Effective Management of Ginger Soft Rot Caused by Penicillium digitatum through Plant Extracts
Research Article
Effective Management of Ginger Soft Rot Caused by Penicillium digitatum through Plant Extracts
Qaiser Shakeel1*, Rabia Tahir Bajwa1, Yasir Iftikhar2, Mustansar Mubeen2, Muhammad Luqman3, Waqas Ashraf1 and Ifrah Rashid4
1Department of Plant Pathology, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Pakistan; 2Department of Plant Pathology, College of Agriculture, University of Sargodha, Pakistan; 3Department of Agriculture Extension, College of Agriculture, University of Sargodha, Pakistan; 4Department of Plant Pathology, Muhammad Nawaz Sharif University of Agriculture, Multan, Pakistan.
Abstract | In-vitro experimentation consisting of crude extracts of different plants and fungicides were performed for determination of antifungal activity against Penicillium digitatum, a plant pathogenic fungi responsible for ginger soft rot. Petri dish were used to conduct bioassay through poisoned food technique with triplicates. The crude extract of all the plants showed a better inhibitory effects on the pathogen’s (P. digitatum) mycelial growth rather than fungicides. Among all the plant extracts, the best result was showed by garlic extract with 87% of mycelial growth inhibition followed by moringa with 82%, the best concentration is 100mg/mL. Besides, Ridomil gold showed the most effective control against ginger soft rot with 84% of mycelial inhibition growth among all the tested fungicides. And found that protective treatment had a best effect than curative treatment. These results revealed that plants are rich source of metabolites with a wide range of antifungal activity against ginger soft rot. Crude extracts of these plants have potential to become effective biopesticides upon purified fractions with no hazardous effects on environment as well as humans.
Received | December 08, 2020; Accepted | March 21, 2021; Published | June 18, 2021
*Correspondence | Qaiser Shakeel, Department of Plant Pathology, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Pakistan; Email: [email protected]
Citation | Shakeel, Q., R.T. Bajwa, Y. Iftikhar, M. Mubeen, M. Luqman, W. Ashraf and I. Rashid. 2021. Effective management of ginger soft rot caused by penicillium digitatum through plant extracts. Sarhad Journal of Agriculture, 37(3): 714-721.
DOI | http:s//dx.doi.org/10.17582/journal.sja/2021/37.3.714.721
Keywords | Ginger soft rot, Penicillium digitatum, Biopesticides, Plant extract, Antifungal activity
Introduction
Ginger (Zingiber officinale Rosc.) is a perennial, herbaceous rhizomic vegetable having great importance in medicine and spice. The export and import value of ginger is globally enhancing every year, whereas soft rot causes significant yield losses, which ranges from 15% to 50% (Indo-Swiss Project Sikkim, 2005; Behera et al., 2020), manage the disease is very important for ginger production.
Penicillium digitatum as the pathogen of ginger soft rot has been found for the first time. Phyto-insecticides gain more popularity in the market due to their multi active services (Isman, 2014) . For instance, commercial plant products including pyrethrum have been known effective against insect pests because they retain the neurotoxicant effects which is responsible for knock down and paralysis resulting in insects mortality (Grdiša and Gršić, 2013). Phyto-pesticides are also responsible for disturbance in significant enzymes production, some of them causes moulting resulting in inhibition of mycelial growth and pathogen development (Ntalli and Menkissoglu-Spiroudi, 2011). Some plant extracts including turmeric and garlic (Curcuma longa and Allium sativum) are found to be effective against red flour beetle (Tribolium castaneum) as they are responsible for causing repellence, poisonousness, mortality and inhibition of offspring emergence (Ali et al., 2014).
Major symptoms of ginger soft rot yellowing of leaves abd stem, stunted growth, brown discoloration of water-conducting tissues, root rottening with black discoloration which ultimately leading to rottening of rhizome (Gollifer and Brown, 1972; Mekbib, 2007; Mohana and Raveesha, 2007; Youssef et al., 2011; Sanzani et al., 2014). The optimum temperature for rapid development of soft rot is 24°C while below 10°C and above 30°C grow slowly (Mekbib, 2007).
