Efficacy of Nano-Cr Particles Supplemented Sunflower Meal Based Diets on Growth Performance, Digestibility and Hematology of Catla catla Fingerlings
Efficacy of Nano-Cr Particles Supplemented Sunflower Meal Based Diets on Growth Performance, Digestibility and Hematology of Catla catla Fingerlings
Syed Makhdoom Hussain1*, Nisar Ahmad1, Azhar Rasul1, Muhammad Mudassar Shahzad2, Muhammad Latif3, Muhammad Zubair Ul Hassan Arsalan1, Muhammad Umair1 and Hafiza Hina Shafqat1
1Department of Zoology, Government College University, Faisalabad
2Department of Zoology, Division of Science and Technology, University of Education Township, Lahore
3Department of Zoology, University of Education, Faisalabad Campus, Faisalabad
ABSTRACT
Molecules at nano size possesses novel properties regarding absorbance and surface activity, so the aim of research was to estimate the impact of Cr nanoparticles on growth performance, nutrient digestibility and hematological parameters of C. catla fingerlings fed nano- Cr particles supplemented sunflower meal based diets. The experiment was consisted on seven test diets on the basis of supplementation of nano Cr graded levels (0, 0.5, 1, 1.5, 2, 2.5 and 3 mg Kg-1). Chromic oxide was used as an inert marker. Fingerlings were fed at the rate of 5% of their live wet weight. Maximum improvement in the growth performance (weight gain 197%, FCR 1.49, SGR 1.21 and survival 100%), nutrient digestibility (crude protein 71% and gross energy 69%) and hematological parameters (WBCs 7.87 ×103mm-3, RBCs 3.04 ×106mm-3 and Platelets 67) were observed in the fingerlings fed with test diet supplemented with 2 mg kg-1 nano Cr while crude fat digestibility (79%) was found maximum at test diet supplemented with 1.5 mg kg-1 of nano Cr which were significantly different from fish fed with control and other test diets. It was concluded that the supplementation of Nano-Cr particles at the rate of 2 mg kg-1 is the best one to improve growth performance, nutrient digestibility and hematological parameters of C. catla fingerlings fed sunflower meal based diets.
Article Information
Received 03 January 2019
Revised 01 March 2019
Accepted 15 March 2019
Available online 31 July 2019
Authors’ Contribution
SMH planned and supervised the research. NA conducted the research. MAS and NA wrote the manuscript. AR and MAS helped in chemical analysis. ML helped in statistical analysis. MZH conducted feeding trial. MU helped in fish feed formulation. HHS collected fish feces.
Key words
Nano- Cr particles, growth, nutrient digestibility, hematology, C. catla
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.5.1943.1952
* Corresponding author: [email protected]
0030-9923/2019/0005-1943 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
INTRODUCTION
The major goal for fish industry is to develop cost effective fish feed (Baruah et al., 2004). Fishmeal has been used for essential nutrients and growth factors in fish feed (Zhou et al., 2004). Due to high cost, uneven supply and increasing demand of fish meal it has become necessary to search for alternative protein sources (Pham et al., 2008; Lech and Reigh, 2012). In fish culture industry, only feed accounts more than 50% of total cost of fish culture (Essa et al., 2004). Positive effects in terms of fish growth have been reported by using plant meal in diet (Hussain et al., 2011; Shahzad et al., 2018). Plant by-products are being used as alternative protein sources because they are cheaper, environment friendly and easily available as compared to fish meal (Gatlin et al., 2007; Dalsgaard et al., 2009). Sunflower meal contains about 45-48% crude protein (Mushtaq et al., 2006) and is being used in feed formulations as it contains endogenous proteolytic enzymes to digest proteins for fish (Kocher et al., 2000). In Pakistan this crop has lowest cost among plant protein sources (Khan et al., 2006). It is important to formulate nutritionally balanced and highly palatable feed which results in maximum growth of fish (Afzal et al., 2004; Tahir et al., 2008).
Nanotechnology is developing continuously as is being applied with a high potential for the betterment of livestock production and animals in general. In nanotechnology structures under 100nm in size are being used, which is 1000 times narrower than the diameter of a human hair. The nanoparticles increase the bioavailability of nutrients and carry them via the gastrointestinal tract (GIT) into the bloodstream. Nanoparticles also improve the bioavailability of ώ- 3 fatty acids, natural antioxidants and trace minerals (Bunglavan et al., 2014). Nanoparticles may increase the bioavailability of nutrients. Nano form of supplementation increases the surface leading increase in absorption and utilization of minerals
(Vijayakumar and Balakrishnan, 2014). Nanoparticles exhibit novel characteristics, such as great specific surface area, high surface activity, high catalytic efficiency and the character of low toxicity (Pelyhe and Miklós, 2013). Nanomaterials possess greater bioavailability than larger molecules (Albrecht et al., 2006; Wang et al., 2007). In feed industry nano-particles are being used in feed processing and dietary supplementations (Powell et al., 2010).
Nanotechnology tools are being used in aquaculture industry for rapid disease detection, and targeted delivery of nutrients (Ashraf et al., 2011). Supplementation of nanoparticles can improve the absorption of minerals. Chromium (Cr) is an essential trace element for humans and animal’s health. It plays important role in insulin receptor activation. Cr is also involved in the metabolism of biological molecules like carbohydrates, nucleic acids, lipids and proteins whereas its deficiency results in growth retardation (Wang and Xu, 2004). Cr nano increases immunoglobulin contents in blood plasma (Wang et al., 2007). A key factor which affects the digestion and absorption of Cr in intestinal tract is particle size and nature of the polymer (Wang and Xu, 2004).
Catla catla is one of the most important fish with maximum market demand and it contributes along with Labeo rohita and Cirrhinus mrigala about 67% of total freshwater fish production in South India (Krishnaveni et al., 2013). The major objectives of this study were to evaluate the effects of Cr NPs supplementation on growth performance, nutrient digestibility and hematological parameters of C. catla fingerlings.
