Effect of Different Dietary Protein Levels on Egg Development and its Response to Inducing Agents during Induced Spawning of Channa marulius
Effect of Different Dietary Protein Levels on Egg Development and its Response to Inducing Agents during Induced Spawning of Channa marulius
Muhammad Hafeez-ur-Rehman1, Farzana Abbas1, Muhammad Ashraf1, Naeem Tariq Narejo2, Khalid Javed Iqbal3, Ghulam Abbas4* and Syedah Andleeb5
1Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Lahore, Pakistan
2Department of Fresh Water Biology and Fisheries, University of Sindh, Jamshoro, Pakistan
3Department of Life Sciences, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
4Center of Excellence in Marine Biology, University of Karachi, Karachi, Pakistan
5Department of Zoology, Govt. Postgraduate Islamia College for Women, Cooper Road, Lahore, Pakistan
ABSTRACT
Brood stock of Channa marulius with an average weight of 948.02±5.72g was randomly stocked in duplicate earthen ponds (90×70×4ft) and fed on 40%, 35% and 30% protein diet @5% of their live body weight. In 40% protein diet treatment, male fish was injected 1st dose with ovaprim + HCG (0.3+0.3ml) and female fish was given 2nd dose after 24 hrs of intervals with ovaprim (0.2ml), while 1st dose ovaprim (0.7ml)+HCG (1.0ml) and 2nd dose ovaprim (0.7ml) was given to the females. The treatment which was given 35% protein diet received 1st HCG+HMG (0.3+0.3ml) and ovaprim (0.2ml) to males and ovaprim (0.5ml)+fresh PG (1.0 ml), 2nd dose ovaprim (0.7ml) and 30% protein containing feed treatment 1st dose with ovaprim+HMG (0.3+0.3ml) and 2ndovaprim (0.2ml) to males and ovaprim (0.3ml) + HMG (1.0ml) with 2nd dose ovaprim (0.7ml kg-1) to females were injected. The highest average fecundity with latency period of 47.70±0.54 h was observed in treatment 3 (40% CP) while treatment 2 (35% CP), in combination of ovaprim + fresh PG showed very short latency period when compared to the ovaprim+HCG injected fish. Treatment 1 and control failed to spawn. Fish fed on 30% protein diet (treatment 1) and the control had the lowest fecundity showing insufficiency of protein required for proper development of the ovary. These studies revealed that 40% protein diet not only improved fish growth and health but also enhanced egg fecundity, gonadal development and spawning.
Article Information
Received 16 December 2015
Revised 02 May 2016
Accepted 02 August 2016
Available online 10 January 2017
Authors’ Contributions
MHR designed the experiment and performed experimental work. FA managed the brooders. GA formulated the feed. MA performed chemical analysis. KJI and SA analyzed the data. MHR wrote the article. NTN helped in preparation of manuscript.
Key words
Dietary protein, Egg development, Inducing agents, Spawning, Channa marulius.
* Corresponding author: [email protected]
0030-9923/2017/0001-0359 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.1.359.365
INTRODUCTION
Channa marulius is an important member of snake headed fishes and is well liked in Pakistan due to high growth potential, peculiar taste and recuperating qualities for sick and feeble both physically and mentally. Though, this fish is carnivorous in nature but comfortably can consume and digest diets composed from different plant by-products with high protein contents. It can be successfully reared in mono as well as in polyculture set up with locally culturable commercial fish varieties.
