Field Evaluation of Selective Systemic Formulations against Sucking Insect Pest Complex and their Natural Enemies on a Transgenic Bt Cotton
Field Evaluation of Selective Systemic Formulations against Sucking Insect Pest Complex and their Natural Enemies on a Transgenic Bt Cotton
Talha Nazir1,2,*, Muhammad Dildar Gogi2, Muhammad Zeeshan Majeed1, Waheed ul Hassan2, Abdul Hanan2 and Muhammad Jalal Arif2
1Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, PR China
2Department of Agriculture Entomology, University of Agriculture, Faisalabad 38040, Pakistan
ABSTRACT
Sucking insect pest complex, comprising of aphids Aphis gossypii Glover, jassids Amrasca devastans Distant, whiteflies Bemisia tabaci Gennadius, thrips Thrips tabaci Lindeman, red cotton bugs Dysdercus koenigii Walk and dusky cotton bugs Oxycarenus hyalinipennis Costa, has been a challenge on transgenic (Bt) cotton crop all over the world. This study assessed the field efficacy of six commercial insecticides viz.; Blaster 72.5%WP (imidacloprid + acephate), Bugatti 50%SC (imidacloprid + bifenthrin), Confidor 20%SL (imidacloprid), Jozer 202SL (imidacloprid + acetamiprid), Pouch 35%SC (pyriproxyfen + etofenprox) and Senator 41.6%EC (imidacloprid + pyriproxyfen) against sucking insect pest complex and also determined their side-effect on natural enemies of these insect pests such as ants, parasitoid wasps, green lacewings and coccinellid beetles. Experimental design was split-plot randomized complete block with three replications for each treatment. Transgenic (Bt) cotton variety (FH-118) was sown. Insecticide formulations were applied according to their field recommended dose rates upon attainment of ETLs of most of the sucking insect pests. Data regarding insect populations were recorded 3 and 7 day post treatment. Results revealed that Confidor proved to be most effective systemic insecticide against all insect pests, followed by Pouch, Senator, Blaster, Bugatti and Jozer. However, Confidor, Jozer, Bugatti, Blaster and Senator showed a top-down effect on beneficial insects causing 50–90% reduction in their populations, while Pouch showed minimum effect (< 50% reduction) on the population of beneficial insects and proved to be relatively safe. All insecticide formulations tested exhibited ≥ 50% reduction in the population of green lacewings. Similarly, whitefly parasitism recorded in the Confidor and Jozer plots was very close to that of control plots for both post-treatment observations. Based on these findings, Confidor and Jozer are recommended to be considered for their integration in sucking pests’ management strategies on transgenic crops such as Bt cotton.
Article Information
Received 05 April 2017
Revised 16 My 2017
Accepted 29 May 2017
Available online 11 September 2017
Authors’ Contribution
TZ and MDG conceived and designed the experiments. TZ, WH and AH conducted the experiments. TZ and MZM did statistical analysis. TZ and MZM prepared manuscript. MDG and MJA provided technical assistance for the study.
Key words
Transgenic cotton, Sucking insect pests, Systemic insecticides, Beneficial insects, In-situ efficacy.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.5.1789.1796
* Corresponding author: [email protected]
0030-9923/2017/0005-1789 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Pakistan is an agricultural country and cotton crop (Gossypium hirsutum L.) ranks at top in the agricultural GDP and economy of this country. The average yield of cotton seed production of Pakistan is nearly 520 kg ha-1 which is considered better than that of the world average cotton seed production. However it is far behind if this quantity and quality of cotton yield is compared with those of other cotton producing countries such as Australia, USA, India and Turkey. This low quantity and quality of cotton fiber and seed is due to many factors (Khan, 2010). These factors include environmental and agronomic factors, incidence of various diseases and insect pest species (Aslam et al., 2004; Ali and Aheer, 2007).