MATERIALS AND METHODS
C. catla fingerlings were purchased from Government Fish Seed Hatchery Faisalabad. Fingerlings were acclimatized for 15 days in Fish Nutrition Laboratory, GCUF and were fed on the basal diet once in a day
Analysis of feed ingredients and Cr-nano particles
The feed ingredients were analyzed by following standard methods (AOAC, 1995). Cr-nano particles were purchased from market (sigma-Aldrich), to confirm their pure crystalline structure and size, they were analyzed by XRD and TEM (TEM-JEOL2100-20171206), respectively (Ramamurthy et al., 2013; Iqbal et al., 2014).
Formation of pellets
All the feed ingredients were ground until they passed through 0.5mm sized sieve. All ingredients were mixed for 5 min, fish oil was gradually added (Table I). Finally, water was added slowly to make suitable dough and pellets were formulated thereafter with the help of pelleting machine by following Lovell (1989).
Preparation of NP stock solution
Preparation and confirmation of stock solutions of NPs was carried out according to Federici et al. (2007). Stock solution of 100% pure NPs dry powder was made by sonication method (for 6-8 h) and from these stock solutions further dilutions were made to ensure our required levels (0, 0.5, 1, 1.5, 2, 2.5 and 3 mg Kg-1) of Cr NPs.
Addition of NPs to basal diets
The diluted Cr solutions were sonicated further for 15 min just before spraying on basal diets according to Ramsden et al. (2009). One-Kg feed was placed in a commercial food mixer and gradually sprayed with the appropriate dilution. The seven test diets were formulated by mixing graded levels (0, 0.5, 1, 1.5, 2, 2.5 and 3 mg Kg-1) of nano Cr. NPs immediately coated the feed pellets. The feed pellets were allowed to dry then ultimately were stored in air tight containers for further use.
Feeding protocol and sample collection
C. catla fingerlings were fed for 90 days on prescribed diets mentioned above at the rate of 5% of their live wet weight. Three replicates were used for each diet and 15 fingerlings were stocked in each replicate. Their feces were collected by alternative opening and closing of valve-I and valve-II following Hussain et al. (2018).
Chemical analysis of feed and feces
Feed ingredients, experimental diets and samples of feces were homogenized and then subsequently analyzed by standard methods (AOAC, 1995). Protein by micro-Kjeldahl apparatus, crude fat by ether extraction method, crude fiber by loss on ignition and gross energy was determined by oxygen bomb calorimeter (Table II).
Chromic oxide contents in diets and feces were estimated by following Divakaran et al. (2002) using UV-VIS 2001 spectrophotometer at 370 nm absorbance.
Growth study
Growth performance of fingerlings was evaluated by following standard methods as defined by Hussain et al. (2015).

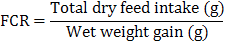
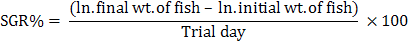
Nutrient digestibility
Apparent nutrient digestibility coefficients (ADC) for experimental diets were calculated by the formula reported in NRC (1993).

Study of hematological parameters
Hematological parameters were evaluated following Peake (1998) and Coyle et al. (2004). Hematocrit was determined using capillary tubes (Brown, 1980). RBCs and WBCs were counted with a haemo-cytometer
(Blaxhall and Daisley, 1973). Hb (Hemoglobin) concentration was determined as described by
(Blaxhall and Daisley, 1973). To calculate mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH) and mean cell volume (MCV) following formulae were used:
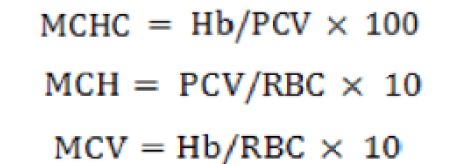
Statistical analysis
One-way analysis of variance was applied to data of haematology, digestibility and growth of fish
(Steel et al., 1996). Tukey’s Honesty Significant Difference Test (p<0.05) was used to compare the differences among various levels (Snedecor and Cochran, 1991). For statistical analysis CoStat Computer Package (Version 6.303, PMB 320, Monterey, CA, 93940 USA) was used.
RESULTS
Results of morphological analysis of the Cr-NPs by using Transmission electron microscope (TEM) are shown in (Fig. 1). TEM confirms the shape and size of Cr NPs, as magnified form depicted in (Fig. 1a) and normal TEM form in Figure (Fig. 1b). In these samples, the scale bar was set to 10 nm in case of magnified TEM image and 100 nm of scale bar in terms of normal form TEM image. TEM image justify the rods form of Cr NPs with about 25-30 nm diameter and about 40-70nm in length almost homogenous structure format. The above results of TEM confirm that Cr NPs used in experimental diets of our study contain size less than 100nm about 25-30 nm. It confirms that they are pure nano particles in their nature.
Table I.- Chemical composition (%) of feed ingredients.
|
Ingredients |
Fish meal |
Rice polish |
Wheat flour |
Sunflower |
|
Dry matter (%) |
91.67 |
94.06 |
92.4 |
93.80 |
|
Crude Protein (%) |
49.03 |
11.87 |
09.73 |
40.81 |
|
Crude Fat (%) |
6.93 |
12.69 |
2.24 |
3.69 |
|
Crude Fiber (%) |
1.23 |
11.91 |
2.73 |
1.94 |
|
Ash(%) |
23.15 |
11.32 |
1.99 |
09.96 |
|
Gross Energy (kcal/g) |
2.49 |
3.41 |
3.06 |
3.64 |
|
Carbohydrates |
19.66 |
52.21 |
82.21 |
43.6 |
The crystal structure and the phase composition of chromium nanoparticles were confirmed by using X-Ray Diffraction (XRD) techniques shown in (Fig. 2). The XRD pattern confirms very clearly that the sample is nano-crystalline in nature as it matches very well with that of the standard chromium powder of chromium nano-particles. Growth parameters such as weight gain (13.47 g), weight gain% (197 %), specific growth rate (1.21) and survival (100%) were observed maximum at 2 mg kg-1 nano Cr as shown in Table III. Second higher value of growth parameters (weight gain 13.08 g, weight gain% 191 % and SGR 1.19) were observed in the fingerlings fed on test diet vI (2.5 mg kg-1 nano Cr). Lowest/best FCR (1.49) was observed in the fingerlings fed at 2 mg kg-1 nano Cr followed by (1.57) the fish fed at 2.5 mg Kg-1 nano Cr. It was observed that these values were statistically different (weight gain 11.49 g, weight gain% 168 %, SGR 1.1 andsurvival 96 %) from the fish fed on control diet. Lowest weight gain (9.64 g), weight gain% (141 %) and SGR 0.98) were noted in the fingerlings that were fed on test diet VII (3 mg kg-1 nano Cr).