Nutrition plays a significant role in the spawning performance of fish (Yaakub and Ali, 1992; Faturoti, 2000; Manissery et al., 2001; Muchlisin et al., 2006; Fasakin, 2007; Hafeez-ur-Rehman et al., 2016). Proper feeding of brood stock before the commencement of spawning can guarantee healthy brood stock and proper gonad development which results successful spawning. Several investigations have reported lot of differences in nutritional requirements of brood stock (NRC, 1983; Izquierdo et al., 2001). Best combination of both live and supplementary feed also influences the gonadal development and fecundity in brood fish. In addition, energy contents (Smith et al., 1979; Takeuchi et al., 1981), proper balance of essential fatty acids, amino acids (Santiago et al., 1983; Watanable et al., 1984a, b; Shim et al., 1989; Santiago and Reyes, 1993) and protein to carbohydrate ratio (Cerda et al., 1994) have pivotal role in the cascade of this process. In the maturation stage, some of the non-saturated fatty acids are present in the ovaries which increases the level of protein and dry matter which is evident of their integral role in reproductive processes (Lie et al., 1993). Feeding management of prepared feed like ration size demands independent and careful manipulation (Tyler and Dunn, 1976; Hislop et al., 1978; Moitra et al., 1979; Springate et al., 1985). Therefore, both quality and quantity of feed are equally important for vegetative and reproductive functions of fish life. For efficient induction of ovulation and spawning in murrels, ovaprim, ovatide, HCG and carp pituitary gland have been used as inducing agents (Thakur, 1976; Haniffa et al., 1996, 2000; Marimuthu and Haniffa, 2007). Although, hypophysation is difficult and complicated technique but sometimes gives good results in some of the fish species. One of the main disadvantage is its proper collection and determination of appropriate stage with maximum potency. Other synthetic hormones such as human chorionic gonadotropin (HCG) (Mollah and Tan, 1983; Zairin et al., 1992; Inyang and Hettiarachchi, 1994), luteinizing hormone (Billard et al., 1984; De Leeuw et al., 1985; Fermin, 1992) and Ovaprim (Alok et al., 1993; Francis, 1996; Haniffa et al., 1996; Hafeez-ur-Rehman et al., 2016) have been variably used with very little success.
The purpose of this study was, therefore, to investigate the role of varying protein levels in artificial feed on growth and reproductive performance of Channa marulius, in terms of fish response to various inducing agents (fresh PG, HCG, ovaprim, HMG) when administered independently and/or in various preconceived or on site manipulated ratios.
MaterialS and Methods
Maintenance of brood stock
Thirty brood fishes of C. marulius having an average weight of 948.02±5.72g were randomly stocked in earthen ponds (90×70×4ft). Three different diets (Table I) were prepared independently containing protein levels of 40%, 35% and 30%, hereafter named as treatment 3, 2 and 1, respectively. Six ponds were randomly allotted to each dietary treatment (2 ponds per treatment). Feed ingredients were analyzed for crude protein, crude fiber, crude lipid, nitrogen free extract, ash and moisture contents (AOAC, 2003). Fish were fed @5% of their live body weight twice a day at 08:00 A.M. and 04:00 P.M for 365 days. Water was regularly monitored for accidental changes in pH and dissolved oxygen.
Table I.- Formulation of experimental diets containing different protein concentration*.
| Ingredients |
T1 30% CP |
T2 35% CP |
T3 40% CP |
|||
|
% |
C.P |
% |
C.P |
% |
C.P |
|
| Fish meal |
15 |
7.5 |
15 |
7.5 |
25 |
12.5 |
| Soybean meal |
20 |
8.4 |
20 |
8.4 |
20 |
8.4 |
| Maize gluten |
20 |
12 |
25 |
15 |
30 |
18 |
| Rice polish |
40 |
4.8 |
35 |
4.4 |
20 |
2.4 |
| Molasses |
4 |
- |
4 |
- |
4 |
- |
| Vitamins |
1 |
- |
1 |
- |
1 |
- |
| Total |
100 |
32.7 |
100 |
35.3 |
100 |
41.3 |
*Control has received the natural food with 30% CP as reference diet that are widely used for major carps.
Selection of brood stock
Sexually mature brooders were selected from each pond on the basis of external maturity signs (Haniffa et al., 1996). Mature males had the soft pectoral fins, the lower jaw of the mouth was slightly hard like sand paper and anal genital papilla was round in shape. Unlike carps marulius females had hard pectoral fins, lower jaw was soft, abdomen was swollen, and genital papilla was swollen and slightly oval in shape. Like carps slight pressure the female belly did not ooze out eggs. Maturity stage in both sexes was, however, further confirmed by opening up their belly and taking out gonads and observing them under microscope. Peripheral dislocation of nucleus verified maturity of eggs and readiness of female for hormonal administration. Confirmation of maturation was followed by collection of desired brood stock and their further preparation for hormonal injections.