Approximately more than 200 species of insect pests attack on cotton crop at its various growth stages (Kannan et al., 2004; Ali and Aheer, 2007; Luttrell et al., 2015; Sarwar and Sattar, 2016). However, eighteen species of insects are considered as the most destructive pests of cotton under agro-climatic conditions of Pakistan (Abbas, 2001; Asi et al., 2008). Among cotton insect pests, sucking insect pest complex comprising of aphid Aphis gossypii Glover, jassid Amrasca devastans Distant, whitefly Bemisia tabaci Gennadius, thrips Thrips tabaci Lindeman, red cotton bug Dysdercus koenigii Walk and dusky cotton bug Oxycarenus hyalinipennis Costa has been a major challenge to cotton crop production in the Indo-Pak region (Ahmad, 1999; Majeed et al., 2016). These insect pests attack cotton crop at its different growth stages from seedling to reproductive or harvesting stage. They damage plants directly by extensive desaping causing stunted plant growth and reduced fruiting bodies. Indirectly, these insect pests harm cotton plants by facilitating the development of sooty mold growth on plant leaves and twigs due to their honeydew secretions. There could be high defoliation and premature fruit and flower dropping in case of heavy infestation (Aslam et al., 2001; Abro et al., 2004; Asi et al., 2008). About 35–50% yield loss is caused by sucking insect pests in cotton crop every year (Bo, 1992; Tayyib et al., 2005; Khan, 2010; Majeed et al., 2016; Shah et al. 2017).
A wide range of systemic pesticides are being employed by cotton growers against sucking insect pest infestation without much success, most probably due to the field evolved resistance in these insect pests against most widely used synthetic conventional insecticides (Ahmad, 1999; Luttrell et al., 2015). Moreover, environmental contamination and non-target effects on beneficial fauna, including insect predators (ants, coccinellid beetles and green lacewings) and parasitoids (wasps), are other contemporary issues of these irrationally used broad spectrum insecticides (Simon-Delso et al., 2015).
Therefore, there is a need to evaluate and screen out more target-specific pesticide formulations against sucking insect pests which would be relatively safer for insect natural enemies. The present study has been conducted to evaluate the efficacy of different insecticide formulations viz.; Blaster, Bugatti, Confidor, Jozer, Pouch and Senator against the above mentioned sucking insect pest complex and their natural enemies under field conditions.
Materials and methods
The experiment was conducted in Youngwala Entomological Research Station (31°44’N and 73°06’E) of the University of Agriculture, Faisalabad (Punjab, Pakistan) during 2015 kharif season from May to September. Study objective was to evaluate the top-down effect of some new insecticide formulations on sucking insect pest complex and their natural enemies under field conditions. Certified seeds of transgenic (Bt) cotton variety ‘FH-118’ was acquired from the Cotton Research Institute of Ayyub Agriculture Research Institute (AARI), Faisalabad, and was sown in 1st week of June 2015 by line-sowing method with row-to-row and plant-to-plant distance of 90 and 40 cm, respectively.
The experiment was laid out in RCBD under split block design having Bt-cotton variety (FH-118) in main plots and the application of recommended doses of six insecticides Blaster 72.5%WP (imidacloprid + acephate), Bugatti 50%SC (imidacloprid + bifenthrin), Confidor 20%SL (imidacloprid), Jozer 202SL (imidacloprid + acetamiprid), Pouch 35%SC (pyriproxyfen + etofenprox) and Senator 41.6%EC (imidacloprid + pyriproxyfen) in sub-plots. Other agronomic practices such as weeding, irrigation and fertilization were applied as per routine recommendations for this cotton variety. Each subplot was divided into three blocks representing three replications of each treatment. The recommended dose rates of six insecticides, as detailed in Table I, were applied three times with an interval of at least 10 days upon the attainment of economic threshold level (ETL) of most of the sucking insect pests. A control treatment receiving spray of water, the same as used for pesticide mixtures, was maintained in each subplot.
