Variations in Soil Properties and Forms of Inorganic Phosphorus within the Rhizosphere of Annual Crops
Variations in Soil Properties and Forms of Inorganic Phosphorus within the Rhizosphere of Annual Crops
Stanley Uchenna Onwudike* and Vivian Chizoba Edoziem
Soil Science and Technology Department, Federal University of Technology Owerri, PMB 1526, Imo State, Nigeria.
Abstract | Phosphorus deficiency is one of the soil factors affecting crop production in Southern part of Nigeria and understanding its distributions in rhizosphere soils will help in its management. This investigation examined some variations in soil properties and inorganic phosphorus forms within the rhizosphere of three annual crops, viz groundnut (Arachis hypogea), bean (Phaseolus vulgaris) and okra (Abelmoschus esculentus) on a loamy sand, typic haplustult soil. The work was sited at the research farm of the University during 2016 planting season and the region lies within the latitude of 5° 38¹N and longitude 6° 97¹ E. At 10 weeks after planting, both the rhizosphere soil (soils within 2 mm region of the test crops) and bulk soil or control (soils away from the root zone) from the experimental plots that were planted with bean, groundnut and okra were sampled, air dried and analyzed in the laboratory. Results showed significant effect (p = 5%) on soil moisture content, soil pH, organic carbon and exchangeable bases between the bulk soil and the rhizosphere. The highest value of saloid bound P (7.77 mg/kg) was found within the bean root zone while the highest value of phosphorus bound to Al (Al - P) (13.27 mg/kg) was found in okra root zone. Highest value of occluded – phosphorus (46.07 mg/kg), Fe -Al–P (40.62 mg/kg) and Ca-P (6.77 mg/kg) were recorded in groundnut root zones. Significant relation occurred between occluded–P with bulk density, exchangeable Ca and ECEC (effective cation exchange capacity), saloid bound P with BS (base saturation), moisture content as well as organic carbon, Al–P with available phosphorus and moisture content while Ca–P and Fe-Al–P had significant relation with available phosphorus and effective cation exchange capacity. As a result of different concentrations of inorganic phosphorus forms within these roots zones, appropriate agronomic management practices such as crop rotation, mixed cropping and shifting cultivation is recommended for sustainable phosphorus management in the studied soil.
Received | March 11, 2018; Accepted | December 15, 2019; Published | March 03, 2020
*Correspondence | Stanley Uchenna Onwudike, Soil Science and Technology Department, Federal University of Technology Owerri, PMB 1526, Imo State, Nigeria; Email: [email protected]
Citation | Onwudike, S.U. and V.C. Edoziem. 2020. Variations in soil properties and forms of inorganic phosphorus within the rhizosphere of annual crops. Sarhad Journal of Agriculture, 36(1): 359-366.
DOI | http://dx.doi.org/10.17582/journal.sja/2020/36.1.359.366
Keywords | Acid soil, Phosphorus fixation, Phosphorus forms, Root zone, Soil characteristics
Introduction
In Southern part of Nigeria, phosphorus is among the limiting macro nutrient elements in soil. Phosphorus deficiency has been attributed to high rainfall and runoff, acidity of the soil with pH range of 5.3 – 5.9 (Onwudike et al., 2015) and phosphorus fixation (Asmare et al., 2015; Onweremadu and Ofoh, 2007). Researchers have advocated agronomic practices for addressing issues regarding soil fertility (Khan et al., 2018) and phosphorus management in the region like application of inorganic fertilizers (Nottidge et al., 2005), animal manure (Ano and Agwu, 2005), mulching (Saroa and Lal, 2004) and their integrated application (Uwah and Iwo, 2011).
Phosphorus occurs in organic form and inorganic form in the soil (Asmare et al., 2015). Inorganic phosphorus forms made up of the easily soluble active forms like the aluminum and iron oxides and phosphorus forms related to calcium (Ano and Agwu, 2005). Inactive phosphorus forms include phosphorus occluded within aluminum and iron oxides and the soluble iron bound phosphorus (Asmare et al., 2015). These phosphorus forms vary in terms of their movement in the soil and the degree of accessibility by plants (Asmare et al., 2015).