Artificial propagation
Two years old, C. marulius were randomly divided into three treatments: T1 (30% CP), T2 (35% CP), T3 (40% CP) and control in duplicates. Each treatment had four females and two males (Jhingran and Pullin, 1985). Four females of 1615±86.98g each were stocked in control circular tanks, while females weighing 1750±68.49g, 1817.5±218.84g and 1702.5±68.49g were stocked in T1, T2 and T3 tanks, respectively. In the same tanks two males with an average weight of 1500±35.35g, 1750±49.49g, 1800±113.13g, and 1800±282.84g were paired with females in control, T1, T2 and T3 tanks, respectively. Fishes were anaesthetized and then weighed for dose calculation. Both male and female fishes simultaneously were administered with various hormonal preparations (Table II). The various hormones injected were; carp fresh pituitary (cPG) of Cyprinus carpio, human chorionic gonadotropin (LG Laboratories-HCG-5000-PK-0506) (5000 IU kg), human menopausal gonadotropin (HMG Massone, FSH-75IU, LH 75 IU) and ovaprim (Syndel Laboratories, Vancouver, BC, Canada). All the fish were administered second dose of ovaprim after 24 h (Table II) and released into their respective concrete circular spawning tanks (2m diameter and 2000 L water holding capacity) provided with continuous fresh aerated well water. Breeding behavior of fishes was observed every 6 h in the first half and then every one h or less. Continuously running water maintained dissolved oxygen and temperature at 5-6mg/l and 28-30°C, respectively.
Table II.- Proximate composition of experimental diets.
| Ingredients (%) | Control |
T1 30% CP |
T2 35% CP |
T3 40% CP |
| Crude protein | 30.8 |
30.5 |
35.21 |
40.34 |
|
Crude fibers |
3.15 |
3.20 |
7.35 |
3.89 |
|
Crude lipid |
3.55 |
3.75 |
4.02 |
5.52 |
|
Dry matter |
85.10 |
86.30 |
89.55 |
92.07 |
|
Moisture |
14.40 |
14.10 |
13.70 |
13.20 |
|
Ash |
17.05 |
18.02 |
16.90 |
14.08 |
|
Nitrogen free extract (NFE) |
42.35 |
45.30 |
36.52 |
26.24 |
|
Gross energy (MJg-1) |
14.40 |
15.20 |
17.97 |
19.33 |
Spawning, fecundity and egg fertilization rate
Numbers of eggs were counted gravimetrically (Haniffa and Sridhar, 2002). One gram of egg sample was randomly withdrawn from the bulk and weighed. Sampling and subsequent weighing was repeated thrice. All the weighed egg samples were counted manually and then averaged.
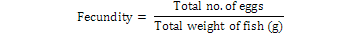
Fertilization of eggs was very much obvious soon after sprinkling but was confirmed after 6-8 h. Egg development was very slow, however it was easily differentiable from unfertilized eggs. Percent fertilization was calculated following (Muir and Robert, 1985):
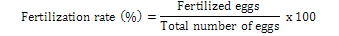
Egg development
During egg development, samples were randomly collected at regular interval of 4 h. The collected eggs were fixed in 4% formalin and examined under a binocular microscope (Nikon Eclipse E400).
Statistical analysis
The data obtained from the induced spawning of C. marulius with administration of different hormone sources was subjected to analyses of variance (ANOVA) to determine if significant difference (P<0.05) occurred among the treatments. The differences among treatment means were differentiated by applying Duncan’s Multiple Range Test (P≤0.05) using SAS-9.2 statistical package.
Results
Crude protein contents of all the diets of this experiment were within the range of 30.5% and 40.34% while crude fibers ranged between 3.15% and 3.89%. Gross energy was highest in the T3 (19.33 MJg-1) and least in control as reference diet (14.40 MJg-1). Table II shows the proximate composition of the experimental diets.
The brooders of C. marulius showed the aggressive behavior 23 h after 2nd dose of hormones. During courtship, the male bent its body close to the female and ejected milt in close proximity to the release of eggs by females with concomitant initiation of fertilization. There was complete harmony and synchronization in breeding activities of both sexes. Water temperature, dissolved oxygen, pH were invariably maintained at 28-30oC, 5.5-6mg/l and 6.7-7.6, respectively with continuous supply of fresh aerated water. Treatment 2 with inclusion of fresh PG though comparatively with low protein diet spawned successfully.