Table II.- Ingredients composition (%) oilseed meal based test diets.
|
Ingredients |
Test Diet-I |
Test Diet-II |
Test Diet-III |
Test Diet-IV |
Test Diet-V |
Test Diet-VI |
Test Diet-VII |
|
Nanoparticles (mg kg-1) |
0 |
0.5 |
1 |
1.5 |
2 |
2.5 |
3.0 |
|
Sunflower meal |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
|
Fish meal |
14.5 |
14.5 |
14.5 |
14.5 |
14.5 |
14.5 |
14.5 |
|
Wheat flour* |
13 |
13 |
13 |
13 |
13 |
13 |
13 |
|
Rice polish |
11 |
11 |
11 |
11 |
11 |
11 |
11 |
|
Fish oil |
7.5 |
7.5 |
7.5 |
7.5 |
7.5 |
7.5 |
7.5 |
|
Vitamin premix** |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Minerals premix*** |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Ascorbic acid |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Chromic oxide |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
* Nano-particles will be added on the cost of wheat flour.
** Vitamin premix/kg= Vitamin D3: 3,000,000 IU; Vitamin A, 15,000,000 IU; Vitamin E, 30000 IU; Vitamin B1, 3000 mg,;Vitamin B6, 4000 mg; Vitamin B12, 40mg; Vitamin B2, 7000 mg; Vitamin C, 15,000 mg; Vitamin K3, 8000 mg; Folic acid, 1500 mg; Calcium pantothenate, 12,000mg; Nicotinic acid, 60,000 mg.
*** Mineral premix/kg= Mn(Manganese), 2000 mg; Ca (Calcium), 155 gm; Zn (Zinc), 3000 mg; Cu, (Copper), 600 mg; Co, (Cobalt), 40 mg; I (Iodine), 40 mg; P (Phosphorous), 135 gm; Fe (Iron), 1000 mg; Mg (Magnesium), 55 gm; Se (Selenium), 3 mg; Na (Sodium), 45 gm.
Table III. Growth performance of C. catla fingerlings fed graded levels of Cr-nano supplemented Sunflower meal based diets.
|
Diets |
Cr -nano (mg kg-1) |
IW (g) |
FW (g) |
WG (g) |
WG (%) |
FI (g) |
WG (fish-1 day-1) g |
FCR |
SGR |
Survival % |
|
Test Diet –I (Control diet) |
0 |
6.83 |
18.32 c |
11.49 d |
168.11 d |
0.23 a |
0.13 d |
1.83 b |
1.10 d |
95.56 a |
|
Test Diet –II |
0.50 |
6.83 |
18.91 bc |
12.08 cd |
176.95 cd |
0.23 a |
0.13 cd |
1.72 c |
1.13 cd |
97.78 a |
|
Test Diet –III |
1.00 |
6.82 |
19.28 abc |
12.46 bc |
182.66 bc |
0.23 a |
0.14 bc |
1.64 cd |
1.15 bc |
95.56 a |
|
Test Diet –IV |
1.50 |
6.83 |
19.76 ab |
12.93 ab |
189.22 ab |
0.23 a |
0.14 ab |
1.59 d |
1.18 ab |
97.78 a |
|
Test Diet –V |
2.00 |
6.83 |
20.29 a |
13.47 a |
197.27 a |
0.22 ab |
0.15 a |
1.49 de |
1.21 a |
100.00 a |
|
Test Diet –VI |
2.50 |
6.86 |
19.94 ab |
13.08 ab |
190.72 ab |
0.23 a |
0.15 ab |
1.57 e |
1.19 ab |
100.00 a |
|
Test Diet –VII |
3.00 |
6.85 |
16.49 d |
9.64 e |
140.65 e |
0.21 b |
0.11 e |
1.97 a |
0.98 e |
97.78 a |
|
*PSE |
0.077 |
0.232 |
0.174 |
2.049 |
0.001 |
0.003 |
0.020 |
0.008 |
1.68 |
|
|
P Value |
.999 ns |
.0000 *** |
.000 *** |
.000 *** |
.000*** |
.0031 ** |
.000 *** |
.000 *** |
.0991 ns |
|
Means within columns having different superscripts are significantly different at P< 0.05. Data are means of three replicates. IW, Initial Weight; FW, Final Weight; WG, Weight gain; FI, Feed Intake; SGR, Specific Growth Rate; FCR, Feed Conversion Ratio; *PSE, pooled; SE= √MSE/n (where MSE = mean-squared error).
Table IV. Percentage of nutrients in test diets of C. catla fingerlings fed graded levels of Cr -nano supplemented Sunflower meal based diets.
|
Diets |
Cr -nano (mg kg-1) |
CP (%) in diet |
EE (%) in diet |
GE (kcal g-1) in diet |
|
Test Diet –I(Control diet) |
0 |
31.40 |
6.94 |
3.87 |
|
Test Diet –II |
0.50 |
31.49 |
6.94 |
3.87 |
|
Test Diet –III |
1.00 |
31.39 |
6.92 |
3.86 |
|
Test Diet –IV |
1.50 |
31.35 |
6.94 |
3.87 |
|
Test Diet –V |
2.00 |
31.43 |
6.92 |
3.88 |
|
Test Diet –VI |
2.50 |
31.36 |
6.92 |
3.86 |
|
Test Diet –VII |
3.00 |
31.20 |
6.91 |
3.86 |
|
*PSE |
0.172171 |
0.0290322 |
0.035367 |
|
|
P Value |
.9387 ns |
.9642 ns |
.9999 ns |
|
Means within columns having different superscripts are significantly different at P< 0.05. Data are means of three replicates. CP, Crude protein; EE, ether extract (crude fat), GE, gross energy. *PSE, pooled SE = √MSE/n (where MSE = mean-squared error).
