et al., 2016). Our results also demonstrated that there is clear inhibition zone created by the antimicrobial activity of the Trichoderma and formation of zone of inhibition and its size may be due to the production of antibiotics like glucanases, chitinases, trichodermol, trichodermin, peptaibols (Harman et al., 2004; Idris et al., 2007; Shoresh et al., 2010; Chowdappa et al., 2013).
The fungal mycelia had lysed with abnormal morphology and mycoparasitism clearly evident in Figure 2. Our results are almost comparable with the findings of (Rai et al., 2016) who reported that among twenty Trichoderma isolates obtained from tomato rhizosphere, nine considerably reduced the linear growth of fungal pathogens including F. Oxysporum (Trillas et al., 2006; Tondje et al., 2007; de los Santos-Villalobos et al., 2013). The results of the laboratory bioassays (Yao et al., 2016) showed that Trichoderma isolate NHA 14 significantly retarded the growth of the P. infestans (Barari, 2016) reported that the inhibition efficacy of twenty eight isolates obtained from healthy tomatoes rhizosphere of Trichoderma against F. oxysporumL-6 under dual culture bioassays, the isolate T. harzianum isolate N-8 proved most effective.
The practice of using organic amendment for the management of soil borne pathogen is an alternate strategy however; inconsistent results hinder its application in modern agriculture
Table 1: Response of different plant parameters on nutrient enriched (P and Zn) compost emended with Trichoderma spp.
| Trichoderma species |
Nutrient enriched compost |
|||||||||||
|
Fusarium oxysporum |
Phytophthora infestans |
|||||||||||
| Plant height (cm) | No. of leaves | Shoot fresh wt. (g) | Shoot dry wt. (g) | Root fresh wt. (g) | Roots dry wt. (g) | Plant height (cm) | No. of leaves | Shoot fresh wt. (g) | Shoot dry wt. (g) | Root fresh wt. (g) | Roots dry wt. (g) | |
| Control |
4.2± 1.07 d |
11.1± 1.6e |
1.3± 1.7 d |
0.7± 0.78d |
0.69± 0.04 d |
0.38± 0.05d |
3.97± 1.17d |
10.01± 1.05 e |
0.98± 0.13 d |
0.30± 0.05 d |
0.38± 0.04 e |
0.20± 0.06d |
|
T. asperellum TH |
6.1± 0.05 b |
25.3± 1.77 c |
9.9± 0.26 b |
1.2± 0.09 c |
2.10± 0.04 c |
1.58± 0.05b |
6.24± 0.04b |
22.54± 0.04 c |
8.76± 0.05 c |
1.29± 0.03a |
1.75± 0.04c |
0.81± 0.07b |
|
T. harzianum HM |
7.4± 0.04 a |
32.5± 1.66 b |
11.4± 0.17a |
1.5± 0.12 b |
2.71± 0.76 b |
1.81± 0.03a |
6.89± 0.06 a |
29.4± 0.04 b |
9.88± 0.02 b |
1.43± 0.03 a |
2.1± 0.03 b |
0.91± 0.03a |
|
T. harzianum HK |
7.6± 0.76 a |
36.0± 1.04 a |
12.3± 0.24a |
1.9± 0.2 a |
2.98± 0.24 a |
1.7± 0.04 a |
7.14± 0.05 a |
33.87± 1.52 a |
10.69± 0.06 a |
1.56± 0.05 a |
2.64± 0.03 a |
0.97± 0.02a |
|
T. harzianum HC |
5.4± 0.06 c |
21.5± 0.04 d |
8.7± 0.14 c |
1.1± 0.08c |
1.57± 0.04 c |
0.94± 0.09 c |
5.23± 0.03 c |
19.43± 0.06 c |
8.11± 0.05 c |
0.95± 0.04 c |
1.51± 0.04 d |
0.61± 0.03 c |
*Small letters are for comparison for within column.