The highest (P<0.05) average fecundity (1427.50±94.67) with latency period of 47.70±0.54 was observed in treatment 3 (40% CP) while treatment 2 (35% C.P) had comparatively low fecundity (1277.50±124.18) with latency period of 49.25±0.12 (Table IV). Channa marulius did not spawn in Treatment 1 and control tank. Fishes in these treatments were dissected, ovaries were removed and eggs were counted
Table III.- Hormone sources and their dosages in both male and female Channa marulius.
| Treatment |
Male body |
Female body |
Male Channa marulius |
|
Female Channa marulius |
||||||
|
Hormone dose (ml kg-1 BW) 1st dose |
Hormone dose (ml kg-1 BW) 2nd dose |
|
Hormone dose (ml. kg-1 BW) 1st dose |
Hormone dose (ml. kg-1 BW) 2nd dose |
|||||||
| Control |
1445.0± 42.03a |
1495.0± 156.02 a |
- |
- |
- |
- |
|
- |
- |
- |
- |
| Treatment - 1 (30% CP) |
1587.5± 175.0 a |
1632.5± 103.61 a |
Ovaprim + HMG |
0.3 +0.3 |
Ovap rim |
0.2 |
|
Ovaprim + HMG |
0.3 +1 |
Ovap rim |
0.7 |
| Treatment -2 (35% CP) |
1702.5± 216.08a |
1613.7± 179.54 a |
HCG + HMG |
0.3 +0.3 |
Ovap rim |
0.2 |
|
Ovaprim +Fresh PG |
0.5 +1 |
Ovap rim |
0.7 |
| Treatment - 3 (40% CP) |
1655.0± 154.1a |
1608.7± 282.15a |
Ovaprim + HCG |
0.3 +0.3 |
Ovap rim |
0.2 |
|
Ovaprim + HCG |
0.7 +1 |
Ovap rim |
0.7 |
Table IV.- Outcomes of induced spawning with administration of different hormone sources.
| Treat ments |
Male body weight (g) |
Female body weight (g) |
Late ncy period (h) |
Spaw ning succ ess |
Fertili zation rate (%) |
Incub ation period (h) |
Fecun dity rate (natural) |
Fecundity (after dissection) |
Ova diam eter (mm) |
| Control |
1445.0± 42.03a |
1495.0± 156.02a |
Nil | Nil | Nil | Nil | Nil |
965.00± 136.06b |
1.47 ±0.03d |
| Treat ment -1 (30% CP) |
1587.5± 175.0a |
1632.5± 103.6a |
Nil | Nil | Nil | Nil | Nil |
1051.88± 95.76a |
1.51 ±0.03c |
| Treat ment -2 (35% CP) |
1702.5± 216.08a |
1613.7± 179.5 a |
49.25± 0.12a |
Comp lete |
49.12± 7.23b |
Nil |
1277.5 ± 124.1b |
Nil |
1.77 ±0.02b |
| Treat ment -3 (40% CP) |
1655.0± 154.1a |
1608.7± 282.1a |
47.7± 0.54b |
Comp lete |
65.25± 5.36 a |
Nil |
1427.5 ± 94.6a |
Nil |
1.8 ±0.03a |
Data figures with different superscript letters are significantly different at P<0.05
after mulching the ovaries and releasing the eggs free. Fecundity was 1051.88±95.76 and 965.00±136.06 in treatment 1 and control, respectively. Though, Treatment 2 (ovaprim in combination with fresh pituitary hormone) showed long latency period but fertilization (%) (49.12±7.23) was low, while Treatment 3 (ovaprim + HCG) showed very short latency period when compared to the pituitary-injected fish (Table IV). Breeding performance of fish was closely observed during whole 48 h of spawning process. Fertilized eggs were yellow in color, spherical, non-adhesive and translucent. Blastodisc divided after 35-60 min into two blastomeres. Segmentation was meroblastic. Second cleavage proceeded after 1-2 h, and then multicellular blastodic was formed.
DISCUSSION
In this study, crude protein contents of different diets used were 30.5%, 35 and 40.34%, crude fiber ranged from 3.15% to 3.89% gross energy was the highest in T3 (19.33 MJg-1) and the lowest in control (14.40 MJg-1). In the present study, Treatment 3 (40% CP and GE 19.33 MJg-1) showed the maximum fecundity and egg fertilization which favorably corroborate with previous studies. These results are also quite in line with Sotolu (2010), Pathmasothy (1985) and Shim et al. (1989) who investigated that increased protein levels from 30% to 40% in the diets increased size and weight of fish, ovary size and hatchability in freshwater fish species. In addition, Cerda et al. (1994) and Muchlisin et al. (2006) reported that rise in dietary protein level in brood stock diets significantly improved weight gain, quantity and quality of eggs and larval viability. In our study, ovaprim+HMG treated females yielded low quality eggs and in treatment 1 and in control even failed to spawn and those spawned did not hatch. Eggs collection after fish dissection were of poor quality. This might be the result of protein malnutrition. Egg clumping and clustering inside the ovary was also common in the females of this treatment.