The percentages of nutrients in feed, feces and their digestibility are given in Tables IV, V and VI, respectively. Results showed that all the respective nutrients were statistically similar in all the test diets fed to fingerlings but significantly different in feces when they fed on different concentrations based diets. Lowest nutrients were discharged through feces when fish fed test diet-IV (2 mg Kg-1) whereas maximum nutrients were released in water when they fed with 3 mg Kg-1 nano Cr based diet. The ADC% of nutrients (CP 71% and GE 69%) were observed at their highest in fingerlings fed 2 mg kg-1 of nano- Cr while highest ADC% of crude fat 79% was observed at 1.5 mg kg-1 of nano- Cr, respectively. These values were significantly different from that of control group (CP: 58%, EE: 55% and GE: 55%). On the other hand lowest values (EE: 54%, GE 50% and CP: 56%) were noted at 3 and 0 mg Kg-1, respectively.
Supplementation of Cr-nano improved the hematological indices of C. catla. Results showed that the best values of RBCs (3.04 ×106mm-3), WBCs (7.87×103mm-3), Hb (8.56 g/100ml), MCV (189.60 fl), PCV (25.08), PLT (67.23), MCH (51.31 pg) and MCHC (33.95 %) were observed in fish that fed on Cr-nano based diet supplemented at 2 mg Kg-1 Cr-nano particles (Table VII). The least values of RBCs (1.30 ×106mm-3), WBCs (6.70 ×103mm-3), Hb (6.13 g/100ml), MCV (90.67), PCV (21.54), PLT (53.83), MCH (35.38 pg) and MCHC (25.63 %) were observed in fish fed at 3 mg kg-1 Cr-nano level based diet. While in control group, these values were: RBCs (2.04 ×106mm-3), WBCs (7.05 ×103mm-3), Hb (7.09 g/100ml), MCV (100.06), PCV (23.04), PLT (57.21), MCH (40 pg) and MCHC (28 %). So it is clear that these parameters started to improve from 0.5 to 2 mg Kg-1 Cr-nano level based diets and thereafter started to decrease on higher (2.5 and 3 mg Kg-1 Cr-nano level) supplementation in sunflower meal based diets.
Overall it was observed that growth, digestibility and hematological indices were started to improve from 0.5 up to 2 mg Kg-1 Cr-nano level based diet and started to decrease at further increase of Cr-nano supplementation.
Table V. Percentage of nutrients in feces of C. catla fingerlings fed graded levels of Cr -nano supplemented sunflower meal based diets.
|
Diets |
Cr-nano (mg kg-1) |
CP (%) in feces |
EE (%) in feces |
GE (kcal g-1) in feces |
|
Test Diet –I (Control diet) |
0 |
14.61 ab |
3.42 a |
1.90 b |
|
Test Diet –II |
0.5 |
15.20 a |
2.84 a |
2.01 ab |
|
Test Diet –III |
1 |
13.49 bc |
2.14 a |
1.82 b |
|
Test Diet –IV |
1.5 |
11.72 d |
1.60 a |
1.57 c |
|
Test Diet –V |
2 |
10.11 e |
2.07 a |
1.33 d |
|
Test Diet –VI |
2.5 |
13.08 cd |
2.78 a |
1.81 b |
|
Test Diet –VII |
3 |
15.23 a |
3.54 a |
2.16 a |
|
*PSE |
0.314822 |
0.0515167 |
0.045513 |
|
|
P Value |
0.0000*** |
0.0000*** |
0.0000*** |
|
Means within columns having different superscripts are significantly different at P< 0.05. Data are means of three replicates. *PSE = pooled SE = √MSE/n (where MSE = mean-squared error).
Table VI. Percentage of nutrients digestibility of C. catla fingerlings fed graded levels of Cr -nano supplemented sunflower meal based diets.
|
Diets |
Cr-nano (mg kg-1) |
CP (%) digestibility |
EE (%) digestibility |
GE(%) digestibility |
|
Test Diet–I(Control diet) |
0 |
57.53 e |
55.08 ab |
55.30 e |
|
Test Diet –II |
0.5 |
55.63 f |
62.40 ab |
52.20 f |
|
Test Diet –III |
1 |
60.07 d |
71.27 a |
56.24 d |
|
Test Diet –IV |
1.5 |
66.25 b |
79.13 a |
63.38 b |
|
Test Diet –V |
2 |
71.15 a |
73.17 a |
69.23 a |
|
Test Diet –VI |
2.5 |
62.07 c |
63.42 ab |
57.35 c |
|
Test Diet –VII |
3 |
56.50 f |
54.41 ab |
50.09 g |
|
*PSE |
0.204686 |
0.213874886 |
0.192056 |
|
|
P Value |
.0000 *** |
.0000 *** |
.0000 *** |
|
Means within columns having different superscripts are significantly different at P< 0.05. Data are means of three replicates. *PSE = pooled SE = √MSE/n (where MSE = mean-squared error).