Table 2: Response of different plant parameters on nitrogen rich compost emended with Trichoderma spp.
| Trichoderma species |
Nitrogen compost |
|||||||||||
|
Fusarium oxysporum |
Phytophthora infestans |
|||||||||||
| Plant height (cm) | No. of leaves | Shoot fresh wt. (g) | Shoot dry wt. (g) | Root fresh wt. (g) | Roots dry wt. (g) | Plant height (cm) | No. of leaves | Shoot fresh wt. (g) | Shoot dry wt. (g) | Root fresh wt. (g) | Roots dry wt. (g) | |
| Control |
4.23± 0.04d |
12.10± 1.6 e |
1.38± 1.3 d |
0.74± 0.12d |
0.79± 0.06 d |
0.47± 0.06 d |
3.99± 0.06 d |
10.66± 0.08 e |
1.12± 0.14 e |
0.33± 0.06 c |
0.44± 0.06c |
0.23± 0.05d |
|
T. asperellum TH |
6.73± 0.06b |
37.32± 1.77c |
12.4± 0.29b |
1.64± 0.13b |
2.46± 0.05 c |
1.11± 0.04 b |
6.33± 0.06 b |
25.31± 0.05 c |
10.13± 0.06 c |
1.21± 0.04 b |
1.88± 0.03b |
0.89± 0.07b |
|
T. harzianum HM |
7.56± 0.05a |
43.43± 1.70b |
13.1± 0.20b |
1.78± 0.13a |
2.38± 0.84 b |
1.92± 0.04 a |
7.00± 0.04 a |
30.5± 0.05 b |
11.36± 0.03 b |
1.58± 0.04 a |
2.02± 0.05a |
1.21± 0.04a |
|
T. harzianum HK |
8.02± 0.08a |
46.0± 1.06a |
15.6± 0.21a |
2.11± 0.23a |
3.26± 0.12 a |
2.1± 0.05 a |
7.36± 0.03 a |
36.54± 0.04 a |
13.44± 0.0 a |
1.73± 0.04 a |
2.72± 0.04a |
1.3± 0.03a |
|
T. harzianum HC |
5.88± 0.07c |
31.77± 0.07d |
10.5± 0.17c |
1.44± 0.07c |
2.12± 0.05 c |
0.83± 0.03 c |
5.32± 0.02 c |
25.55± 0.03 c |
9.43± 0.04 d |
1.37± 0.02 b |
1.54± 0.03b |
0.67± 0.03c |
*Small letters are for comparison for within column.
Table 3: Response of different plant parameters on carbon rich compost emended with Trichoderma spp.
| Trichoderma species |
Carbon compost |
|||||||||||
|
Fusarium oxysporum |
Phytophthora infestans |
|||||||||||
| Plant height (cm) | No. of leaves | Shoot fresh wt. (g) | Shoot dry wt. (g) | Root fresh wt. (g) | Roots dry wt. (g) | Plant height (cm) | No. of leaves | Shoot fresh wt. (g) | Shoot dry wt. (g) | Root fresh wt. (g) | Roots dry wt. (g) | |
| Control |
6.10± 0.05 d |
16.04± 0.72e |
1.44± 0.11 e |
0.83± 0.05 d |
0.79± 0.07c |
0.47± 0.05e |
5.59± 0.04d |
14.66± 0.07e |
1.18± 0.12d |
0.63± 0.07d |
0.62± 0.08c |
0.31± 0.07c |
|
T. asperellum TH |
7.80± 0.13 b |
43.55± 1.47c |
16.3± 0.42c |
1.92± 0.18b |
2.77± 0.08b |
1.26± 0.05c |
6.62± 0.07b |
33.43± 0.04c |
14.16± 0.05c |
1.33± 0.03b |
1.99± 0.05b |
1.11± 0.04a |
|
T. harzianum HM |
9.91± 0.014a |
58.43± 1.90b |
18.0± 0.27b |
2.37± 0.19a |
3.14± 0.94a |
2.09± 0.06b |
8.00± 0.05a |
45.5± 0.03b |
16.34± 0.02b |
1.67± 0.02a |
2.44± 0.04a |
1.33± 0.05a |
|
T. harzianum HK |
9.95± 0.36a |
62.0± 0.07a |
22.2± 0.34a |
2.54± 0.21a |
3.53± 0.15a |
2.4± 0.08a |
8.64± 0.04a |
49.66± 0.04a |
19.67± 0.03a |
1.96± 0.04a |
2.86± 0.06a |
1.7± 0.8a |
|
T. harzianum HC |
6.97± 0.09c |
38.77± 0.09d |
15.1± 0.18d |
1.54± 0.06c |
2.52± 0.06b |
0.98± 0.04d |
5.54± 0.03c |
28.64± 0.02d |
13.56± 0.03c |
1.10± 0.03c |
1.77± 0.04b |
0.78± 0.04b |
*Small letters are for comparison for within column.