Researchers have evaluated phosphorus chemistry under different soil conditions. Soil properties like soil organic carbon (OC), pH of the soil, exchangeable Ca, and aluminum has been shown to influence phosphorus adsorption in acid soil (Onweremadu and Ofoh, 2007). Variation in the concentration of inorganic phosphorus forms has been reported on soils under different management system (Asmare et al., 2015). Tahereh and Hosseinpur (2017) recorded variations in the concentration of non-occluded phosphorus, residual phosphorus and occluded phosphorus fractions within plant root region and the non rhizosphere soil while variations in the phosphorus forms within the root zone of perennial crops have also been documented (Chime et al., 2016; Hagen-Thorn et al., 2004).
Inorganic phosphorus forms are the forms of phosphorus that have been precipitated or fixed in un-dissolvable compounds with aluminum and iron. The precipitation of P by Fe or Al results to Fe–P and Al–P, respectively, as stated in the works of Ohaeri and Eshett (2011). When P is fixed in the interior crystals of Fe and Al, it results to Occluded–P while inorganic phosphorus forms that are loosely–bound are referred to as saloid–bound P. These inorganic P forms are unavailable to plants and constitute the major component of the total phosphorus in the soil (Agbenin, 2003). Soil pH and parent material affects the concentration of inorganic phosphorus forms in the soil (Ohaeri and Eshett, 2011). Understanding the concentrations of these inorganic forms of P will help in adopting those agronomic practices that will reduce P fixations and hence increase its available form.
Therefore, information on the concentrations of inorganic phosphorous forms under a given environmental situation and management practices and their interactions with each other is vital for sustainable management of phosphorus in agricultural system. Unfortunately, there is a paucity of information on the inorganic phosphorus forms within the rhizosphere of annual crops grown in acid soil. This work, therefore, evaluated variations in soil properties and inorganic phosphorus forms within the rhizosphere of selected annual crops in southern part of Nigeria (latitude 5° 38¹N and longitude 6° 97¹ E).
Materials and Methods
The experiment was conducted at the FUTO Teaching and Research Farm of Federal University of Technology, Owerri (FUTO) Nigeria (latitude 5° 38¹N and longitude 6° 97¹ E). The area is characterized by annual average precipitation of 1950 mm - 2500 mm and temperature of 27°C - 32°C per year (Okorie, 2015; Obi and Salako, 1995). Soils in the region are Typic Haplustult as reported by FDALR (1985), with coastal plain sand (Benin formation) as the parent material according to Onweremadu et al. (2011). The region has rainforest vegetation; although highly altered by anthropogenic activities (Onwudike et al., 2017).
A farm of two years fallow was mapped out and manually cleared using a local hoe and shovel. Ten soil samples were sampled from an area measuring 221m2 at random with a soil auger within 0–30 cm depth for pre-planting soil physicochemical parameters (Table 1). The soil was texturally loamy sand with high sand fraction (Table 1) which could be due to coastal plain sand (parent material) that formed the soil. The soils had low soil moisture content with low pH and low in potassium, nitrogen, exchangeable bases and moderate in phosphorus concentration according to critical limits for interpreting fertility level of analytical parameters (Esu, 1991).
The location was divided into three plots, each plot measuring 9 m2. Plots were tilled using a spade. 5 t/ha of poultry manure was applied on each bed on April 4, 2016 and left to incubate for 7 days. On April 12, 2016 two seeds of the test crops (bean seed, groundnut and okra seeds) were planted per hole on each plot with one crop type on each plot. The planting distance was 50 cm x 50 cm. Each plot was replicated four times given a total of 12 plots. On April 20, 2016 the crops were thinned to one plant per stand. Weeding was carried out on May, 14 and June 7, 2016. Pests were controlled by hand picking.