Fish fed on 30% protein containing diet (treatment 1) and the control had the lowest fecundity showing insufficiency of protein required for proper development of the ovary. Cerda et al. (1994) and Al Hafedh et al. (1999) reported similar findings stressing the importance of quality diet. Although, studies with tilapia and grass carp showed no relationship with dietary protein and egg size (Gunasekara et al.,1997; Khan et al., 2004), but the findings of Manissery et al. (2001) contradicted these studies and showed that dietary protein level may affect quality of common carp eggs.
Mean egg diameter of all fish which ovulated in the four experiments ranged from 1.47±0.03mm to 1.84±0.03 mm. Germinal vesicle broke down and an increase in the size of oocytes due to hydration indicated the changes in the nucleus and cytoplasm during final maturation (Goetz, 1983; Guraya, 1986). The lowest ova diameter (1.47±0.03 mm) was observed in the control tank. The highest ova diameter (1.84±0.03) was observed in ovaprim+HCG injected C.marulius while those injected with ovaprim+fresh PG had comparatively smaller ova diameter (1.77±0.02 mm). The early action of steroidogenesis through hormonal influence might have resulted in increased ova diameter. Due to early steroidogenesis, the batch of oocytes might not have obtained sufficient yolk and hence resulted in reduced diameter of ova.
Latency period of brooders in treatment 3 (40% CP) was 47.70±0.54 h where ovaprim+HCG was injected while it was 49.25±0.12 h in treatment 2 (35% CP) which received ovaprim + fresh pituitary hormones. The egg fertilization rate was 65.25% and 49.125% in former and later treatment. Oladosu et al. (1993) artificially induced H. bidorsalis (mean weight 707.72±4.28.22g) for breeding with carp pituitary produced 33460±20.571 eggs. Contradictory to the previous studies showing low fertilized eggs similar in current studies might have been due to the weight differences of breeders. The latency period reported by many researchers are 23-24 h for C. striata (Haniffa et al. 2000), 6-25 h for C. punctatus (cf. Banerji, 1974), 22-25 h for Heteropneustes fossilis, (Kohli and Goswami, 1987), 14 h (Rao et al., 1989) and 16-20 h (Munshi and Hughes, 1991) for Clarias gariepinus. With regard to pituitary extract Parameswaran and Murugsesan (1976b) reported 28-100% fertilization in C. striatus and Kohli and Goswami (1987) observed 45% fertilization in H. fossilis. Fertilization was high in diet containing 40% CP and injected with ovaprim and HCG when compared with brooders fed diet containing 30% CP. After 59 h of release during hatching fungus severely attacked developing eggs and converted into clusters losing their integrity.
Egg development followed the same trend as has been reported by Marimuthu and Haniffa (2007) and Parameswaran and Murugsesan (1976b). In H. fossilis, the first cleavage was started in about 30 min after fertilization and 16 celled stages in about 70-80 min and morulla stage was observed in about 100 min (Thakur, 1976). In C. punctate, however, on the other hand fertilized eggs attained 16 cell stages after 45 min (Banerji, 1974). During the development of C. marulius eggs, blastodisc divided after 35-60 min into two blastomeres. Segmentation was meroblastic. Second cleavage preceded after 1-2 h, and then multicellular blastodic was formed. These findings are in agreement with the results of Haniffa et al. (2000) and Khan et al. (2004).
CONCLUSIONS
This study revealed that 40% CP containing diet not only improved health of brood stock but also enhanced female fecundity. This level of protein in diet improved growth, fish maturation, gonadal development and spawning of mature fish far better than 35% crude protein containing feed. So it can be concluded that 40% protein is required for proper breeding. Induced spawning on this fish species was a revolutionary success in the history of fisheries and aquaculture in Pakistan. Further studies are, however, required to investigate the possible factors which hampered egg development and invited pathogens which spoiled all the eggs before hatching.