Table I.- List of insecticide formulations evaluated in the study.
| Insecticidal formulation |
Dose (ml or g/acre) |
| Confidor 20%SL (imidacloprid) |
250 |
| Pouch 35%SC (pyriproxyfen + etofenprox) |
125 |
| Senator 41.6%EC (imidacloprid + pyriproxyfen) |
125 |
| Blaster 72.5%WP (imidacloprid + acephate) |
250 |
| Bugatti 50%SC (imidacloprid + bifenthrin) |
200 |
| Jozer 202SL (imidacloprid + acetamiprid) |
110 |
Data regarding population of insects (both pests and beneficials) were collected by manual pest scouting on per plant basis from five randomly selected plants according to Mario’s method and by net sweeping on per sweep basis. The specimens collected by net sweepings were identified as pest or beneficial ones. The data of population dynamics of insects was collected 1 day before, and 3 and 7 days post-treatment applications. The collected data was transformed into percent population reduction or increase by the following formula:
% Population reduction =
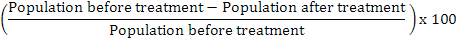
Data was analyzed by statistical software (Statistix 8.1v, Tallahassee, FL) using two-way factorial analysis of variance for finding out the effect of treatments (insecticides), different time intervals and replications on population level of insect pests and their natural enemies. Before ANOVA, data normality was checked by Shapiro-Wilk test and data were log-transformed (log (value+1)) in case of not a normal distribution of residuals. Comparisons of means were performed using Fischer’s least significant difference (LSD) test at P = 0.05.
Results and discussion
In-situ screening of pesticidal formulations against particular insect pests has been a vital and inevitable part of integrated pest management programs all over the world. This study determined the field efficacy of some novel binary formulations of different insecticidal compounds mostly recommended against sucking insect pests. All formulations tested in this study caused significant reduction in the population of aphids, jassids, thrips, whiteflies and bugs after 3 and 7 days of pesticides application (Tables II, III, IV, V, VI, VII) but, at the same time, suppressed insect predators and parasitism as well (Tables VIII, IX, X, XI, XII).
Among insecticides, Confidor 20%SL (imidacloprid) was the most effective and caused significant reduction in the population of aphids (56 and 88%), jassids (45 and 78%), whiteflies (39 and 67%), thrips (50 and 76%), red cotton bugs (47–63%) and dusky cotton bugs (62 and 70%) respectively at 3 DPT and 7 DPT (Tables II, III, IV, V, VI, VII). These findings are in accordance with the results of many previous studies (Shivanna et al., 2011; El-Naggar and Zidan, 2013; Hossain et al., 2013; Afzal et al., 2014; Asif et al., 2016). All these studies did a comparative assessment of different insecticides with various modes of actions and found considerable and significant reduction of different sucking insect pests of cotton by imidacloprid formulations. Moreover, imidacloprid (Confidor) gave maximum reduction of aphids as compared to other sucking insect pests. These results are in agreement with those of Shivanna et al. (2011) and Afzal et al. (2014) who also reported the maximum efficiency of imidacloprid against aphid populations.
Table II.- Means for population reduction percentage of aphid Aphis gossypiiat 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (Mean ± SE) |
|||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
|||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
|
| Blaster |
55.69C ± 0.48 |
75.05D ± 0.32 |
26.79B ± 0.59 |
37.47D ± 0.47 |
56.89C ± 0.27 |
75.74D ± 0.35 |
46.46 |
62.75 |
| Bugatti |
59.44B ± 0.53 |
89.14C ± 0.27 |
29.76A ± 0.65 |
68.01C ± 0.53 |
61.39B ± 0.41 |
85.10C ± 0.41 |
50.20 |
79.75 |
| Confidor |
67.13A ± 0.64 |
93.72A ± 0.17 |
32.42A ± 0.77 |
77.33A ± 0.61 |
69.01A ± 0.35 |
93.12A ± 0.53 |
56.19 |
88.10 |
| Jozer |
60.52B ± 0.59 |
91.58B ± 0.21 |
31.00A ± 0.71 |
74.61B ± 0.58 |
61.97B ± 0.30 |
90.66B ± 0.48 |
51.16 |
85.62 |
| Pouch |
47.21D ± 0.39 |
58.57F ± 0.13 |
20.17C ± 0.47 |
21.37F ± 0.40 |
49.01D ± 0.36 |
54.33F ± 0.25 |
38.80 |
44.76 |
| Senator |
48.61D ± 0.44 |
67.50E ± 0.19 |
24.75B ± 0.55 |
26.33E ± 0.43 |
49.78D ± 0.21 |
58.33E ± 0.29 |
41.05 |
50.72 |
| Control |
08.33E ± 0.32 |
09.33G ± 0.11 |
07.33D ± 0.40 |
09.00G ± 0.33 |
09.66E ± 0.17 |
09.00G ± 0.19 |
08.44 |
09.11 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column. DPT = days post-treatment.