(Bonanomi et al., 2010). It is valuable that, composts which rich in carbon, nitrogen and zinc as well as phosphorous used as raw material emended with Trichoderma in this work was suppressive to F. oxysporum and P. infestans pathogens of tomato and promote the plant growth. The present results suggested that carbon rich compost with Trichoderma is most effective than the nitrogen rich and nutrient enriched compost against F. oxysporum and P. infestans. The maximum disease suppression can be achieved using optimum ratio of C: N in the compost and that compost emended with Trichoderma can give efficient control as well as promote the growth of the plant. In addition, compost characterized by mixture of different substrates authorized an equal large diversity of microorganism to establish in which some are contributing in disease suppression (Pascual et al., 2002).
Composts inoculation with antagonists may enhance its effectiveness and consistency to control plant pathogens (Noble and Coventry, 2005). Our results demonstrated that different plant parameters are greatly influenced with the amendments of Trichoderma in different composts while the carbon enriched compost was most effective. Earlier reports reported that compost prepared from swedge sludge with low carbon and nitrogen ratio was not suppressive to Fusarium wilt (Hoitink et al., 1987). It has been also reported that be deficient in suppression of biocontrol agent against soil borne pathogens in such low carbon and nitrogen ratio is due to presence of high concentration of ammonia (Hoitink et al., 1987). Numerous studies were done with the application of compost inoculated with bio control agents to control soil-borne diseases (Lumsden et al., 1983; Borrero et al., 2004). Researchers are more focused in formulating correct combination of composts with antagonists for the control of plant pathogens (Abdel-Kardar et al., 2013; Saxena et al., 2015). In this regards, combination of compost with different agriculture waste proved effective in suppressing R. solani in cucumber young seedlings (Trillas et al., 2006) and Fusarium wilt of tomato was suppressed by Trichoderma spp. and sewage sludge compost (Cotxarrera et al., 2002). Similarly, the suppressiveness of R. solani was enhanced with the addition of Trichoderma T-22 in the compost/peat mix against the soil borne plant pathogens (Pugliese et al., 2011) are also support the present findings. (Li et al., 2017) reported that T. asperellum strain CHF 78 increased plant growth i.e. dry weight and plant height and mineral uptake significantly thereby reduced the wilt disease in tomatoes. T. harzianum strain notably enhanced seedling growth in tomatoes in reducing the lesion size (Chowdappa et al., 2013). Fusarium wilt of melon was control (Lopez-Mondejar et al., 2010) by the combine application of citrus composed with T. harzianum T-78 is another example supports our findings. The addition of T. asperellum strain T34 restored suppressive capacity of the compost against Fusarium wilt of carnation as compared with control (Sant et al., 2010). The addition of Trichoderma isolates has direct impact on different plant parameters as in our experiments, T. harzianum HK found to be most effective for root and shoot development. The results reported by (Rabeendran et al., 2000) are in conformity with our studies as the authors showed that T. longipile and T. tomentosum increased leaf, shoot and root dry weight of cabbage seedlings in field trial (58-71%; 91-102%; 100-158% respectively). Tomato plant had 35% less disease intensity when grown in perlite enriched with T34 and also showed increased leaf area, plant height and uptake of nutrient (Fernández et al., 2014) as compared with plants grown in perlite without Trichoderma. The melon wilt can be effectively controlled with the application of T. polysporum and liquid compost with 100% increase (Gava and Pinto, 2016) in the production of commercial fruits. (El Khaldi et al., 2016) tested two compost with different nitrogen source against R. solani on potato and found highest disease incidence and severity with cattle manure compost than with sheep manure compost. The fortification of vrmi compost with Trichoderma greatly influenced the length and weight of plant parts (shoot, root) in mungbean (Saxana et al., 2015) are also in line with the present findings. The disease intensity was significantly lower with low percentage of melon stem infected with F. oxysporum f. sp. melonis in the compost (Blaya et al., 2013) inoculated with T. harzianum. Different Trichoderma isolates have proved effective against P. infestans on various host plants, isolate Th-Sks was proved most virulent antagonist against P. infestans on tomato and promoted plant height and fruits yield during field trial (Sain and Pandey, 2016) are consistent with our studies. Moreover, six isolates of T. asperellum showed higher mycoparasitic activity against Phytophthora ramorum under the field conditions (Widmer, 2014). Trichoderma strains isolated form Moroccan agro systems and inoculated with composts was proved effective for the biocontrol of Fusarium wilt of tomato (Taghdi et al., 2015). The reduction in both the diseases in the present study by fortification of compost and biocontrol interaction might be due to mechanisms of antibiosis, competition for substrate, microbiostasis, colonization, propagule destruction and induced systemic resistance (Blaya et al., 2013). Trichoderma isolates have the ability to live as endophyte in plant roots and enhanced the IAA production that might increase the root weight in the said study which was also supported by (López-Bucio et al., 2015) additionally, the siderophores produced by Trichoderma isolates enhanced uptake of nutrients (Rudresh et al., 2005), this may be the reason of increased shoot weight, plant height and number of leaves in amended compost which was more pronounced in T. harzianum HK in our experiments.