Table 1: Soil characteristics of the area.
| Soil property | Values |
| Sand ( g/kg) | 810.2 |
| Silt ( g/kg) | 76.4 |
| Clay ( g/kg) | 113.4 |
| Textural class | Loamy sand |
|
Bulk density (g/cm3) |
1.32 |
| Total porosity (%) | 50.2 |
| Moisture content (g/kg) | 160.3 |
|
pH (H2O) |
4.50 |
| Organic carbon content (g/kg) | 3.18 |
| Total nitrogen concentration(g/kg) | 0.12 |
| Available phosphorus (mg/kg) | 19.9 |
| Exchangeable Calcium (cmol/kg) | 0.08 (16mg/kg) |
| Exchangeable Magnesium (cmol/kg) | 0.01(1.2mg/kg) |
| Exchangeable Potassium (cmol/kg) | 0.01(3.9mg/kg) |
| Exchangeable. Sodium (cmol/kg) | 0.05(11.5mg/kg) |
| Total exchangeable. acidity (TEA) (cmol/kg) | 0.08 |
| Effective cation exchange capacity (ECEC)(cmol/kg) | 0.33 |
| Percentage base saturation (%BS) | 45.45 |
Ranges of exchangeable cations in cmol./kg; Ca: <2 = very low, 2–5= low, 5-10=moderate, 10-20 =high >20 = very high; Mg: <0.3 = very low, 0.3 – 1.0= low, 1.0- 3.0=moderate, 3.0 -8.0 =high >8.0= very high. K: < 0.2 = very low, 0.2 – 0.3= low, 0.3-0.6=moderate, 0.6 -1.2 =high 1.2 – 2.0 = very high. Na <0.1 = very low, 0.1– 0.3= low, 0.3 – 0.7=moderate, 0.7 – 2.0 =high >2.0 = very high (FAO, 2006).
On June 22, 2016 (nine weeks after planting) soil samples from treatment plots were collected by the following methods. First, three plants from each experimental plot were tagged and carefully pulled out. Three soil samples were sampled 2 mm within the root zone (rhizosphere soil). The samples were mixed together to represent the rhizosphere soil. Secondly, three composite samples from the treatment plot, 10 cm away from the roots of the plants (bulk soil), were sampled and mixed together. The samples were carefully bagged, numbered and then prepared for analyses.
The particle size fractions of soils (sand, silt and clay) were determined according to Gee and Or (2002) method. The bulk density of the soil before and after the study was determined with the method of Grossmans and Reinch (2002). Total porosity was obtained by using the formula adopted by Grossmans and Reinch (2002) as:

Where;
dsi = particle density which is taken as 2.65 g/cm3 and BDi = bulk density.
Gravimetric moisture (GM) content of the studied soil was computed using the formula.
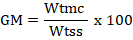
Here, Wtmc = gravimetric moisture in the soil ; Wtss = weight of analyzed soil sample.
pH of the soil sample was measured with soil pH metre according to Hendershot et al. (1993). Chromic acid wet oxidation (CAWO) method of Nelson and Sommers (1982) was used in the determination of soil organic carbon while Bremner and Yeomans (1988) method was adopted to determine soil total nitrogen. Available phosphorus was obtained using Olsen and Sommers (1982) method while EDTA was used to extract Mg and Ca ions and exchangeable K and Na was determined according to Thomas (1982). Exchangeable bases (EB) were determined according to Thomas (1982). In determining exchangeable acidity (EA), Mclean (1982) procedure was adopted while ECEC was computed by summing the total exchangeable bases (TEB) and total exchangeable acidity (TEA).
Percentage base saturation (BS) was generated using the formula:
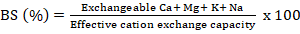
Determination of inorganic phosphorus forms
The following inorganic phosphorus forms were determined: saloid –P, Al – P, Ca – P, occluded – P and Fe – Al – P. Saloid – P was obtained by measuring 10 g of soil sample into 100 ml centrifuge tube and mixed with I N NH4Cl, shaken for 30 minutes and centrifuged at 2000 rpm for 10 minutes. The suspension was decanted into 10 ml plastic container and the P in the solution was read with spectrophotometer at 880nm wavelength (Ohaeri and Eshett, 2011). Al–P was determined using the residues from the saloid P which were put in 60 ml plastic container and 10 ml of NH4F was added, shaken for an hour and centrifuged at 2000 revolution per minute for 10 minutes. The P in the supernatant was determined using spectrophotometer at 880nm wavelength as described by Ohaeri and Eshett (2011). Occluded P was determined using soil residue from Al – P. Here 10 ml of NaCl was mixed with residue from Al – P, centrifuged at 2000 rpm for 5 minutes. The supernatant was later decanted, shaken for 15 minutes after mixing with 10 ml of 0.3N sodium nitrate and the P in the supernatant was read using spectrophotometer at 800 nm wavelength as stated in the works of Ohaeri and Eshett (2011). Fe and Al-P was determined using the residue from occluded P which was washed with 10 ml NaCl and centrifuged at 2000 rpm for 5 minutes. The supernatant was then decanted and added to 10 ml of 0.1 NaOH, centrifuged at 1500 revolution per minute for a period of 15 minutes. The phosphorus form was obtained using spectrophotometer at 850 nm wavelength in the supernatant. Ca–P was determined using soil residue from Fe and Al–P which was mixed with 10 ml NaCl and centrifuged at 2000 rpm for 5 minutes. The supernatant was later separated, centrifuged at 2000 revolution per minute for 10 minutes and the P in the supernatant was read at 880 nm wavelength using a UV/VIS Unicam spectro-colorimete.