Table III.- Means for population reduction percentage of jassid Amrasca devastans at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
|||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
|||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
|
| Blaster |
50.39C ± 0.34 |
67.72D ± 0.26 |
25.70C ± 0.42 |
37.45C ± 0.33 |
38.64C ± 0.65 |
82.06C ± 0.40 |
38.24 |
62.41 |
| Bugatti |
51.43C ± 0.38 |
75.30C ± 0.39 |
28.68B ± 0.46 |
38.03C ± 0.39 |
40.19BC ± 0.73 |
90.34B ± 0.44 |
40.10 |
67.89 |
| Confidor |
59.17A ± 0.47 |
86.05A ± 0.30 |
30.93A ± 0.53 |
51.93A ± 0.47 |
46.06A ± 0.81 |
96.16A ± 0.53 |
45.39 |
78.05 |
| Jozer |
54.19B ± 0.43 |
79.68B ± 0.35 |
29.73AB ±0.51 |
42.55B ± 0.43 |
42.81B ± 0.77 |
95.40A ± 0.51 |
42.24 |
72.54 |
| Pouch |
40.16E ± 0.27 |
52.11F ± 0.18 |
19.63D ± 0.32 |
22.35E ± 0.24 |
28.89E ± 0.51 |
70.52D ± 0.33 |
29.59 |
48.33 |
| Senator |
43.55D ± 0.31 |
60.12E ± 0.21 |
23.98C ± 0.36 |
28.05D ± 0.26 |
33.72D ± 0.59 |
70.52D ± 0.37 |
33.75 |
52.00 |
| Control |
08.33F ± 0.21 |
09.00G ± 0.14 |
07.00E ± 0.25 |
08.33F ± 0.18 |
08.00F ± 0.42 |
08.00E ± 0.26 |
07.78 |
08.49 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table IV.- Means for population reduction percentage of white fly Bemisia tabaci at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
|||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
|||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
|
| Blaster |
43.83D ± 0.42 |
56.62D ± 0.36 |
22.95B ± 0.62 |
34.30D ± 0.37 |
31.96D ± 0.26 |
46.57C ± 0.39 |
32.91 |
45.83 |
| Bugatti |
45.26C ± 0.37 |
64.37C ± 0.42 |
25.93A ± 0.67 |
48.51C ± 0.33 |
34.52C ± 0.39 |
50.13B ± 0.44 |
35.24 |
54.34 |
| Confidor |
50.64A ± 0.30 |
79.63A ± 0.47 |
27.71A ± 0.77 |
60.29A ± 0.17 |
38.60A ± 0.30 |
61.91A ± 0.53 |
38.98 |
67.28 |
| Jozer |
47.66B ± 0.33 |
77.90B ± 0.51 |
26.77A ± 0.71 |
54.61B ± 0.25 |
36.38B ± 0.35 |
60.25A ± 0.49 |
36.94 |
64.25 |
| Pouch |
34.90F ± 0.21 |
42.81F ± 0.26 |
17.53C ± 0.48 |
18.28F ± 0.18 |
23.74F ± 0.19 |
26.50E ± 0.26 |
25.39 |
29.20 |
| Senator |
39.33E ± 0.26 |
49.83E ± 0.33 |
21.61B ± 0.57 |
25.21E ± 0.21 |
28.69E ± 0.21 |
33.80D ± 0.31 |
29.88 |
36.28 |
| Control |
07.00G ± 0.18 |
08.66G ± 0.21 |
07.33D ± 0.41 |
08.66G ± 0.14 |
09.00G ± 0.13 |
07.00F ± 0.16 |
07.78 |
8.11 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table V.- Means for population reduction percentage of thrips Thrips tabaci at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
|||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
|||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
|
| Blaster |
48.50C ± 0.26 |
61.68D ± 0.31 |