Conclusions and Recomendations
The present research work demonstrated the integrated management strategies to control diseases of tomato caused by F oxysporum and P. infestans. The important feature of study was the utilization of antagonist with the carbon, nitrogen and nutrient enriched compost which has potential of controlling tomato diseases as well as promoting the plant growth. Therefore, use of different compost emended with Trichoderma spp. may be incorporated with other control strategies to minimize the losses caused by F. oxysporum and P. infestans of tomato crop under commercial tomato production systems.
Novelty Statement
Trichoderma species are more frequently used as antagonist against different foliar, seed borne and soil-borne pathogens. The study finds out the efficacy of carbon, nitrogen and nutrient enriched compost inoculated with Trichoderma species against P. infestans and F. oxysporum. It proves different compost enriched bio-control agents enhances efficacy against plant pathogens.
Author’s Contribution
Muhammad Usman Ghazanfar conceived the idea and facilitated, guided and supervised the experiment. Mr. Mubashar Raza planned and executed the experiment and also noted the results. Imran Hamid did statistical analysis while Waqas Raza wrote and finalized the manuscript.
References
Abad, G. 2008. Methods for identification of phytophthora species. In workshop: Fighting phytophthora: how to detect, investigate, and manage phytophthora. APS Cenntennial Meeting. http://www. phytophthoradb. org/pdf/2008APS_ abad. pdf (consultado enero de, 2011).
Abbasi, P.A. and G. Lazarovits. 2005. Effects of AG3 phosphonate formulations on incidence and severity of Pythium damping-off of cucumber seedlings under growth room, micro plot and field conditions. Can. J. Plant Pathol. 27 (3): 420-429. https://doi.org/10.1080/07060660509507241
Abbasi, P.A. and G. Lazarovits. 2006. Seed treatment with phosphonate (AG3) suppresses Pythium damping-off of cucumber seedlings. Plant Dis. 90 (4): 459-464. https://doi.org/10.1094/PD-90-0459
Abbasi, P.A., K.L. Conn and G. Lazarovits. 2004. Suppression of Rhizoctonia and Pythium damping-off of radish and cucumber seedlings by addition of fish emulsion to peat mix or soil. Can. J. Plant Pathol. 26 (2): 177-187. https://doi.org/10.1080/07060660409507129
Abdel-Kader, M.M., F. Abdel-Kareem, N.S. El-Mougy and R.S. El-Mohamady. 2013. Integration between compost, Trichoderma harzianum and essential oils for controlling peanut crown rot under field conditions. J. Mycol. https://doi.org/10.1155/2013/262130
Abo-Elyousr, K.A.M., I.I.S. Abdel-Hafez and I.R. Abdel-Rahim. 2014. Isolation of Trichoderma and evaluation of their antagonistic potential against Alternaria porri. J. Phytopathol. 162 (9): 567-574. https://doi.org/10.1111/jph.12228
Ayukawa, Y., K. Komatsu, T. Kashiwa, K. Akai, M. Yamada, T. Teraoka and T. Arie. 2016. Detection and differentiation of Fusarium oxysporum f. sp. lycopersici race 1 using loop-mediated isothermal amplification with three primer sets. Lett. Appl. Microbiol. 63(3): 202-209. https://doi.org/10.1111/lam.12597
Barari, H. 2016. Biocontrol of tomato Fusarium wilt by Trichoderma species under in vitro and in vivo conditions. Cerce. Agronom. Mold. 49 (1): 91-98. https://doi.org/10.1515/cerce-2016-0008
Beecher, G.R. 1998. Nutrient content of tomatoes and tomato products. Proc. Soc. Exp. Biol. Med. 218 (2): 98-100. https://doi.org/10.3181/00379727-218-44282a