Ginger soft rot can be control by synthetic fungicide effectively but it also shows a bad effect on the environment, human as well as other organisms. Plant extract is an alternative non-chemical pesticide due to its non-toxic effect, availability and eco-friendly nature. Plant extract has great antimicrobial and cytotoxic effects on microorganism’s e.g. chemical of mustard allyl isothiocyanate is capable to reduce the growth of P. expansum (Ikeura et al., 2011; Ali et al., 2018).
The main purpose of this work is to manage (P. digitatum) on ginger by using various plant extract and their comparison with fungicidal control.
Materials and Methods
Sample collection and isolation
Ginger with symptoms of fungal infection was collected from the local market and cold storages of Bahawalpur District and brought to the Plant Pathology Laboratory at University College of Agriculture & Environmental Sciences (The Islamia University of Bahawalpur). The samples were washed with tap water, surface sterilized for two minutes with 2% Clorox and then rinsed with sterilize distilled water (SDW) for three times. Slices of infected tissues ranging from three to five millimeter were cut from the margins with sterile scalpel and lodged in petri plates having (15 mL/plate) PDA (potato dextrose agar) medium, then incubated at 28ºC for 5 days under 12 h photoperiod. Hyphal tip method was used for purification of the fungus (Tutte, 1969) and the purified isolates were preserved on PDA slants for further use.
Pathogenicity test
Wound inoculation method was followed to confirm the pathogenicity of isolated fungi in laboratory (Moalemiyan et al., 2007). Healthy gingers were washed with water and surface sterilized by 5% NaoCl for 60 sec. following 3 times rinsing with SDW. Fungus spore suspension was prepared from 7 days old culture by mixing 15 mL SDW in petri plates. Injuries were made on gingers with sterilized needle and inoculated with 105 spores/mL, untreated gingers served as controlled and incubated at 28ºC for 7 days. To confirm the pathogenicity (Koch, 1876), P. digitatum was re-isolated from the infected part and characterized to confirm its pathogenicity.
Preparation of aqueous extracts
In-vitro extract from leaves of four plants viz. Garlic (Allium sativum), Sohanjna (Moringa oleifera), Neem (Azadirachta indica) and Akk (Calotropsis gigantea) were collected from the various parts of Bahawalpur District. Healthy and fresh leaves of plants (50 g) washed thoroughly and crushed by adding 50 mL of SDW in waring grinder for 10 min. The resultant saturate was dribbled through two layers of muslin cloth and then the extract was centrifuged at 4000 rpm for 30 min. The filtrate was strained through filter polycarbonate membrane 0.22 mm, and then ethyl acetate was used twice for the extraction of the filterate and the final crude extract was obtained after drying the filterate in a vacuum (Shakeel et al., 2016). The extract was aseptically conserved at 4ºC for further use (Satish et al., 1999). The prepared extract was termed as (100% concentration) crude extract.
Preparation of plant extract dilutions
For the preparation of dillutions of all the plant extracts, 100 mg of plant extracts was dissolved in 1 mL of SDW and further diluted by serial dilution method to obtain required dilutions i.e. 12.5mg/mL, 25mg/mL, 50mg/mL. The inoculation volume was 0.5 mL/plate containing 20 mL of media while the control treatment remained as it. There were five treatments (including control) and three replications of each treatment.
In-vitro management of P. digitatum using fungicides
Fungicides namely Ridomil gold, Bio magic and Anthem were purchased from the local market to evaluate their efficiency on growth of mycelial diameter of P. digitatum by Poisoned food technique (Grower and Moore, 1962; Falck, 1907). Three different concentration of fungicides viz Ridomil gold (Mancozaib 64% w/w), Bio magic (Bacillus subtilis 10 million CFU/g) and Anthem (Pyroxasulfone 22.61% w/w) were distributed individually in sterilized molten PDA medium. 0.25 mg, 0.5 mg and 1 mg of fungicides were dissolved in SDW to make 250, 500 ppm and 1,000 ppm concentration. Petri dishes were incubated at 28ºC for 5 days. The growth diameter of targetd pathogen was measeured and compared with control to inhibition percentage of fungicides.