Table 2: Soil physical properties in the bulk and rhizosphere region.
| Treatment | Bulk density | Total porosity | Gravimetric moisture |
|
g/cm3 |
% | g/kg | |
| Bean rhizosphere | 1.01 |
61.93a |
124.13a |
| Groundnut rhizosphere | 1.09 |
58.71b |
113.57b |
| Okra rhizosphere | 1.13 |
57.20b |
108.13b |
| Bulk soil | 1.21 |
54.16c |
98.03c |
|
LSD(0.05) |
ns | 2.71 | 9.56 |
Means having same letters are insignificant at 5%, ns: Not significant, SCR: silt clay ratio, TC: textural class.
Results were analyzed statistically with Genstat software. Mean values were separated with least significant difference at the probability of 5%. P forms and soil parameters were correlated to ascertain the relationship among them.
Results and Discussion
Soil physical properties of the study area
The lowest value of soil bulk density was recorded in bean root zone while the highest was in bulk soil. Significant effect (p= 0.05) was observed on soil total porosity and moisture content in the root zones as compared to the bulk soil. These significant effects on these physical properties could be as a result of variations in soil organic matter (Table 3) where higher organic carbon was found at the rhizosphere soil region than in the bulk soil region. Bulk density is a fundamental soil physical parameter which affects water holding capacity, infiltration rate and soil aeration and this has been reported to have negative correlation with soil carbon and vegetative covers (Moraa and Lazarob, 2014; Laiye et al., 2016). Reduction in the value of bulk density increased total porosity and soil gravimetric moisture (Onwudike et al., 2015). Bulk density of bulk soil after the study was lower than that before the study due the vegetative cover of the legumes that prevented the impact of raindrop on soil since this could influence soil compaction (Laiye et al., 2016). Significant effects on total porosity and gravimetric moisture retention in the rhizosphere soil as compared to bulk soil could be attributed to higher organic matter accumulation in the rhizosphere soil since organic matter increased soil aggregation and microbial population. The activities of these soil microbes improve soil porosity and gravimetric moisture retention (Onwudike et al., 2015).
Soil chemical properties of the study location
Significant effects were observed in some chemical properties between the rhizosphere soil and the non rhizosphere soil (Table 3). No significant effect (p= 0.05) was recorded on soil pH between the rhizosphere soils and the bulk soil. There was 51.6% increase in organic carbon content in bean rhizosphere soil than the bulk soil (Table 3). Similarly, soil total nitrogen was increased by 65.1% in bean rhizosphere soil while available phosphorus was increased by 29.5% in groundnut rhizosphere. Higher carbon content in the rhizosphere could contribute to more available phosphorus in the rhizosphere when compared to the value before the study (Table 1). The rhizosphere soils contained higher total exchangeable acidity and exchangeable bases than the bulk soil.