Blaya, J., R. López-Mondéjar, E. Lloret, J.A. Pascual and M. Ros. 2013. Changes induced by Trichoderma harzianumin suppressive compost controlling Fusarium wilt. Pest. Biochem. Physiol. 107 (1): 112-119. https://doi.org/10.1016/j.pestbp.2013.06.001
Bonanomi, G., V. Antignani, M. Capodilupo and F. Scala. 2010. Identifying the characteristics of organic soil amendments that suppress soil borne plant diseases. Soil Biol. Biochem. 42 (2): 136-144. https://doi.org/10.1016/j.soilbio.2009.10.012
Borrero, C., M.I. Trillas, J. Ordovás, J.C. Tello and M. Avilés. 2004. Predictive factors for the suppression of fusarium wilt of tomato in plant growth media. Phytopathol. 94 (10): 1094-1101. https://doi.org/10.1094/PHYTO.2004.94.10.1094
Chaerani, R. and R.E. Voorrips. 2006. Tomato early blight (Alternaria solani): the pathogen, genetics, and breeding for resistance. J. Gen. Plant Pathol. 72 (6): 335-347. https://doi.org/10.1007/s10327-006-0299-3
Chowdappa, P., S.P.M. Kumar, M.J. Lakshmi and K.K. Upreti. 2013. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control. 65 (1): 109-117. https://doi.org/10.1016/j.biocontrol.2012.11.009
Cotxarrera, L., M.I. Trillas-Gay, C. Steinberg and C. Alabouvette. 2002. Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato. Soil Biolo. Biochem. 34 (4): 467-476. https://doi.org/10.1016/S0038-0717(01)00205-X
Davis, J.R., O.C. Huisman, D.O. Everson and A.T. Schneider. 2001. Verticillium wilt of potato: a model of key factors related to disease severity and tuber yield in southeastern Idaho. Amer. J. Potato Res. 78 (4): 291-300. https://doi.org/10.1007/BF02875694
de los Santos-Villalobos, S., D.A. Guzmàn-Ortiz, M.A. Gomez-Lim, J.P. Délano-Frier, S. de-Folter, P. Sànchez-Garía and J.J. Peña-Cabriales. 2013. Potential use of Trichoderma asperellum (Samuels, Liechfeldtet Nirenberg) T8a as a biological control agent against anthracnose in mango (Mangifera indica L.). Biol. Control. 64 (1): 37-44. https://doi.org/10.1016/j.biocontrol.2012.10.006
El Khaldi, R., M. Daami-Remadi and M. Cherif. 2016. Biological control of stem canker and black scurf on potato by date palm compost and its associated fungi. J. Phytopathol. 164 (1): 40-51. https://doi.org/10.1111/jph.12423
El-Komy, M.H., A.A. Saleh, A. Eranthodi and Y.Y. Molan. 2015. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. The Plant Pathol. J. 31 (1): 50-60. https://doi.org/10.5423/PPJ.OA.09.2014.0087
Fernández, G., G. Segarra and M.I. Trillas. 2014. Physiological effects of the induction of resistance by compost or Trichoderma asperellum strain T34 against Botrytis cinerea in tomato. Biol. Control. 78: 77-85. https://doi.org/10.1016/j.biocontrol.2014.06.012
Gava, C.A. T. and J.M. Pinto. 2016. Biocontrol of melon wilt caused by Fusarium oxysporum Schlect f. sp. melonis using seed treatment with Trichoderma spp. and liquid compost. Biol. Control. 97: 13-20. https://doi.org/10.1016/j.biocontrol.2016.02.010
Gavrilescu, M. and Y. Chisti. 2005. Biotechnology-a sustainable alternative for chemical industry. Biotechnol. Adv. 23 (7-8): 471-499. https://doi.org/10.1016/j.biotechadv.2005.03.004
Hajieghrari, B., M. Torabi-Giglou, M.R. Mohammadi and M. Davari. 2008. Biological potential of some Iranian Trichoderma isolates in control of soil borne plant pathogenic fungi. Afr. J. Biotechnol. 7 (8): 967-972.