Assesment of treatment time effect of various plant extract and fungicides on penecillium digitatum disease index
The examined constituents included 0.5 mL of aqueous solution of garlic and moringa with 200 mg/mL while Ridomil gold and Anthem with 2,000 mg/mL. The treatments were: (i) the control in which ginger was not treated with any treatment. (ii) the protective treatments in which ginger was inoculated individually with plant extract and fungicides 24 h before the inoculation of P. digitatum (iii) the competition treatments in which ginger was inoculated individually with plant extract and fungicides along with P. digitatum at the same time. (iv) the curative treatments in which ginger was inoculated individually with plant extract and fungicides 24 h after the inoculation of P. digitatum (Shakeel et al., 2016). Each treatment has three replications.
Comparison between effect of plant extract and fungicides
The two most effective plant extract viz garlic and moringa and the two most effective fungicides namely Ridomil gold and Anthem were selected and experiment was made for their comparison to select the best one among all. Different concentrations (50, 100 and 200 mg/mL) were made for plant extract while in case of fungicides 500 ppm, 1,000 ppm and 2,000 ppm concentration were made for the experiment. Each treatment has three replications.
Preparation of poisoned food plates
For inhibition of mycelial growth Poisoned Food Technique was used in Potato Dextrose Agar (Groover and Moore, 1962; Shahi et al., 1999). After autoclaving the PDA it was retained at 40°C in a water bath. The prepared extracts and fungicides were individually poured into sterilized molten PDA in different concentrations. The medium was transferred into petri plates of 60×15mm. Mycelial discs of 5 mm were transferred in the center of all petri plates after solidification of the media. The discs were taken from the mature culture plate i.e., one week old culture of the pathogen. There were eight treatments including control and there was three replications of each treatment. Controlled petri dishes remained untreated.
Petri dishes were incubated at 28°C for 7 days. Untreated Dishes were filled with mycelial growth. (Groover and Moore, 1962; Fang, 1998). After incubation, the mycelial growth diameter of P. digitatum was measured in mm (Singh and Tripathi, 1999). Regarding inhibition percentage of pathogenic growth and the antifungal activity of the extracts and fungicides were computed through the given formula:
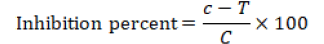
Where; C= Average increase of fungal diameter in control and T= Average increase of fungal diameter in treatment (Mohana and Raveesha, 2007).
Due to the treatment of different fungicides at different concentration the percent inhibition in growth was calculated as follows:
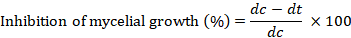
Where; dc= average of fungal mycelial diameter in control, and dt= average of fungal mycelial diameter in treatment (Taskeen-Un-Nisa et al., 2011).
Statistical analysis
Data from in-vitro antifungal assays was exposed to an ANOVA study, followed by a least significance difference (LSD) test. Statistical analyses were performed with SAS/STAT Software for Windows.
Results and Discussion
Pathogenicity test
Symptoms started to appear after 24-36 h. Early symptoms showed slightly discolored spots. After 6-7 days, extensive green sporulation of fungus surrounded by a dense whitish band of mycelial growth was appeared on the surface of ginger. Slide was prepared for the observation of hyphal structure of the fungus under the microscope. On taxonomic and morphological basis the pathogen was identified as P. digitatum that was the causal agent of soft rot.
Effecacy of plant extract regarding inhibition of mycelial growth
The antifungal activity of plant extract at various concentrations was evaluated through food poisoned method on PDA media. The result showed that the most effective mycelial inhibition percentage (87%) on PDA media amended with the garlic extract and moderate activity was observed in extract of Moringa (82%), Akk (41%) was found as low significant for mycelial inhibition and Neem (17%) showed least mycelial inhibition of P. digitatum (Figure 1). The LSD analysis of data revealed that aqueous extract of garlic significantly inhibited the hyphal growth and spore germination rate of the fungus. Inhibition percent of tested pathogen on PDA media showed that garlic was highly effective and Neem showed least effectiveness. The best control was observed at the concentration of 100mg/mL (Figure 2).