Statement of conflict of interest
Authors have declared no conflict of interest.
references
Al-Hafedh, Y.S., Siddiqui, A.Q. and Saiady, M.Y., 1999. Effects of dietary protein levels on gonad maturation, size and age at first maturity, fecundity and growth of Niletilapia. Aquacult. Int., 7: 319-332. https://doi.org/10.1023/A:1009276911360
AOAC, 2003. Official methods of analysis of the association of official’s analytical chemists, 17th edn. Association of official analytical chemists, Arlington, Virginia.
Alok, D., Krishna, T., Talwar, G.P. and Grag, I.C., 1993. Induced spawning of catfish, Heteropneustes fossilis (Bloch), using D-Lys6 salomon gonadotropin releasing hormone analogue. Aquaculture, 115: 159-167. https://doi.org/10.1016/0044-8486(93)90366-7
Banerji, S.R., 1974. Hypophysation and life history of Channa punctatus (Bloch). J. Inland Fish. Soc. India, 6: 62-73.
Billard, R., Reinaud, P., Hollebecq, M.G. and Breton, B., 1984. Advancement and synchronization of spawning in Salmogairdneri and Sitaltrutta following administration of LHRHa combined or not with pimozide. Aquaculture, 43: 57-66. https://doi.org/10.1016/0044-8486(84)90009-7
Cerda, J., Carrillo, M., Zanuy, S., Ramos, J. and Higuera, M., 1994. Influence of nutritional composition of diets on sea bass (Diacentrarchus labrax L.), reproductive performance and egg and larval quality. Aquaculture, 128: 345-361. https://doi.org/10.1016/0044-8486(94)90322-0
DeLeeuw, R., Goods, H.J.T., Richter, C.J.J. and Edind, E.H., 1985. Pimozide-LHRHa induced breeding in the African catfish, Clarias gariepinus (Burchell). Aquaculture, 44: 299-302.
Faturoti, E.O., 2000. Beneath the ripples and sustainable fish production. Inaugural Lecture Series, University of Ibadan. pp. 54.
Fasakin, E.A., 2007. Fish as food yesterday, today and forever. Inaugural Lecture Series 48, The Federal University of Technology, Akure. pp.52.
Fermin, J.D.T., 1992. Induction of oocyte maturation and ovulation in the freshwater Asian catfish, Clarias macrocephalusby LHRHa and pimozide. J. appl. Ichthyol., 80: 90-98. https://doi.org/10.1111/j.1439-0426.1992.tb00671.x
Francis, T., 1996. Studies on the effect of pituitary hormone and feeds on the reproduction of Heteropneustes fossilis (Bloch). Ph.D. thesis, Tamilnadu Veterinary and Animal Sciences University, Madras, India. pp. 236.
Goetz, F.W., 1983. Hormonal control of oocyte final maturation and ovulation in fishes. In: Fish physiology (eds. W.S. Hoar, D.J. Randall and E.M. Donaldson), Vol. 9, Academic Press, New York. pp. 117-170. https://doi.org/10.1016/s1546-5098(08)60303-9
Gunasekara, R.M., Shim, K.F. and Lam, T.J., 1997. Influence of protein content on the distribution of amino acids in oocytes, serum and muscle of Nile tilapia, Oreochromis niloticus (L.). Aquaculture, 152: 205-221. https://doi.org/10.1016/S0044-8486(96)01526-8
Guraya, S., 1986. Ovum maturation. In: The cell and molecular biology of fish oognesis, monographs in developmental biology (ed. H.W. Saver), Vol. 18, New York, pp. 155-164.
Hafeez-ur-Rehman, M., Ashraf, M., Abbas, F., Iqbal, K.J., Qureshi, I.A. and Andleeb, S., 2016. Effect of different synthetic hormones and/or their analogues on induced spawning in Channa marulius. Pakistan J. Zool., 47: 745-752.
Haniffa, M.A., Merlinrose, T. and Shaik, M.J., 2000. Induced spawning of the striped murrel Channastriatus using pituitary extracts, human chorionic gonadotropin, luteinizing hormone releasing hormone analogue and ovaprim®. Acta Ichthegol. Piscat., 30: 53-60. https://doi.org/10.3750/AIP2000.30.1.04
Haniffa, M.A., Shaik, M.J. and Merlin Rose, T., 1996. Induction of ovulation in Channa striatus (Bloch) by SGnRH. Fishing Chimes, 16: 23-24.