Harman G.E., C.R. Howell, A. Viterbo, I. Chet and M. Lorito. 2004. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2(1): 43-56. https://doi.org/10.1038/nrmicro797
Harman, G.E. 2006. Overview of mechanisms and uses of Trichoderma spp. Phytopathol. 96 (2): 190-194. https://doi.org/10.1094/PHYTO-96-0190
Hoitink, H.A.J., M. Dughtrey and H. Tayama. 1987. Control of cyclamen Fusarium wilt: a preliminary report. Ohio Florists’ Assoc. Bull. 693: 1-3.
Idris, E.E.S., D. Iglesias, M. Talon and R. Borriss. 2007. Tryptophan dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microb. Interact. 20 (6): 619-626. https://doi.org/10.1094/MPMI-20-6-0619
Lazarovits, G. 2001. Management of soil-borne plant pathogens with organic soil amendments: a disease control strategy salvaged from the past. Can. J. Plant Pathol. 23 (1): 1-7. https://doi.org/10.1080/07060660109506901
Li, Y.T., S.G. Hwang, Y.M. Huang and C.H. Huang. 2017. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot. https://doi.org/10.1016/j.cropro.2017.03.021
Leslie, J.F., B.A. Summerell and S. Bullock. 2006. The fusarium laboratory manual, 1st edition. Wiley Blackwell. https://doi.org/10.1002/9780470278376
López-Bucio, J., R. Pelagio-Flores and A. Herrera-Estrella. 2015. Trichoderma as biostimulant: exploiting the multilevel properties of a plant beneficial fungus. Sci. Hort. 196: 109-123. https://doi.org/10.1016/j.scienta.2015.08.043
Lopez-Mondejar, R., A. Bernal-Vicente, M. Ros. F. Tittarelli, S. Canali, F. Intrigiolo and J.A. Pascual. 2010. Utilisation of citrus compost-based growing media amended with Trichoderma harzianum T-78 in Cucumismelo L. seedling production. Bioreso. Technol. 101 (10): 3718-3723. https://doi.org/10.1016/j.biortech.2009.12.102
Lumsden, R.D., J.A. Lewis and P.D. Millner. 1983. Effect of composted sewage sludge on several soilborne pathogens and diseases. Phytopathol. 73 (11): 1543-1548. https://doi.org/10.1094/Phyto-73-1543
Mader, P., A. Fliessbach, D. Dubois, L. Gunst, P. Fried and U. Niggli. 2002. Soil fertility and biodiversity in organic farming. Sci. 296 (5573): 1694-1697. https://doi.org/10.1126/science.1071148
Majeed, A., Z. Muhammad, Z. Ullah, R. Ullah and H. Ahmad. 2017. Late blight of potato (Phytophthora infestans) I: fungicides application and associated challenges. Turk. J. Agric. Food Sci. Technol. 5 (3): 261-266. https://doi.org/10.24925/turjaf.v5i3.261-266.1038
Martínez-Medina A., I. Fernández, M.J. Sánchez-Guzmán, S.C. Jung, J.A. Pascual and M.J. Pozo. 2013. Deciphering the hormonal signaling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Front. Plant Sci. 4: 1-12. https://doi.org/10.3389/fpls.2013.00206
Marzano, M., A. Gallo and C. Altomare. 2013. Improvement of biocontrol efficacy of Trichoderma harzianum vs. Fusarium oxysporum f. sp. lycopersici through UV-induced tolerance to fusaric acid. Biol. Control. 67 (3): 397-408. https://doi.org/10.1016/j.biocontrol.2013.09.008
Mwangi, M.W., E.O. Monda, S.A. Okoth and J.M. Jefwa. 2011. Inoculation of tomato seedlings with Trichoderma harzianum and arbuscular mycorrhizal fungi and their effect on growth and control of wilt in tomato seedlings. Brazilian J. Microbiol. 42(2): 508-513. https://doi.org/10.1590/S1517-83822011000200015
Nawrocka, J. and U. Małolepsza. 2013. Diversity in plant systemic resistance induced by Trichoderma. Biol. Control. 67 (2): 149-156. https://doi.org/10.1016/j.biocontrol.2013.07.005