Effect of fungicides on the mycelial growth inhibition
The result revealed that highly significant percent inhibition (84%) of mycelial growth of P. digitatum was observed in PDA media amended with the Ridomil gold fungicide and moderate activity was observed in Anthem fungicide (66%). The bio-fungicide Bio magic (30%) was found as least significant for mycelial inhibition of P. digitatum (Figure 3). The LSD analysis of the data revealed that ridomil gold significantly inhibited mycelial growth and spore germination of P. digitatum Percent inhibition of tested fungi on PDA media revealed that ridomil gold was highly effective and Bio magic showed the least effectiveness (Figure 4).
Assesment of treatment time effect of various plant extract and fungicides on Penicillium digitatum disease index
The time for treatment of plant extract and fungicides affected the mycelial growth inhibition of P. digitatum (Figure 5). The maximum inhibition in P. digitatum growth and development was observed in protective treatment. Competition treatment was the second best treatment regarding the reduction of P. digitatum while the curative treatment was the least effective. Among all plant extract and fungicides, garlic extract proved to be best for managing P. digitatum (Figure 5).
Comparison between effect of plant extract and fungicides
In the comparison of plant extract and fungicide, the garlic with the concentration of 200 mg/mL was the highly significant in the reduction of growth of P. digitatum (Figure 6). Moringa with the concentration 200 mg/mL and Ridomil gold fungicide with the concentration of 2,000 ppm was the 2nd best in controlling of P. digitatum. Anthem showed least mycelial inhibition of P. digitatum as compared to others (Figure 6).
Crude extract of plant and fungicides were tested for their efficacy against P. digitatum. The efficiency of tested materials ranged from 5% to 90% in growth inhibition of P. digitatum. The main source of ginger soft rot is very common and undefined in their host range. It is a main issue of broad array of horticultural crops (Chaube and Pundhir, 2005; Onu et al., 2002). There is no single adequate method for the management of P. digitatum is available (Babadoost, 2004). Currently, synthetic fungicides are known to be the most successful technique of disease management. Although, there are lots of concerns related to use of synthetic chemicals which makes them unfit to be considered in long term solution. Major concerns related to the use of synthetic chemicals are environmental pollution, public health problems, poisonous effect on non-targeted individuals, reduction in crop quality and above all resistance development in agricultural pests and disease causing agents (Kagala, 2004; Rai et al., 2006; Rahman et al., 2010). WHO has prohibited several effective pesticides because of their hazardous effect on environment as well as on humans (Barnard et al., 1997). Considering these limitations of synthetic pesticides, we are in a dearly need to find alternatives for managing rhizome soft rot of ginger (Pandey et al., 2010). Most suitable, environmentally safe option instead of synthetic pesticides is use of natural plant products for managing phytopathogenic issues. Effectiveness of plant extract has already been reported in different studies (Sankarasubramanian et al., 2008; Mishra et al., 2009; Yanar et al., 2011; Talibi et al., 2012). Many researchers has reported the antifungal activity of certain plant extracts against various phyto-pathogens (Taskeen-un-Nisa et al., 2011; Amadioha, 2000; Okigbo and Emoghene, 2004; Okigbo and Nmeka, 2005). Assessment of plant extract against other fungi responsible for causing rot i.e. Pythium aphanidermatum etc. has already been published under in-vivo and in-vitro conditions (Sagar et al., 2007; Haouala et al., 2008; Suleiman and Emua, 2009). Current investigation, different plant extract and fungicides with their inhibitory activity on other fungi were used. Present study results cleared revealed the effectiveness of different plant extract against P. digitatum under in-vitro condition. The encouraging mycelial inhibition percentage (around 90% in the present study) indicates that plant under consideration has wide fungicidal activity. For advance study of fractionation, basis could be formed for detecting active fractions. The current study was conducted to assess the different plant extracts to demonstrate the fact that the plants still serve as a reservoir of several pharmaceuticals that could be isolated and used against various plant diseases. It provides an eco-friendly alternative plant disease control method to chemicals.