Increase in soil organic carbon in the rhizosphere soils could be responsible for the increase in the exchangeable bases in contrast to the bulk soil since organic matter serves as reservoirs for exchangeable cations in the soil (Onwudike et al., 2015; Collignon et al, 2011). Total exchangeable bases were increased by 5.96% in the groundnut rhizosphere, though not significant in the three experimental plots while ECEC was increased by 16.5% in the groundnut
Table 3: The chemical properties of the soils.
| Treatment | pH | Organic carbon | total nitrogen | available P |
← Exch. → |
TEA | TEB | ECEC | BS | |||||
| Ca | Mg | K | Na | |||||||||||
|
(H2O) |
g/kg | g/kg | mg/kg |
← Cmol/kg → |
% | |||||||||
| Bean rhizosphere | 5.15 |
2.50a |
0.43a |
25.53a |
0.44 |
0.66a |
0.13 |
0.13a |
1.29a |
1.36 |
2.65a |
51.47a |
||
| Groundnut rhizosphere | 5.42 |
2.36a |
0.36a |
36.07b |
0.54 |
0.64a |
0.16 |
0.17b |
1.27a |
1.51 |
2.78a |
54.30a |
||
| Okra rhizosphere | 5.52 |
2.06b |
0.30a |
24.80a |
0.58 |
0.48b |
0.16 |
0.20c |
1.24a |
1.43 |
2.66a |
53.53a |
||
| Bulk soil | 5.64 |
1.21c |
0.15b |
25.40a |
0.42 |
0.71a |
0.15 |
0.14a |
0.90b |
1.42 |
2.32b |
61.43b |
||
|
LSD(0.05) |
ns | 0.19 | 0.11 | 8.79 | ns | 0.12 | ns | 0.02 | 0.23 | ns | 0.25 | 6.23 | ||
Means with the same letters are not significant at 5%, ns: Not significant; Exch.: exchangeable, TEA: total exchangeable acidity, TEB: total exchangeable bases, ECEC: effective cation exchange capacity, BS: base saturation.
rhizosphere. The reason could be due to high organic carbon content within the rhizosphere region of these crops and again, organic matter serves as reservoir of exchangeable bases in the soil (Onwudike et al., 2015), therefore, variations in the organic matter content within the roots of these crop species could be responsible for the significant effects on the total exchangeable bases within the rhizosphere of the studied crops.
Concentration of inorganic phosphorus forms in the studied soils
Results showed that there was 60.1% increase in saloid bound P at the bean rhizosphere, 34.5% increase at the okra rhizosphere and 30.65% increase at the groundnut rhizosphere (Figure 1). Also, there was 37.7% increase of Al–P in the okra rhizosphere soil, 24.8% increase of Al–P in the bean rhizosphere soil while in the groundnut rhizosphere, Al – P was decreased by 38%. Ca–P was increased by 97.8% in the groundnut rhizosphere soil, 96.6% increase in the bean rhizosphere soil and 57% increase in the okra rhizosphere soil when compared to bulk soil.
The concentration of occludded–P and Fe–Al–P are presented in Figure 2. In the groundnut rhizosphere soil, occludded–P was significantly (p = 0.05) increased by 34.1%. There was 25.8% increase of occludded–P in the okra rhizosphere soil and 23.5% increase in the bean rhizosphere soil when compared to bulk soil. Similarly, Fe –Al–P was significantly (p = 0.05) increased by 80.7% at the groundnut rhizosphere, 44.9% increase at the okra rhizosphere and 14% decrease at the bean rhizosphere when compared to bulk soil.
Significant differences in inorganic P forms in the rhizosphere soils could be attributed to differences in soil pH and organic matter concentrations. According to Ohaeri and Eshett, 2011, soil pH and organic matter influence the mineralization of organic P into inorganic constituents.
Phosphorus is a vital plant nutrient whose organic forms constitute 20–80% of total P in the soil. However, these organic forms must be mineralized into inorganic forms before plants could use them. Hence, knowing the distribution of these inorganic forms which are mineralized from organic constituents within the root zone will assist in enhancing soil quality (Ye et al., 2015).