Noble, R. and E. Coventry. 2005. Suppression of soil-borne plant diseases with composts: A review, Biocontrol Sci. Technol. 15 (1): 3-20. https://doi.org/10.1080/09583150400015904
Nowicki, M., M.R. Foolad, M. Nowakowska and E.U. Kozik. 2012. Potato and tomato late blight caused by Phytophthora infestans: An overview of pathology and resistance breeding. Plant Dis. 96 (1): 4-17. https://doi.org/10.1094/PDIS-05-11-0458
Oladiran, A.O. and L.U. Lwu. 1993. Studies on the fungi associated with tomato fruit rots and effects of environment on storage. Mycopathol. 121 (3): 157-161. https://doi.org/10.1007/BF01104071
Olanya, O.C., R.P. Larkin and C.W. Honeycutt. 2015. Incidence of Phytophthora infestans(Mont.) de Bary on potato and tomato in Maine, 2006-2010. J. Plant Prot. 55 (1): 58-68. https://doi.org/10.1515/jppr-2015-0009
Otadoh, J.A., S.A. Okoth, J. Ochanda and J.P. Kahindi. 2011. Assessment of Trichoderma isolates for virulence efficacy on Fusarium oxysporum f. sp. phaseoli. Trop. Subtrop. Agroecosyst. 13 (1): 99-107.
Panthee, D.R. and F. Chen. 2010. Genomics of fungal disease resistance in tomato. Curr. Genom. 11 (1): 30-39. https://doi.org/10.2174/138920210790217927
Pascual, J.A., T. Hernandez, C. Garcia, S. Lerma and J.M. Lynch. 2002. Effectiveness of municipal waste compost and its humic fraction in suppressing Pythium ultimun. Microb. Ecolo. 44 (1): 59-68. https://doi.org/10.1007/s00248-001-0040-x
Peralta, I.E. and D.M. Spooner. 2007. History, origin and early cultivation of tomato (Solanaceae). In: Mattoo, A.K: Genetic improvement of solanaceous crops Vol. 2: Tomato, Sci. Publ. 2006. pp. 1-24. https://doi.org/10.1201/b10744-2
, M., B.P. Liu, M.L. Gullino and A. Garibaldi. 2011. Microbial enrichment of compost with biological control agents to enhance suppressiveness to four soil-borne diseases in greenhouse. J. Plant Dis. Prot. 118 (2): 45-50. https://doi.org/10.1007/BF03356380
Rabeendran, N., E.E. Jones, D.J. Moot and A. Stewart. 2006. Biocontrol of Sclerotinialettuce drop by Coniothyrium minitans and Trichoderma hamatum. Biol. Control. 39 (3): 352-362. https://doi.org/10.1016/j.biocontrol.2006.06.004
Rai, S., P.L. Kashyap, S. Kumar, A.K. Srivastava and P.W. Ramteke. 2016. Identification, characterization and phylogenetic analysis of antifungal Trichoderma from tomato rhizosphere. Springer Plus. 5 (1): 1939. https://doi.org/10.1186/s40064-016-3657-4
Rodriguez-Kabana, R. 1967. An improved method for assessing soil-fungus population density. Plant and Soil. 26 (2): 393-396. https://doi.org/10.1007/BF01880191
Rosado, I.V., M. Rey, A.C. Codón, J. Govantes, M.A. Moreno-Mateos and T. Benítez. 2007. QID74 Cell wall protein of Trichoderma harzianum is involved in cell protection and adherence to hydrophobic surfaces. Fung. Gen. Biol. 44 (10): 950-964. https://doi.org/10.1016/j.fgb.2007.01.001
Rudresh, D.L., M.K. Shivaprakash and R.D. Prasad. 2005. Tricalcium phosphate solubilizing abilities of Trichoderma spp. in relation to P uptake and growth and yield parameters of chickpea (Cicerarietinum L. Cana. J. Microbiol. 51 (3): 217-222. https://doi.org/10.1139/w04-127
Sain, S.K., and A.K. Pandey. 2016. Biological spectrum of Trichoderma harzianum Rifai isolates to control fungal diseases of tomato (Solanum lycopersicon L.). Arch. Phytopathol. Plant Prot. 49 (19-20): 507-521. https://doi.org/10.1080/03235408.2016.1242393