Conclusions and Recommendations
Crude extract of plants are effective against P. digitatum which has potential to cause economic losses. These plant extracts which were showing effective results have potential to be included in organic farming for development of effective biological fungicides. Garlic extract with 87% effectiveness proved to be lethal for management of disease following moringa extract (82%). The best concentration for their maximum management was 100 mg/mL. Moreover it was proved that prevention is batter than cure as protective treatment was most effective among all treatments. These results revealed that plants are rich source of metabolites with a wide range of fungicidal activity against P. digitatum. The crude extract of these plants has potential to become effective biopesticides upon purified fractions with no hazardous effects on environment as well as humans. Furthermore, exploitations of natural plant extracts, which hinders the pathogenic growth, would be a more accurate and economical method to produce commercial pesticides for the protection of agricultural crops, with a major concern about plant disease management.
Acknowledgements
Author(s) are highly thankful to department of Plant Pathology, University of Agriculture, Faisalabad for their contribution during course of study.
Novelty Statement
This is first time that attempt has been made to address the post-harvest losses of ginger at market level.
Author’s Contribution
Qaiser Shakeel: Designed and conducted ex-periments.
Rabia Tahir Bajwa: Conducted experiments.
Yasir Iftikhar: Supervised experiments.
Mustansar Mubeen: Designed and conducted ex-periments.
Muhammad Luqman: Statistical analysis.
Waqas Ashraf: Part of paper write-up.
Ifrah Rashid: Conducted experiments.
Conflict of interest
The authors have declared no conflict of interest.
References
Ali, S., M.A. Farooqi, A. Sajjad, M.I. Ullah, A.K. Qureshi, B. Siddique, W. Waheed, M. Sarfraz and A. Asghar. 2018. Compatibility of entomopathogenic fungi and botanical extracts against the wheat aphid, Sitobion avenae (Fab.) (Hemiptera: Aphididae). Egypt J. Biol. Pest Cont., 28: 97. https://doi.org/10.1186/s41938-018-0101-9
Ali, S., S.M.H. Muhammad, A. Muneer, H. Faisal, F. Muhammad, H. Dilbar, S. Muhammad and G. Abdul. 2014. Insecticidal activity of turmeric (Curcuma longa) and garlic (Allium sativum) extracts against red flour beetle, Tribolium castaneum: A safe alternative to insecticides in stored commodities. J. Entomol. Zool. Stud., 3: 201-205.
Amadioha, A.C., 2000. Fungitoxic effects of some leaf extracts against Rhizopus oryzae causing tuber rot of potato. Arch. Phytopathol. Pflan. pp. 1-9
Babadoost, M., 2004. Phytophthora blight: A serious threat to cucurbit industries. Available at: http://www.apsnet.org/online/featue/cucurbit/links.asp, https://doi.org/10.1094/APSnetFeature-2004-0404.
Barnard, C., M. Padgitt and N.D. Uri. 1997. Pesticide use and its measurement. Int. Pest Contr., 39: 161-164.
Behera, S., P. Sial, H. Das and K. Pradhan. 2020. Pythium soft rot management in ginger (Zingiber officinale Roscoe). A review. Curr. J. App. Sci. Tech., 39(35): 106-115. https://doi.org/10.9734/cjast/2020/v39i3531061
Chaube, H.S. and V.S. Pundhir. 2005. Crop diseases and their management. Prentice-Hall of India. pp. 702.
Falck, R., 1907. Wachtumgesetze, wachstum Laktorehnund temperature wertder holzersterenden. Myceture, 32: 38-39.
Fang, Z.D., 1998. Research Methodology for Plant Diseases 8–12, third ed. China Agriculture Press, Beijing, China.