Table VII. Hematological parameters of C. catla fingerlings fed graded levels of Cr -nano supplemented Sunflower meal based diets.
|
Diets |
Cr-nano (mg kg-1) |
RBC (106mm-3) |
WBC (103mm-3) |
PLT |
Hb (g/100ml) |
PCV (%) |
MCHC (%) |
MCH (pg) |
MCV (fl) |
|
Test Diet –I(Control diet) |
0 |
2.04 d |
7.05 cd |
57.21 d |
7.09 b |
23.04 c |
28.16 de |
40.00 d |
100.06 f |
|
Test Diet –II |
0.5 |
2.64 c |
7.27 bc |
61.76 c |
7.82 ab |
23.20 c |
28.55 cde |
36.10 e |
125.25 e |
|
Test Diet –III |
1 |
2.69 bc |
7.63 ab |
64.58 b |
8.03 a |
24.18 ab |
29.35 bcd |
40.06 d |
167.18 d |
|
Test Diet –IV |
1.5 |
2.73 bc |
7.69 ab |
63.90 b |
8.15 a |
24.22 ab |
31.83 ab |
42.82 c |
170.83 c |
|
Test Diet –V |
2 |
3.04 a |
7.87 a |
67.23 a |
8.56 a |
25.08 a |
33.95 a |
51.31 a |
189.60 a |
|
Test Diet –VI |
2.5 |
2.85 ab |
7.74 ab |
63.90 b |
8.32 a |
23.95 bc |
31.27 abc |
45.89 b |
186.84 b |
|
Test Diet –VII |
3 |
1.30 e |
6.70 d |
53.83 e |
6.13 c |
21.54 d |
25.63 e |
35.38 e |
90.67 g |
|
*PSE |
0.040 |
0.105 |
0.1900 |
0.158 |
0.202 |
0.607 |
0.25 |
0.252 |
|
|
P Value |
.0000*** |
.0000 *** |
.0000 *** |
.0000 *** |
.0000 *** |
.0000 *** |
.0000 *** |
.0000 *** |
|
Means within columns having different superscripts are significantly different at P< 0.05. Data are means of three replicates. WBC, White blood cell; RBC, Red Blood Cell; PCV, Packed cell volume; Hb, hemoglobin concentration; PLT, Platelet; MCV, Mean corpuscular volume; MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration. *PSE = pooled SE = √MSE/n (where MSE = mean-squared error).
DISCUSSION
All the growth parameters such as weight gain, weight gain %, FCR, SGR and survival rate were improved by the supplementation of Cr nanoparticles. These findings aresupported by Ashouri et al. (2015). They used Nano-Se at the levels of 0, 0.5, 1 and 2 mg Kg-1 for 8 weeks and found that all levels were significantly higher than that of control one in all parameters except survival which was 100% in all four treatments. They also observed that weight gain (96%) of fish remained highest at 1 mg Kg-1 of nano-Se significantly different from basal diet (60%).
Faiz et al. (2015) also presented similar results with current findings. They found 85% difference in WG of grass carp juvenile when they fed nanoparticles of zinc oxide. They used two levels that of 30mg Kg-1 and 60mg Kg-1 while best results were at the first one; 30mg Kg-1.
Zhou et al. (2009) also found a significant improvement in growth parameters such as weight gain (10.88g), FCR (1.63) and relative gain rate (77.52) in crucian carp fed on nano-Se (0.5mg Kg-1) as compared to control one (WG 7.62g, FCR 1.73 and RGR 52.75%). Srinivasan et al. (2016)
found that all the growth parameters (WG 1.61g, SGR 1.30%, FCR 0.86) and survival rate (90.83%) of giant fresh water prawn were significantly improved when they were fed on iron oxide nanoparticles as compared to that of control diet (WG 0.74g, SGR 0.99%, FCR 1.34 and SR 80.03%). The above said improved results were found at Fe2O3 nano particles supplementation at the rate of 20 mg/Kg while the others levels were 10, 30, 40 and 50 mg/Kg. These differences in results for growth indices may be due to the many factors such as processing methods of feed, types of feed ingredients used, pH of stomach and methods used for feed drying. Bagheri et al. (2015)
found that the use of nano-Se at the level of 0.5 mg Kg-1 caused the best feed conversion ratio (1.67) in chicken while FCR of control group was 1.73. Also, the level of 0.5 mg/kg Nano-Se showed the most average daily weight gain (52g) in comparison to the control diet (45g) at the end of experimental period (p<0.05).
This improvement is due to the special metabolism pathway and deposition mechanism of NPs in carps due to which soluble proteins can interact with nanoparticles to form halo (crona). Nano-protein cronas can interfere with protein folding and can enhance protein cross linking (Zhou et al., 2009; Onuegbu et al., 2018). When concentration of NPs crosses the optimum levels then feed starts to lose palatability which may be the possible reason of decrease of carcass parameters on higher levels of supplementation (Onuegbu et al., 2018). Cr is involved in protein metabolism. It also plays an important role as an integral component of the glucose tolerance factor, which improves the potential the action of insulin and regulates glucose metabolism (Sirriat et al., 2012). On the other hand Ramsden et al. (2009) observed no effect on growth of rainbow trout when exposed to TiO2 nanoparticles. Wang et al. (2015) reported that the growth was decreased with increasing doses of CuSO4 or Cu-NPs. The fish (Epinephelus coioides) were divided in three groups: control, exposed to 20 and 100 g CuL−1 CuSO4 or Cu-NPs for twenty five days. Dobrochna et al. (2018) reported that rainbow trout fingerlings treated by AgNPs and CuNPs groups showed lower growth rate as compared to the control one.
Current research suggests that nano particles are very important for useful and proper nutrient digestibility of C. catla fingerlings. Similarly Kumari et al. (2013) reported that supplementation of nano encapsulated trypsin improved digestibility of L. rohita. They used two levels (0.01% and 0.02%) of nano encapsulated trypsin and found better results on 0.01%. Eguia et al. (2009) also observed a significant increase in digestibility of all nutrients when piglets were treated with 50mg Kg-1 CuNPs supplemented diet as compared to control one. However contrary to our results there was no significant difference of crude protein digestibility of both groups. On the other hand Wang et al. (2015) found that crude protein and crude lipid decreased with an increase in CuSO4 and Cu-NPs dose, ultimately causing decrease in growth performance of fish.