Table 4: Relationship between phosphorus forms and soil l properties.
| Soil Property | (Occluded – P) | (Soloid – P) | (Al – P) | (Ca - P) | (Fe - Al – P) |
| AP | 0.3815 | -0.2382 | -0.6201* | 0.5946* | 0.8151* |
| BD | -0.6623* | -0.0912 | -0.0174 | -0.4165 | -0.4927 |
| BS | -0.2496 | -0.5105* | -0.1051 | -0.4381 | -0.1822 |
| Ca | 0.6117* | -0.1378 | 0.4356 | 0.1023 | 0.3585 |
| Clay | -0.1889 | -0.5253* | -0.1579 | -0.5502* | 0.0978 |
| ECEC | 0.5607* | 0.3535 | -0.0072 | 0.5858* | 0.5381* |
| MC | 0.0432 | 0.5619* | 0.6149* | -0.0726 | -0.4720 |
| Mg | -0.3502 | 0.0972 | -0.4725 | 0.1799 | -0.1026 |
| OC | 0.6703* | 0.6728* | -0.0155 | 0.6697* | 0.3847 |
|
pH (H2O) |
-0.4042 | -0.6086* | -0.1602 | -0.5689* | 0.0354 |
| Sand | 0.4742 | 0.8212** | 0.3909 | 0.2466 | -0.0384 |
| TEA | 0.4110 | 0.4648 | 0.0516 | 0.5364* | 0.3544 |
| TP | 0.6697* | 0.1112 | 0.0297 | 0.4009 | 0.4791 |
*and **: significant at 0.01 and 0.01 probability level respectively; AP : available P; BD: Bulk density; BS: Base saturation; ECEC: effective cation exchange capacity; MC: moisture content; OC organic carbon; TEA: total exchangeable acidity; TP: total porosity.
Relationship between inorganic P and soil physico-chemical properties
Results showed that significant positive and negative interactions existed with soil properties and the studied P forms (Table 4). Positive relationship between P forms and effective cation exchange capacity (ECEC), available phosphorus (AP), organic matter (OM) and moisture content (MC) could be as a result of a decrease in soil acidity, increase in organic matter accumulation and availability of exchangeable bases in the rhizospheric region as compared to bulk soil. Previous research of Grayston et al. (1996) and Jones et al. (2009) have shown that proton extrusion exudates through plant roots which reduce soil pH in the rhizospheric region could also influence soil attributes in the rhizosphere. Similarly, in this acidic nutrient-poor soil, the positive significant relation with organic carbon and percentage base saturation may be as a result the rhizos depositions within the root zone which helps to make nutrient available to plants (Collignon et al., 2011). Significant negative relation between occluded–P and bulk density means that increase in soil compaction reduces the rate of mineralization of organic P to occluded – P. This is because high soil bulk density reduces soil porosity, soil aeration and biodiversity. Similarly, increase in soil organic carbon increases occluded – P and saloid bound– P in the rhizospheric soil.
Conclusions and Recommendations
The study has shown that total porosity, gravimetric moisture content, organic carbon, total nitrogen, available phosphorus, effective cation exchange capacity and base saturation differ significantly between the rhizosphere soil and the bulk soil of bean, groundnut and okra crops. Different concentrations of occluded P, Ca–P, Fe–Al–P and saloid–P were observed in the rhizosphere of the legume crops studied. Higher concentration of Ca–P, Fe –Al - P and occluded–P were recorded in groundnut root zone while higher concentration of saloid–P was found in bean root zone. Therefore farmers are advised to practice mixed cropping that involves groundnut or bean plant with other crops as well as practicing crop rotation so that these P forms will be adequately utilized by plants and this will help in the management of phosphorus in the soil.
Acknowledgements
The authors acknowledged the help of Soil Science laboratory technologists who help to analyze the soil sample as well as the assistance of Farm Manager, FUTO Teaching and Research Farm for allocation of land for this study and providing other logistic.
Novelty Statement
There are variations in the concentration of inorganic phosphorus forms (saloid bound P, occluded – phosphorus, Fe – Al – P and Ca – P) within the root zones of bean, groundnut and okra plants. Soil physicochemical properties varied between the rhizosphere soil and bulk soil.
Author’s Contribution
OSU was the supervisor of the work while ECV was the researcher. OSU designed the work, corrected the manuscript, did statistical analysis and interpreted the results. ECV gathered the research materials, collected soil samples and run laboratory analysis.
References
Agbenin, J.O., 2003. Extractable iron and aluminum effects on phosphate sorption in a savanna alfisol. Soil Sci. Soc. Am. J. 67: 589–595. https://doi.org/10.2136/sssaj2003.0589
Ano, A. and J.A. Agwu. 2005. Effect of animal manures on selected soil chemical properties. Niger. J. Soil Sci., 15: 14–19.