Sant, D., E. Casanova, G. Segarra, M. Avilés, M. Reis and M.I. Trillas. 2010. Effect of Trichoderma asperellum strain T34 on Fusarium wilt and water usage in carnation grown on compost-based growth medium. Biol. Control. 53 (3): 291-296. https://doi.org/10.1016/j.biocontrol.2010.01.012
Saxena, J., S. Choudhary, S. Pareek, A.K. Choudhary and M.A. Iquebal. 2015. Recycling of organic waste through four different composts for disease suppression and growth enhancement in mung beans. Clean Soil Air Water. 43 (7): 1066-1071. https://doi.org/10.1002/clen.201300748
Segarra, G., E. Casanova, M. Avilés, I. Trillas. 2010. Trichoderma asperellum strain T34 controls Fusarium wilt disease in tomato plants in soilless culture through competition for Iron. Microb. Ecol. 59 (1): 141-149. https://doi.org/10.1007/s00248-009-9545-5
Shoresh, M., F. Mastouri and G.E. Harman. 2010. Induced systemic resistance and plant responses to fungal bio-control agents. Ann. Rev. Phytopathol. 48: 21-43. https://doi.org/10.1146/annurev-phyto-073009-114450
Soylu, E.M., S. Soylu and S. Kurt. 2006. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathol. 161 (2): 119-128. https://doi.org/10.1007/s11046-005-0206-z
Srivastava, R., A. Khalid, U.S. Singh and A.K. Sharma. 2010. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol. Control. 53 (1): 24-31. https://doi.org/10.1016/j.biocontrol.2009.11.012
, M.C., C.I. Selection and characterization of Argentine isolates of Trichoderma harzianum for effective biocontrol of Septoria leaf blotch of wheat. W. J. Microbiol. Biotechnol. 32 (3): 49-58. https://doi.org/10.1007/s11274-015-1989-9
Tondje, P.R., D.P. Roberts, M.C. Bon, T. Widmer, G.J. Samuels, A. Ismaiel, A.D. Begoude, T. Tchana, E. Nyemb-Tshomb, M. Ndoumbe-Nkeng, R. Bateman, D. Fontem and K.P. Hebbar. 2007. Isolation and identification of mycoparasitic isolates of Trichoderma asperellum with potential for suppression of black pod disease of cacao in Cameroon. Biol. Control. 43 (2): 202-212. https://doi.org/10.1016/j.biocontrol.2007.08.004
Trillas M.I., E. Casanova, L. Cotxarrera, J. Ordovás, C. Borrero and M. Avilés. 2006. Composts from agricultural waste and the Trichoderma asperellum strain T-34 suppress Rhizocotonia solani in cucumber seedlings. Biol. Control. 39 (1): 32-38. https://doi.org/10.1016/j.biocontrol.2006.05.007
Weil, R.R. and F. Magdoff. 2004. Significance of soil organic matter to soil quality and health. Soil organic matter in sustainable agriculture. CRC Press, Boca Raton, FL, 1-43. https://doi.org/10.1201/9780203496374.ch1
White T.J., T.D. Bruns, S.B. Lee and J.W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a Guide to Methods and Applications (eds Innis MA, Gelfand DH, Sninsky JJ, White TJ), pp. 315-322. Acad. Press, London, UK. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Widmer, T.L. 2014. Screening Trichoderma species for biological control activity against Phytophthora ramorum in soil. Biol. Control. 79:43-48. https://doi.org/10.1016/j.biocontrol.2014.08.003
Biological control of potato late blight using isolates of Trichoderma. Am. J. Potato Res. 93 (1): 33-42. https://doi.org/10.1007/s12230-015-9475-3
To share on other social networks, click on any share button. What are these?