Hematological parameters are very important to study out the proper health of fish because the blood provides information about the fish physiology. In present study we found that all of the important hematological parameters like WBCs, RBCs, Platelets, MCV, PCV, Hb, MCH and MCHC were observed at their highest level in the fish fed with supplementation of 2 mg kg-1 of nano particles. These results are coinciding with the findings of Behera et al. (2014) that nano-Fe improves the hematological indices of L. rohita. Similar findings were reported by Prochorov et al. (2011). Bagheri et al. (2015) reported that Nano-Se supplementation of chicken diets was effective in increasing the hematological parameters of chicken. Khan et al. (2016) reported an improvement in blood indices of fish fed the Se-NP-supplemented diet (0.68 Se-NPs mg Kg-1) compared to the control group (0 Se-NPs mg Kg-1) of fish. Similarly, Khalafalla et al. (2011), Sadeghian et al. (2012) and Le et al. (2013) found that se-nano improved the immunity of fish and RBC count of the fish which is also parallel to our findings. The reason behind the improvement of hematological parameters may be the strong antioxidant property of nano particles which provide stability and integrity of cells inside the animal’s body and protect them from hemolysis, however, at a high levels, it can become toxic and produce harmful effects (Khan et al., 2016). The hematopoietic effects of Cr could be related to the protection of cell membrane and intracellular organelles by the antioxidant effects of NPs and thus increase the life span of RBC and leukocytes (Alimohamady et al., 2013).
CONCLUSION
Our research work provides sufficient evidence that supplementation of the Cr nano particles are helpful for the proper growth performance, nutrients digestibility and improvement of hematological parameters of C. catla fingerlings fed sunflower meal based diets. It was also concluded that 2 mg Kg-1 supplementation levels of Cr-nano particles is the optimum level for the improvement of all above said factors and higher supplementations could not cause further improvement.
REFERENCES
Afzal, K.M., Jafri, A.K. and Chadha, N.K., 2004. Growth and body composition of rohu, Labeo rohita (Hamilton), fed compound diet: winter feeding and rearing to marketable size. J. appl. Ichthyol., 20: 265-270. https://doi.org/10.1111/j.1439-0426.2004.00550.x
Albrecht, M.A., Evans, C.W. and Raston, C.L., 2006. Green chemistry and the health implications of nanoparticles. Green Chem., 8: 417–432. https://doi.org/10.1039/b517131h
Alimohamady, R., Hassan, A.H., Aliasghar B.A. and Dezfoulian, A.H., 2013. Influence of different amounts and sources of selenium supplementation on performance, some blood parameters, and nutrient digestibility in lambs. Biol. Trace Element Res., 154: 45–54. https://doi.org/10.1007/s12011-013-9698-4
Allan, G.L. and Rowland, S.J., 1992. Development of an experimental diet for silver perch (Bidyanus bidyanus). Austasia Aquacul., 3: 39-40.
Ashouria, S., Keyvanshokooh, S., Salatia, A.P., Joharib, S.A. and Zanoosic, H.P., 2015. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture, 446: 25–29. https://doi.org/10.1016/j.aquaculture.2015.04.021
Ashraf, M., Aklakur, M.D., Sharma, R., Ahmad, S. and Khan, M., 2011. Nanotechnology as a novel tool in fisheries and aquaculture development: A Review. Iran. J. Energy Env., 2: 258-261.
AOAC., 1995. Official methods of analysis, 15th Ed. Association of Official Analytical chemists, Washington, D.C. USA., pp. 1094.
Bagheri, M., Golchin-gelehdooni, S., Mohamadi, M. and Tabidian, A., 2015. Comparative effects of nano, mineral and organic selenium on growth performance, immunity responses and total antioxidant activity in broiler chickens. Int. J. Bio., Pharm. Allied Sci., 4: 583-595.
Behera, T., Swain, P., Rangacharulu, P. V. and Samanta, M., 2014. Nano-Fe as feed additive improves the hematological and immunological parameters of fish, Labeo rohita H. Appl. Nanosci., 4: 687–694. https://doi.org/10.1007/s13204-013-0251-8
Baruah, K., Sahu, N.P., Pal, A.K. and Debnath, D., 2004. Dietary phytase: An ideal approach for cost effective and low-polluting aqua-feed. NAGA, World Fish Center Quart., 27: 15-19.
Behera, T., Swain, P., Rangacharulu, P.V. and Samanta, M., 2014. Nano-Fe as feed additive improves the hematological and immunological parameters of fish, Labeo rohita H. Appl. Nanosci., 4: 687–694. https://doi.org/10.1007/s13204-013-0251-8
Blaxhall, P.C. and Daisley, K.W., 1973. Routine haematological methods for use with fish blood. J. Fish Biol., 6: 771-781. https://doi.org/10.1111/j.1095-8649.1973.tb04510.x
Brown, B.A., 1980. Routine hematology procedures. Hematology: Principles and procedures 71112. https://doi.org/10.1016/j.aquaculture.2008.09.007
Bunglavan, S.J., Garg, A.K., Dass, R.S. and Shrivastava, S., 2014. Use of nanoparticles as feed additives to improve digestion and absorption in Livestock. J. Livest. Res. Int., 2: 36-47.
Coyle, S.D., Durborow, R.M. and Tidwell, J.H., 2004. Anesthetics in aquaculture (No. 3900). Publication 3900, Stoneville, Mississippi: Southern Regional Aquaculture Center.
Dalsgaard, J., Ekman, K.S., Pedersen, P.B. and Verlhac, V., 2009. Effect of supplemented fungal phytase on performance and phosphorus availability by phosphorus-depleted juvenile rainbow trout (Oncorhynchus mykiss) and on the magnitude and composition of phosphorus waste output. Aquaculture, 286: 105-112.