Asmare, M.G., Y. Heluf. Markku and Y. Birru. 2015. Phosphorus status, inorganic phosphorus forms and physicochemical properties of acid soils of Farta District, North –west Highlands of Ethiopia. Appl. Environ. Soil Sci. pp. 11. https://doi.org/10.1155/2015/748390
Bremner, J.M and J.C. Yeomans. 1988. Laboratory techniques for determining of different forms of nitrogen. In: J R. Wilson (ed.). Adv. Nitrogen Cycling Agric. Ecosyst. pp. 339 – 414.
Chime, I.U.D., E.F. Akhabue and I.K. Gideon. 2016. Rhizosphere soil propertiesand growth attributes of four tree species in a four year arboretum at the University of Port Harcourt, Nigeria. Niger. J. Agric. Food Environ., 12(2): 74–80.
Collignon, C., C. Calvaruso and M.P. Turpault. 2011. Temporal dynamics of exchangeable K, Ca and Mg in acidic bulk soil and rhizosphere under Norway spruce (Piceaabies karst) and beech (Fagussulvatica L.) stands. Plant Soil. 349: 355–366. https://doi.org/10.1007/s11104-011-0881-0
Esu, I.E., 1991. Detailed soil survey of NIHORT farm at Bunkure, Kano State, Nigeria. Inst. Agric. Res., Ahmadu Bello Univ. Zaria.
FAO. 2006. Guidelines for soil description. Fourth edition, Food Agric. Organ. U. N. pp. 97.
Federal Department of Agriculture and Land Resources FDALR. 1985. Reconnaissance soil survey of Anambra State of Nig. Soil Reports 1985. Fed. Dept. Agric. Land Res. Lagos- Nigeria.
Gee, G.W. and D. Or. 2002. Particle size analysis. In: Methods of soil analysis. Dan. D.J. and Topps, G.C. (Ed.). Part 4, Physical Methods. Soil Sci. Soc. of America Book Series. No. 5, ASA and SSSA Madison, WI, pp. 225–293.
Grayston, S.J.D., Vaughan and D. Jones. 1996. Rhizosphere carbon flows in trees in comparison with annual plants: The importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol., 5: 29–56. https://doi.org/10.1016/S0929-1393(96)00126-6
Grossmans, R.B and T.G. Reinch. 2002. Bulk density and linear extensibility. In: Methods of soil analysis. Part 4 Physical Methods. Dane, J.H and G.C Topp (eds.). Soil Science Society of Am. Book Series, No 5 ASA and SSA Madison, W.I., pp. 201–228. https://doi.org/10.2136/sssabookser5.4.c9
Hagen-Thorn, A., I. Callesen, K. Armolaitis and B. Nihlgaard. 2004. The impact of six European tree species on the chemistry of mineral top soil in forest plantation on former agricultural land. For. Ecol. Manage., 195: 373–384. https://doi.org/10.1016/j.foreco.2004.02.036
Hendershot, W.H, H. Lalande and M. Duquette. 1993. Soil reaction and exchangeable acidity. In: Carter, M.R (Ed.). Soil sampling and methods of soil analysis. Can. Soc. Soil Sci., Lewis Publ., London. pp. 141-145.
Jones, D.L., C. Nguyen, R.D. Finlay. 2009. Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil, 1: 5–33. https://doi.org/10.1007/s11104-009-9925-0
Khan, I., Z. Shah, W. Ahmad, F. Khan and M. Sharif. 2018. Integrated nutrient and tillage management improve organic matter, micronutrient content and physical properties of alkaline calcareous soil cultivated with wheat. Sarhad J. Agric., 34(1): 144-157. https://doi.org/10.17582/journal.sja/2018/34.1.144.157
Laiye, Q.U, H. Yuanyuan, M.A. Keming, Z. Yuxin and B. Arjen. 2016. Effects of plant cover on properties of rhizosphere and inter-plant soil in a semiarid valley, SW China. Soil Biol. Biochem., 9(4):1–9. https://doi.org/10.1016/j.soilbio.2015.11.004
Mclean, E.D., 1982. Soil pH and lime requirements. In: Page A.L. (Ed). Methods of soil analysis part 2. Chemical and microbiological properties (2nd Ed.). Agronomy series No. SSSA. Maidison, Wis. USA., 199-234.