Haniffa, M.A. and Sridhar, S., 2002. Induced spawning of spotted murrel (Channa punctatus) and catfish (Heteropneustes fossilis) using human chorionic hormone and synthetic hormone Ovaprim. Vet. Arch., 72: 51-56.
Hislop, J.R.G., Robb, A.P. and Gauld, J.A., 1978. Observations on effects feeding level on growth and reproduction in haddock, Melanogrammus aeglefinus (L.) in captivity. J. Fish Biol., 13: 85-98. https://doi.org/10.1111/j.1095-8649.1978.tb03416.x
Inyang, N.M. and Hettiarachchi, M., 1994. Efficacy of human chorionic gonadotropin (HCG) and crude extract of fish and frog in oocyte maturation and ovulations in African catfish Clarias gariepinus Burchell. Aquacult. Fish. Manage., 25: 245-258.
Izquierdo, M.S., Fern´Andez-Palacios, H. and Tacon, A.G.J. 2001. Effect of brood stock nutrition on reproductive performance of fish. Aquaculture, 197: 25-42. https://doi.org/10.1016/S0044-8486(01)00581-6
Jhingran, V.G. and Pullin, R.S.V., 1985. A hatchery manual for the common, chinese and indian major carps. ICLARM Stud. Rev. II, 191 p. Asian Development Bank, Manila, Philippines and International Center for Living Aquatic Resources Management, Manila, Philippines.
Khan, M.A., Jafri, A.K. and Chadha, N.K., 2004. Growth, reproductive performance, muscle and egg composition in grass carp, Ctenopharyngodon idella (Valenciennes), fed Hydrilla or formulated diets with varying protein levels. Aquacult. Res., 35: 1277-1285. https://doi.org/10.1111/j.1365-2109.2004.01150.x
Kohli, M.P.S. and Goswami, U.C., 1987. Spawning behaviour of a freshwater air breathing Indian catfish Heteropneustes fossilis (Bloch). Matsya, 12: 180-183.
Lie, O., Mangor-Jenson, A. and Hemre, G.I., 1993. Brood stock nutrition in cod (Gadus morhua) effect of dietary fatty acids. Fiskeridir. Skr., Ser. Ernaer., 6: 11-19.
Manissery, J.K., Krishnamurthy, D., Gangadhara, B. and Nandeesha, M.C., 2001. Effects of varied levels of dietary protein on the breeding performance of common carp Cyprinus carpio. Asian Fish. Sci., 14: 317-322.
Marimuthu, K. and Haniffa, M.A., 2007. Embryonic and larval development of the striped snakehead Channa striatus. Taiwania, 52: 84-92.
Moitra, A., Pandey, A., Ghosh, T.K. and Munshi, J.S.D., 1979. Spawning behaviour, post-embryonic development and culture of Anabas test estudineus (Bloch). Symposium on Inland Aquaculture held at CIFRI, Barrackpoore, West Bengal, Abstract No. 3: 2-3.
Mollah, M.F.A. and Tan, E.S.P., 1983. HCG-induced spawning of the catfish Clarias macrocephalus (Gunter). Aquaculture, 35: 239-247. https://doi.org/10.1016/0044-8486(83)90094-7
Muchlisin, Z.A., Hashim, R. and Chong, A.S.C., 2006. Influence of dietary protein levels on growth and egg quality in brood stock female Bagrid catfish (Mystusnemurus Cuv. and Val.). Aquacult. Res., 37: 416–418. https://doi.org/10.1111/j.1365-2109.2005.01382.x
Muir, F.J. and Robert, J.R., 1985. Recent advances in aquaculture. Croom Helm, London and Sydney; Westview Press, Boulder, Colorado. pp. 282. https://doi.org/10.1007/978-1-4684-8736-7
Munshi, D.J.S. and Hughes, G.M., 1991. Air breathing fishes of India. Oxford and IBH Publishing Co. Pvt. Ltd. New Delhi-110001. pp. 338.
National Research Council (NRC), 1983. Nutrient requirements of fish. National Academy Press, Washington. pp. 114.
Oladosu, G.A., Ayinla, O.A., Adeyemo, A.A., Yakubu, A.F. and Ajani, A.A., 1993. A comparative study of the reproductive capacity of the African catfish species Heterobranchus bidorsalis (Geoffroy), Clarias gariepinus (Burchell) and their hybrid “Heteroclarias”. Afr. Reg. Aquacult. Centre Tech. Paper, 92: 1–5.