Divakaran, S., Leonard, G.O. and Lan, P.F., 2002. Note on the methods for cetermination of chromic oxide in shrimp feeds. J. Agric. Fd. Chem., 50: 464-467. https://doi.org/10.1021/jf011112s
Dobrochna, A., Jerzy, S., Teresa, O., Magda, F., Małgorzata, R., Yuichiro, M. and Kacper, M., 2018. Effect of copper and silver nanoparticles on trunk muscles in rainbow trout (Oncorhynchus mykiss, Walbaum, 1792). Turkish J. fish. aquat. Sci., 18: 781-788. https://doi.org/10.4194/1303-2712-v18_6_04
Eguia, A.G., Fu, C.M., Lu, F.Y. and Lien, T.F., 2009. Effects of nanocopper on copper availability and nutrients digestibility, growth performance and serum traits of piglets. Livest. Sci., 12: 122–129. https://doi.org/10.1016/j.livsci.2009.06.009
Essa, A.M., Mabrouk, A.H. and Zaki, A.M., 2004. Growth performance of grass carp, Ctenopharyngodon idella and hybrid grass carp fingerlings fed on different types of aquatic plants and artificial diet in concrete basins. Egyptian J. aquat. Res., 30: 341-348.
Faiz, H., Zuberi, A., Nazir, S., Rauf, M. and Younas, N., 2015. Zinc oxide, zinc sulphate and zinc oxide nanoparticles as source of dietry zinc: comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int. J. Agric. Biol., 17: 568-574. https://doi.org/10.17957/IJAB/17.3.14.446
Federici, G., Shaw, B.J. and Handy, R.D., 2007. Toxicity of titanium dioxide nanoparticles to rainbow trout, (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol., 84: 415–430. https://doi.org/10.1016/j.aquatox.2007.07.009
Gatlin, D.M., Barrows, F.T., Brown, P., Dabrowski, K., Gaylord, T.G., Hardy, R.W., Herman, E., Hu, G., Krogdahl, Å., Nelson, R., Overturf, K., Rust, M., Sealey, W., Skonberg, D., Souza, E.J., Stone, D., Wilson, R. and Wurte, L.E., 2007. Expanding the utilization of sustainable plant products in aqua-feeds. Aquacul. Res., 38: 551-579. https://doi.org/10.1111/j.1365-2109.2007.01704.x
Hussain, S.M, Ahmad, N., Javid, A., Shahzad, M.M., Hussain, M. and Arsalan, M.Z., 2018. Effects of phytase and citric acid supplemented corn gluten (30%) meal based diets on the mineral digestibility of Cirrhinus mrigala fingerlings. Turkish J. Fish. aquat. Sci., 18: 501-507.
Hussain, S.M., Afzal, M., Javid, A., Hussain, A.I., Ali, Q., Mustafa, I. and Ullah, M.I., 2015. Efficacy of phytase supplementation on growth performance and mineral digestibility of Labeo rohita fingerlings fed on cottonseed meal based diet. Pakistan J. Zool., 3: 699-709.
Hussain, S.M., Afzal, M., Rana, S.A., Javed, A. and Iqbal, M., 2011. Effect of phytase supplementation on growth performance and nutrient digestibility of Labeo rohita fingerlings fed on corn gluten meal-based diets. Int. J. Agric. Biol., 13: 916-922.
Iqbal, M. Z., Fengping, W., Riaz, H., Tahir, I., Israt, A. Yasir, R. M. and Shujjat, A., 2014. Synthesis and characterization of SnO2 nanorods for energy storage applications. Adv. Sci., Engineer. Med., 6: 1–6. https://doi.org/10.1166/asem.2014.1563
Khalafalla, M.M.E., Eweedahl, N.M., Salem, M.F. and Sallam, A.E., 2011. Effects of different levels of selenium supplementation on growth performance, feed utilization, spawning performance and reproduction of the Nile tilapia (Oreochromis niloticus). Egyptian J. aquat. Biol. Fish., 15: 75-91.
Khan, S.H., Sardar, R. and Siddique, B., 2006. Influence of enzymes on performance of broilers fed sunflower-corn based diets. Pakistan Vet. J., 26: 109-114.
Khan, U.K., Zuberi, A., Nazir, S., Fernandes, K.B.J., Jamil, Z. and Sarwa, H., 2016. Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turkish J. Zool., 40: 704-712. https://doi.org/10.3906/zoo-1510-5
Kocher, A., Choct, M., Porter, M.D. and Broz, J., 2000. The effects of enzyme addition to broiler diets containing high concentrations of canola or sunflower meal. Poult. Sci., 79: 1767-1774. https://doi.org/10.1093/ps/79.12.1767
Krishnaveni, K., Palanivelu, k. and Velavans, S., 2013. Spiritualizing effect of probiotic and spirulina on growth and biochemical performance in common carp (Catla catla). Int. J. Res. Zool., 3: 27-31.
Kumari, R., Gupta, S., Singh, A.R., Ferosekhan, S., Kothari, D.C., Pal, A.K. and Jadhao, S.B., 2013. Chitosan nano encapsulated exogenous trypsin biomimics zymogen-like enzyme in fish gastrointestinal tract. PLoS ONE, 8: e74743. https://doi.org/10.1371/journal.pone.0074743
Le, K.T., Fotedar, R. and Partridge, G., 2013. Selenium and vitamin E interaction in the nutrition of yellowtail kingfish (Seriola lalandi): physiological and immune responses. Aquacul. Nutri., 20: 303-313. https://doi.org/10.1111/anu.12079
Lech, P.L. and Reigh, R.C., 2012. Plant products affect growth and digestive efficiency of cultured Florida pompano (Trachinotus carolinus) fed compounded diets. Open Access peer-Reviewed Scient. J., 7: 1-11. https://doi.org/10.1371/journal.pone.0034981
Lovell, R.T., 1989. Fish nutrition and feeding. Van Nostrand-Reinhold Co., New York. https://doi.org/10.1007/978-1-4757-1174-5
Mushtaq, T., Sarwar, M., Ahmad, G., Nisa, M.U. and Jamil, A., 2006. The influence of exogenous multi-enzyme preparation and graded levels of digestible lysine in sunflower meal-based diets on the performance of young broiler chicks two weeks posthatching. Poult. Sci., 85: 2180-2185. https://doi.org/10.1093/ps/85.12.2180
NRC, 1993. Nutrient requirements of fish. Committee on Animal Nutrition, Board on Agriculture, National Research Council, National Academy Press, Washington DC, USA.