26.67B ± 0.49 |
36.07D ± 0.24 |
40.04D ± 0.18 |
54.29C ± 0.33 |
38.40 |
50.68 |
| Bugatti |
49.00C ± 0.39 |
76.02C ± 0.48 |
29.56A ± 0.54 |
55.44C ± 0.34 |
42.05C ± 0.21 |
65.02B ± 0.41 |
40.20 |
65.49 |
| Confidor |
55.59A ± 0.30 |
88.55A ± 0.35 |
31.49A ± 0.64 |
68.36A ± 0.30 |
63.55A ± 0.30 |
70.90A ± 0.30 |
50.12 |
75.94 |
| Jozer |
51.90B ± 0.35 |
82.62B ± 0.43 |
30.47A ± 0.59 |
59.58B ± 0.27 |
44.07B ± 0.24 |
65.34B ± 0.39 |
42.15 |
69.18 |
| Pouch |
38.77E ± 0.17 |
47.00F ± 0.24 |
21.12C ± 0.42 |
19.59F ± 0.16 |
31.81F ± 0.13 |
41.80D ± 0.22 |
30.57 |
36.13 |
| Senator |
42.81D ± 0.21 |
54.00E ± 0.26 |
25.21B ± 0.46 |
26.55E ± 0.20 |
36.38E ± 0.15 |
41.80D ± 0.27 |
34.80 |
40.78 |
| Control |
07.00F ± 0.12 |
08.33G ± 0.20 |
08.66D ± 0.37 |
09.00G ± 0.13 |
06.00G ± 0.11 |
09.00E ± 0.17 |
07.22 |
08.78 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table VI.- Means for population reduction percentage of red cotton bug Dysdercus koenigii at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
|||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
|||||
|
3 DP |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT | |
| Blaster |
Not appeared |
16.68D ± 0.31 |
25.00D ± 0.40 |
45.04D ± 0.43 |
67.45D ± 0.47 |
30.86 |
46.23 | |
| Bugatti |
19.46C ± 0.27 |
35.00C ± 0.51 |
47.91C ± 0.55 |
70.10C ± 0.61 |
33.69 |
52.55 | ||
| Confidor |
36.29A ± 0.17 |
44.70A ± 0.47 |
58.50A ± 0.53 |
82.25A ± 0.53 |
47.40 |
63.48 | ||
| Jozer |
29.08B ± 0.21 |
39.71B ± 0. 43 |
54.89B ± 0.58 |
75.04B ± 0.56 |
42.35 |
57.36 | ||
| Pouch |
10.96F ± 0.13 |
08.33E ± 0.31 |
18.50F ± 0.31 |
62.29E ± 0.38 |
14.73 |
35.31 |
||
| Senator |
14.84E ± 0.15 |
09.16E ± 0.35 |
30.35E ± 0.36 |
65.79D ± 0.41 |
22.60 |
37.48 | ||
| Control |
06.33G ± 0.10 |
02.57F ± 0.25 |
06.00G ± 0.26 |
09.00F ± 0.27 |
06.17 |
05.67 |
||
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table VII.- Means for population reduction percentage of dusky cotton bug Oxycarenus hyalinipennis at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
|||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
|||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
|
| Blaster |
Not appeared |
21.00D ± 0.34 |
29.00D ± 0.23 |
36.96D ± 0.21 |
75.83D ± 0.33 |
28.98 |
52.42 |
|
| Bugatti |
28.69C ± 0.38 |
42.33C ± 0.28 |
40.41C ± 0.29 |
79.94C ± 0.26 |
34.55 |
61.14 |
||
| Confidor |
46.00A ± 0.47 |
52.25A ± 0.35 |
56.97A ± 0.17 |
83.85A ± 0.17 |
61.59 |
69.55 |
||
| Jozer |
37.89B ± 0.42 |
46.36B ± 0.31 |
45.39B ± 0.25 |
82.81B ± 0.21 |
41.64 |
64.59 |
||
| Pouch |
10.20F ± 0.25 |
08.33E ± 0.16 |
05.66F ± 0.13 |
32.11F ± 0.15 |
07.93 |
20.22 |