Gollifer, D.E. and J.F. Brown. 1972. Virus diseases of Colocasia esculenta in the British Solomon Islands. Plant Dis. Rep., 56: 597-599.
Grdiša, M. and K. Gršić. 2013. Botanical insecticides in plant protection. Agric. Conspect. Sci., 2: 85-93.
Grover, R.K.and J.D. Moore. 1962. Toximetric studies of fungicides against brown rot organisms, sclerotinia-fruticola and S-Laxa. Phytopathol., 52(9): 876.
Haouala, R., S. Hawala, A. ElA-yeb, R. Khanfir and N. Boughanmi. 2008. Aqueous and organic extracts of Trigonella foenum-graecum L. inhibit the mycelia growth of fungi. J. Environ. Sci., 20: 1453-1457. https://doi.org/10.1016/S1001-0742(08)62548-6
Ikeura, H., N. Somsak, F. Kobayashi, S. Kanlayanarat and Y. Hayata. 2011. Application of selected plant extracts to inhibit growth of Penicillium expansum on apple fruits. Plant Pathol. J., 10: 79-84 https://doi.org/10.3923/ppj.2011.79.84
Indo-Swiss Project Sikkim. 2005. Experiences in collaboration ginger pests and diseases, intercooperation India Programme Series 1, Intercooperation Delegation, Hyderabad, India. pp. 57.
Isman, M.B., 2014. Botanical insecticides: A global perspective. Proceedings of the Biopesticides: State of the art and future opportunities. ACS Symp. Ser., 2: 21-30. https://doi.org/10.1021/bk-2014-1172.ch002
Kagale, S., T. Marimuthu, B. Thayumanavan, R. Nandakumar and R. Samiyappan. 2004. Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiol. Mol. Plant P., 65: 91-100. https://doi.org/10.1016/j.pmpp.2004.11.008
Koch, R., 1876. Untersuchungen über Bakterien: V. Die Ätiologie der Milzbrand-Krankheit, begründet auf die Entwicklungsgeschichte des Bacillus anthracis. [Investigations into bacteria: V. The etiology of anthrax, based on the ontogenesis of Bacillus anthracis] (PDF). Cohns Beiträge zur Biologie der Pflanzen (in German). 2 (2): 277–310.
Mekbib, S.B., 2007. Literature review. University of Pretoria etd
Mishra, A.K., A. Mishra, H.K. Kehri, B. Sharma and A.K. Pandey. 2009. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Clin. Microbiol. Antimicrob. 8: 9. https://doi.org/10.1186/1476-0711-8-9
Moalemiyan M., N. Maftoonazad, H.S. Ramaswamy and A.C. Kushalappa. 2007.Effect of pectin-based edible emulsion coating on changes in quality of avocado exposed to Lasiodiplodia theobromae infection, Carbohyd. Polymers, 68:341-349,
Mohana, D.C. and K.A. Raveesha. 2007. Anti-fungal evaluation of some plant extracts against some plant pathogenic field and storage fungi. J. Agric. Techn., 4(1): 119-137.
Ntalli, N.G. and U. Menkissoglu-Spiroudi. 2011. Pesticides of botanical origin: A promising tool in plant protection. Pesticides-formulations, effects, FATE, pp. 1-23.
Okigbo, R.N. and A.O. Emoghene. 2004. Antifungal activity of leaf extracts of some plant species on Mycosphaerella fijiensis Morelet, the causal organism of black sigatoka disease of banana (Musa acuminata) KMITL Sci. J., 4: 20-31.
Okigbo, R.N. and I.A. Nmeka. 2005. Control of yam tuber with leaf extracts of Xylopia aethiopica and Zingiber officinale. Afr. J. Biotechnol., 4(8): 804–807.
Onu, L.I. and G.I. Okafor. 2002. Effect of physical and chemical factor variations on the efficiency of mechanical slicing of Nigerian ginger (Zingiber officinale rose). J. Food Eng., 56: 43-47. https://doi.org/10.1016/S0260-8774(02)00146-2
Pandey, A.K., L.P. Awasthi, J.P. Srivastva and N.K. Sharma. 2010. Management of rhizome rot disease of ginger (Zingiber officinale Rose L.). J. Phytol., 2(9): 18-20.