Onuegbu, U.C., Aggarwal, A. and Singh, B.N., 2018. ZnO nanoparticles as feed supplementation on growth performance of cultured African catfish fingerlings. J. scient. indust. Res., 77: 213-18.
Peake, S., 1998. Sodium bicarbonate and clove oil as potential anesthetics for nonsalmonid fishes. N. Am. J. Fish. Manage., 4: 919-924. https://doi.org/10.1577/1548-8675(1998)018<0919:SBACOA>2.0.CO;2
Pelyhe, C., and Miklós, M., 2013. Myths and facts about the effects of nano selenium in farm animals – Mini-Review. Eur. Chem. Bull., 2: 1049-1052.
Pham, M.A., Lee, K.J., Dang, T.M., Lim, S.J., Ko, G.Y., EO, J. and Oh, D.H., 2008. Improved apparent digestibility coefficient of protein and phosphorus by supplementation of microbial phytase in diets containing cottonseed and soybean meal for juvenile olive flounder (Paralichthys olivaceus). J. Anim. Sci., 21: 1367-1375. https://doi.org/10.5713/ajas.2008.80053
Powell, J.J., Faria, N., McKey, T.E. and Pele, C.L., 2010. Origin and fate of nanaoparticles and microparticles in the gastrointestinal tract. J. Autoimmun., 34: 226-233. https://doi.org/10.1016/j.jaut.2009.11.006
Prochorov, A.M., Pavlov, G.V., Godwin, A.C. and Okpattah, K.A.V., 2011. The effect of nano-disperse iron on the biological parameters of fish. abstract in 10th Foresight Conf. Mol. Nanotechnol.
Ramamurthy, C.H., Sampath K.S., Arunkumar, P., Kumar M.S., Sujatha, V., Premkumar, K. and Thirunavukkarasu, C., 2013. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioproc. Biosyst. Eng., 36:1131–1139. https://doi.org/10.1007/s00449-012-0867-1
Ramsden, C.S., Smith, T.J., Shaw, B.J. and Handy, R.D., 2009. Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology, 18: 939–951. https://doi.org/10.1007/s10646-009-0357-7
Sadeghian, S., Kojouri, G.A. and Mohebbi, A., 2012. Nanoparticles of selenium as species with stronger physiological effects in sheep in comparison with sodium selenite. Biol. Trace Elem. Res., 146: 302-308. https://doi.org/10.1007/s12011-011-9266-8
Shahzad, M.M., Hussain, S.M., Jabeen, F., Hussain, A.I., Javid, A., Asrar, M. and Arsalan, M.Z.H., 2018. Improvement in mineral digestibility and whole body composition of Catla catla fingerlings fed phytase supplemented MOSM based diet. Pakistan J. Zool., 50: 1909-1920. http://dx.doi.org/10.17582/journal.pjz/2018
Sirirat, N., Lu, J.J., Hung, A.T.Y., Chen, S.Y., and Lien, T.F., 2012. Effects different levels of nanoparticles chromium picolinate supplementation on growth performance, mineral retention, and immune responses in broiler chickens. J. agric. Sci., 4: 48-58. https://doi.org/10.5539/jas.v4n12p48
Snedecor, G.W. and Cochran, W.G., 1991. Statistical methods. 8th Ed. Iowa State University. Press, Americans. USA, pp. 503.
Srinivasan, V., Saravana, B.P., Rajkumar, G., Satgurunathan, T. and Muralisankar, T., 2016. Effects of dietary iron oxide nanoparticles on the growth performance, biochemical constituents and physiological stress responses of the giant freshwater prawn Macrobrachium rosenbergii post-larvae. Int. J. Fish. aquat. Stud., 4: 170-182.
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1996. Principles and procedures of statistics, McGraw Hill International Book Co. Inc., New York. USA 3: 336-352.
Tahir, M.Z.I., Ahmed, I., Mateen, A., Ashraf, M., Naqvi, Z.H. and Ali, H., 2008. Studies on partial replacement of fish meal with oilseeds meal in the diet of major carps. Int. J. Agric. Biol., 10: 455-458.
Vijayakumar, M.P. and Balakrishnan, V.B., 2014. Evaluating the bioavailability of calcium phosphate nanoparticles as mineral supplement in broiler chicken. Indian J. Sci. Tech., 7: 1475–1480. https://doi.org/10.17485/ijst/2015/v8i7/69354
Wang, T., Long, X., Cheng, Y., Liu, Z. and Yan, S., 2015. A comparison effect of copper nanoparticles versus copper sulphate on juvenile Epinephelus coioides: growth parameters, digestive enzymes, body composition, and histology as biomarkers. Int. J. Genom., 783021. https://doi.org/10.1155/2015/783021
Wang, H.L., Wang, M.Q., Xu, Z.R., Zha, L.Y. and Lindemann, M.D., 2007. Effects of chromium nanocomposite supplementation on blood metabolites, endocrine parameters and immune traits in finishing pigs. Anim. Feed Sci. Tech., 139: 69-80. https://doi.org/10.1016/j.anifeedsci.2006.12.004
Wang, M.Q. and Xu, Z.R., 2004. Effect of chromium nanoparticle on growth performance, carcass characteristics, pork quality and tissue chromium in finishing pigs. Asian-Austral. J. Anim. Sci., 17: 1118-1122. https://doi.org/10.5713/ajas.2004.1118
Zhou, X., Wang, Y., Gu, Q. and Li, W., 2009. Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture, 291: 78–81. https://doi.org/10.1016/j.aquaculture.2009.03.007
Zhou, Q.C., Tan, B.P., Mai, K.S. and Liu, Y.J., 2004. Apparent digestibility of selected feed ingredients for juvenile cobia Rachycentron canadum. Aquaculture, 241: 441-451. https://doi.org/10.1016/j.aquaculture.2004.08.044
To share on other social networks, click on any share button. What are these?