||
| Senator |
15.25E ± 0.29 |
09.54E ± 0.19 |
31.43E ± 0.15 |
69.88E ± 0.19 |
23.34 |
39.71 |
||
| Control |
08.33G ± 0.19 |
04.15F ± 0.11 |
05.34F ± 0.11 |
08.33G ± 0.13 |
06.84 |
06.24 |
||
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table VIII.- Means for population reduction percentage of ant species at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT 7 DPT |
|
| Blaster |
36.06C ± 0.39 |
77.38D ± 0.47 |
51.49D ± 0.39 |
62.54D ± 0.80 |
53.45B ± 0.71 |
74.14C ± 0.63 |
47.00 71.35 |
| Bugatti |
38.50B ± 0.44 |
81.07C ± 0.65 |
52.61C ± 0.34 |
67.66C ± 0.56 |
54.84B ± 0.65 |
75.40C ± 0.57 |
48.65 74.71 |
| Confidor |
51.00A ± 0.53 |
94.75A ± 0.53 |
64.22A ± 0.17 |
88.33A ± 0.17 |
58.96A ± 0.53 |
82.54A ± 0.53 |
58.06 88.54 |
| Jozer |
38.50B ± 0.48 |
88.50B ± 0.59 |
62.29B ± 0.25 |
77.66B ± 0.29 |
57.41A ± 0.58 |
78.33B ± 0.46 |
52.73 81.49 |
| Pouch |
06.55D ± 0.27 |
12.45F ± 0.34 |
39.71F ± 0.17 |
50.33E ± 0.34 |
45.42C ± 0.35 |
50.00E ± 0.44 |
30.56 37.59 |
| Senator |
34.59C ± 0.33 |
69.75E ± 0.41 |
50.65E ± 0.21 |
62.02D ± 0.26 |
52.77B ± 0.43 |
72.28D ± 0.72 |
46.00 68.01 |
| Control |
06.00D ± 0.21 |
08.00G ± 0.28 |
6.66G ± 0.11 |
08.66F ± 0.50 |
08.00D ± 0.27 |
10.00F ± 0.36 |
06.88 08.88 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table IX.- Mean for population reduction percentage of wasps at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
|||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
|||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
|
| Blaster |
39.95D ± 0.49 |
69.39C ± 0.51 |
39.46C ± 0.56 |
51.54C ± 0.68 |
23.70C ± 0.36 |
40.15C ± 0.35 |
34.37 |
53.69 |
| Bugatti |
42.84C ± 0.41 |
70.37C ± 0.43 |
41.62B ± 0.50 |
55.28B ± 0.50 |
25.90C ± 0.30 |
41.71C ± 0.28 |
36.78 |
55.78 |
| Confidor |
61.00A ± 0.30 |
96.65A ± 0.30 |
48.22A ± 0.30 |
61.00A ± 0.30 |
46.83A ± 0.17 |
66.45A ± 0.17 |
52.01 |
74.70 |
| Jozer |
57.00B ± 0.36 |
87.95B ± 0.36 |
42.66B ± 0.42 |
56.00B ± 0.39 |
30.16B ± 0.23 |
49.18B ± 0.21 |
43.27 |
64.37 |
| Pouch |
06.00F ± 0.21 |
21.17E ± 0.25 |
34.65E ± 0.20 |
38.69E ± 0.21 |
18.81D ± 0.50 |
18.24E ± 0.20 |
19.82 |
26.03 |
| Senator |
38.00E ± 0.25 |
66.76D ± 0.59 |
37.71D ± 0.25 |
47.96D ± 0.25 |
21.13C ± 0.43 |
36.17D ± 0.41 |
32.28 |
50.29 |
| Control |
05.96F ± 0.18 |
08.00F ± 0.19 |
08.00F ± 0.16 |
10.00F ± 0.15 |
07.33E ± 0.57 |
09.33F ± 0.13 |
7.09 |
9.11 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table X.- Means for population reduction percentage of coccinellid beetles at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT 7DPT |
|
| Blaster |
31.66D ± 0.35 |
77.36C ± 0.33 |
53.84C ± 0.35 |