Rahman, A., A.M. Hossain and S.C. Kang. 2010. Control of phytopathogenic fungi by the essential oil and methanolic extracts of Erigeron ramosus (Walt.) B.S.P. Eur. J. Plant Pathol., 128: 211-219. https://doi.org/10.1007/s10658-010-9645-6
Rai, M. and M. Carpinella. 2006. Naturally occurring bioactive compounds. Elsevier, Amsterdam pp. 502.
Sagar, S.D., S. Kulkarni and Y.R. Hegde. 2007. Management of rhizome rot of ginger by botanicals. Int. J. Plant Sci., 2(2): 155-158.
Sankarasubramanian, H., S. Duraiswamy, R. Ramalingam, E.G. Ebenezar and K. Seetharaman. 2008. Use of plant extracts and biocontrol agents for the management of brown spot disease in rice. Biocontrol., 53: 555–567. https://doi.org/10.1007/s10526-007-9098-9
Sanzani, S.M., L. Schena and A. Ippolito. 2014. Effectiveness of phenolic compounds against citrus green mould. Molecules, 19(8): 12500-12508. https://doi.org/10.3390/molecules190812500
Satish, S., K.A. Raveesha and G.R. Janardhana. 1999. Antibacterial activity of plant extracts on phytopathogenic Xanthomonas campestris pathovars. Lett. App. Microbiol., 28(2): 145-147. https://doi.org/10.1046/j.1365-2672.1999.00479.x
Shahi, S.K., A.C. Shukla, A.K. Bajaj, G. Medgely and A. Dikshit. 1999. Broad spectrum antimycotic drug for the control of fungal infection in human beings. Curr. Sci., pp. 836-839
Shakeel, Q., A. Lyu, J. Zhang, M. Wu, S. Chen, W. Chen and L. Yang. 2016. Optimization of the cultural medium and conditions for production of antifungal substances by Streptomyces platensis 3-10 and evaluation of its efficacy in suppression of clubroot disease (Plasmodiophora brassicae) of oilseed rape. Biol. Cont., 101: 59-68. https://doi.org/10.1016/j.biocontrol.2016.06.007
Singh J. and N.N. Tripathi. 1999. Inhibition of storage fungi of blackgram (Vigna mungo L.) by some essential oils. Flavour Fragr. J., 14:1-4.
Suleiman, M.N. and S.A. Emua. 2009. Efficacy of four plant extracts in the control of root rot disease of cowpea (Vigna unguiculata [L.] Walp). Afri. J. Biotechnol., 8(16): 3806-3808.
Talibi, I., L. Askarne, H. Boubaker, E.H. Boudyach, F. Msanda, B. Saadi and A.A.B. Aoumar. 2012. Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent ofpost harvest citrus sour rot. Crop Prot., 35: 41–46. https://doi.org/10.1016/j.cropro.2011.12.016
Taskeen-Un-Nisa, W.A., M.Y. Bhat, S.A. Pala and R.A. Mir. 2011. In vitro inhibitory effect of fungicides and botanicals on mycelial growth and spore germination of Fusarium oxysporum. J. Biopest., 4(1): 53-56.
Tutte, J., 1969. Plant pathological methods Fungi and bacteria Burgess publishing company, U.S.A. pp. 229.
Yanar, Y., A. Gokce, I. Kadioglu, H. Cam and M. Whalon. 2011. In vitro antifungal evaluation of various plant extracts against early blight disease (Alternaria solani) of potato. Afr. J. Biotechnol., 4(8): 811-815.
Youssef, K., A. Ligorio, S.M. Sanzani, F. Nigro and A. Ippolito. 2011. Investigations of Penicillium spp. population dynamic in citrus packinghouse. J. Plant Pathol. 93(4): 61–62.
To share on other social networks, click on any share button. What are these?