Moraa, J.I. and R. Lazarob. 2014. Seasonal changes in bulk density under semi-arid patchy vegetation. Geoderma, 235(236): 30 – 36. https://doi.org/10.1016/j.geoderma.2014.06.022
Nelson, D.W. and I.E. Sommers. 1982. Total organic carbon and matter. In: Page, A.L. (ed.). Methods of soil analysis. Part 2 chemical and microbiological properties (2nd ed.). Agron. Ser. No. 9, ASA, SSA, Madison, Wis. USA., 570.
Nottidge, D.O, S.O. Ojeniyi and D.O. Asawalam. 2005. Comparative effect of plant residues and NPK fertilizer on nutrient status and yield of maize in a humid ultisol. Niger. J. Soil Sci. 15: 1–8.
Obi, M.E. and F.K. Salako. 1995. Rainfall parameters influencing erosivity in Southeastern Nigeria. CATENA, 24: 275–328. https://doi.org/10.1016/0341-8162(95)00024-5
Ohaeri J.E. and E.T. Eshett. 2011. Phosphorus forms and distribution in selected soils formed over different parent materials in Abia State of Nigeria. Agro-Sci. J. Trop. Agric., Food, Environ. Ext., 10(3): 28–37. https://doi.org/10.4314/as.v10i3.4
Okorie, F.C., 2015. Analysis of 30 years rainfall variability in Imo State of Southeastern Nigeria. Hydrological sciences and water security: Past, Present and Future (Proc. 11th Kovacs Colloquium, Paris, France, June 2014). IAHS Publ. 366. https://doi.org/10.5194/piahs-366-131-2015
Olsen, S.R. and I.E. Sommers. 1982. Soil available phosphorus. In: Methods of Soil Analysis, Part 2. Agron., Mono. ASA and SSSA Madison, USA.
Onweremadu, E.U. and M.C. Ofoh. 2007. Sorptivity characteristics of soil phosphorus in relation to land utilization types. Nat. Sci., 5: 27–38.
Onweremadu, E.U, E.E. Ihem, S.U. Onwudike, B.N. Ndukwu, C.M. Idigbor and C.C. Asiabaka. 2011. Evaluation of selected physical properties of soils as influenced by legumes and prominol-P fertilization. J. Emerg. Trends Eng. Appl. Sci. (JETEAS), 2(2): 198–202.
Onwudike, S.U, E.E. Ihem, I.F. Irokwe and G. Onwuso. 2015. Variability in the Physico-chemical properties of soils of similar lithology in three land use types in Ahiazu Mbaise, Imo State Nigeria. J. Agric. Crops. 1(3): 38-43.
Onwudike, S.U., L. Agbani, E.E. Ihem and U. Onyegbule. 2017. Influence of land use types on soil properties and micronutrient concentrations on soils of similar lithology in Owerri, Southeastern Nigeria. MAYFEB J. Agric. Sci., 4: 1-9.
Saroa, G.S and R. Lal. 2004. Mulching effects on phosphorus and sulphur concentrations in a Maimian soil in central Ohio, USA. J. Land Degrad. Dev., pp. 351–365. https://doi.org/10.1002/ldr.599
. 2017. Phosphorus availability and some biological properties in the bean (Phaseolus vulgaris) Rhizosphere, Commun. Soil Sci. Plant Anal., 48(5): 501-510. https://doi.org/10.1080/00103624.2016.1253712
Thomas, G.W. 1982. Exchangeable cations. In: Methods of soil analysis. Part 2, ASA and SSSA, Madison, WI. pp. 159 – 166.
Uwah, D.F. and G.A. Iwo. 2011. Effectiveness of organic mulch on the productivity of maize (Zea mays L.) and weed growth. J. Anim. Plant Sci., 21(3): 525-530.
Ye, D.T., T. Li and H. Yu. 2015. Phosphorus accumulation of polygonum hydropiper, soil P fractions and phosphatase activity as affected by swine manure. Appl. Soil Ecol., 86: 10–18. https://doi.org/10.1016/j.apsoil.2014.10.002
To share on other social networks, click on any share button. What are these?