Parameshwaran, S. and Murugesan, V.K., 1976b. Observation on the hypophysation of Murrels (Ophiocephalidae). Hydrobiologia, 50: 81-87. https://doi.org/10.1007/BF00016845
Pathmasothy, S., 1985. The effect of three diets with variable protein levels on ovary development and fecundity in Leptobarbus hoevenii. In: Fish nutrition research in Asia, I.D.R.C., Ottawa, pp.107-112.
Rao, G.R.M., Janakiram, K. and Muduli, H.K., 1989. National seminar on forty years of freshwater aquaculture in India. Abstract, 111/3: 7-9.
Santiago, C.B., Camacho, A.S. and Laron, A.M., 1983. Effects of varying dietary crude protein levels on spawning frequency and growth Sarotherodon niloticus breeders. Fish. Res. J. Philipp., 8: 9-18.
Santiago, C.B. and Reyes, O.S., 1993. Effects of dietary lipid source on reproductive performance and tissue lipid levels of Nile tilapia Oreochromis niloticus (Linnaeus) brood stock. J. appl. Ichthyol., 9: 33-40. https://doi.org/10.1111/j.1439-0426.1993.tb00385.x
Shim, K.F., Landesman, L. and Lam, T.J., 1989. Effect of dietary on growth, ovarian development and fecundity in the dwarf gourami Colisa lalia (Hamilton). J. Aquacult. Trop., 4: 111-123.
Smith, C.E., Obsorne, M.D. Piper, R.G., Dwyer, W.P., 1979. Effect of diet composition on performance of rainbow trout brood stock during a three year period. Progr. Fish Cult., 41: 185-188. https://doi.org/10.1577/1548-8659(1979)41[185:EODCOP]2.0.CO;2
Sotolu, A.O., 2010. Effects of varying dietary protein levels on the breeding performance of Clarias gariepinus brood stocks and fry growth rate. Livest. Res. Rural Dev., 22: 67-71.
Springate, J.R.C., Bromage, N.R., Nandeesha, C. and Cumararanatunga, P.R.T., 1985. The effect of different ration on fecundity and egg quality in the rainbow and trout (Salmo gairdeneri). In: Nutrition and feeding in fish (eds. C.B. Cowey, A.M. Mackie and J.A. Bell), Academic Press, London. pp. 371-393.
Takeuchi, T., Watanable, T., Ogino, T., Satio, M., Nishimura, K. and Nose, T., 1981. Effects of low protein, high calorie diets and deletion of trace elements from a fishmeal diet on reproduction of rainbow trout. Bull. Jap. Soc. Fish. Sci., 47: 645-654. https://doi.org/10.2331/suisan.47.645
Thakur, N.K., 1976. On the spawning behavior of Clarias batrachus (Linn). Japan. J. Ichthyol., 23: 178-180.
Tyler, A.V. and Dunn, R.S., 1976. Ration growth and measures of somatic and organ condition in relation to meal frequency in winter flounder, Pseudopleuronectes americanus with hypotheses regarding population homeostasis. J. Fish. Res. Bd. Canada, 33: 63-75. https://doi.org/10.1139/f76-008
Watanable, T., Arakawa, T., Kitajima, C. and Fujita, S., 1984a. Effect of nutritional quality of brood stock diets on reproduction of red sea bream. Bull. Jap. Soc. Fish. Sci., 50: 495-501. https://doi.org/10.2331/suisan.50.495
Watanable, T., Itoh, A., Kitajima, C. and Fujita, S., 1984b. Effect of dietary protein levels on reproduction of red sea bream. Bull. Jap. Soc. Fish. Sci., 50: 1015-1022. https://doi.org/10.2331/suisan.50.1015
Yaakob, W.A.A. and Ali, A.B., 1992. Simple method for backyard production of snakehead (Channastriata Bloch) fry. Naga, 15: 22-23.
Zairin, M., Furukawa, K. and Aida, K., 1992. Induction of ovulation by HCG infection in the tropical walking catfish Clarias batrachus reared under 23-25°C. Nippon Suisan Gakkaishi, 599: 1681-1685. https://doi.org/10.2331/suisan.58.1681
To share on other social networks, click on any share button. What are these?