57.45D ± 0.39 |
41.52D ± 0.41 |
56.94D ± 0.36 |
42.34 63.92 |
| Bugatti |
46.83C ± 0.29 |
81.30B ± 0.26 |
54.13C ± 0.29 |
60.14C ± 0.32 |
44.00C ± 0.35 |
63.82C ± 0.28 |
48.32 68.42 |
| Confidor |
59.33A ± 0.17 |
91.90A ± 0.17 |
65.70A ± 0.17 |
77.66A ± 0.17 |
57.00A ± 0.17 |
78.39A ± 0.17 |
60.67 82.65 |
| Jozer |
55.16B ± 0.22 |
85.69B ± 0.21 |
56.88B ± 0.23 |
67.66B ± 0.24 |
45.00B ± 0.29 |
67.08B ± 0.22 |
52.35 73.48 |
| Pouch |
13.50F ± 0.20 |
23.72E ± 0.45 |
50.35E ± 0.15 |
47.56F ± 0.51 |
30.84F ± 0.21 |
31.73F ± 0.51 |
31.56 34.34 |
| Senator |
26.39E ± 0.41 |
72.63D ± 0.39 |
51.32D ± 0.19 |
55.25E ± 0.46 |
40.83E ± 0.47 |
55.74E ± 0.43 |
39.51 61.21 |
| Control |
05.66G ± 0.11 |
07.66F ± 0.20 |
07.33F ± 0.11 |
09.33G ± 0.11 |
06.66G± 0.12 |
08.66G ± 0.11 |
06.55 08.55 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table XI.- Means for population reduction percentage of green lacewing Chrysoperla carnea at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Population reduction percentage (mean ± SE) |
||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT 7 DPT |
|
| Blaster |
30.09C ± 0.58 |
63.10C ± 0.77 |
30.34D ± 0.67 |
63.18C ± 0.67 |
38.03D ± 0.61 |
67.66D ± 0.68 |
32.83 64.65 |
| Bugatti |
33.50B ± 0.55 |
63.50C ± 0.68 |
37.69C ± 0.60 |
69.18B ± 0.62 |
44.47C ± 0.55 |
73.41C ± 0.63 |
38.55 68.70 |
| Confidor |
37.84A ± 0.45 |
81.00A ± 0.51 |
46.93A ± 0.49 |
78.27A ± 0.50 |
53.17A ± 0.47 |
81.95A ± 0.49 |
45.98 80.41 |
| Jozer |
34.33B ± 0.51 |
74.33B ± 0.60 |
44.47B ± 0.51 |
70.17B ± 0.56 |
48.82B ± 0.51 |
77.19B ± 0.55 |
42.54 73.89 |
| Pouch |
27.66D ± 0.71 |
53.00D ± 0.89 |
24.13F ± 0.79 |
53.72E ± 0.76 |
33.36E ± 0.73 |
59.62F ± 0.79 |
28.38 55.45 |
| Senator |
29.0CD ± 0.65 |
62.81C ± 0.82 |
27.08E ± 0.73 |
56.68D ± 0.71 |
33.83E ± 0.69 |
61.49E ± 0.75 |
29.97 60.33 |
| Control |
08.33E ± 0.78 |
10.37E ± 0.91 |
09.33G ± 0.84 |
11.33F ± 0.81 |
07.69F ± 0.80 |
10.13G ± 0.83 |
08.45 10.61 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
Table XII.- Means for parasitism of whiteflies by Encarsia spp. at 3 and 7 days post treatment after 1st, 2nd and 3rd application of different insecticides.
| Insecticide formulation |
Parasitism of whiteflies (mean ± SE) |
|||||||
|
First application |
Second application |
Third application |
Avg. of 3 appl. |
|||||
|
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
3 DPT |
7 DPT |
|
| Blaster |
38.24D ± 0.71 |
56.51D ± 0.66 |
35.59D ± 0.69 |
62.20D ± 1.41 |
33.39C ± 1.79 |
61.78D ± 0.70 |
35.74 |
60.16 |
| Bugatti |
46.86C ± 0.67 |
64.56C ± 0.59 |
43.44C ± 0.63 |
70.67C ± 1.34 |
41.42B ± 1.75 |
69.58C ± 0.89 |
43.90 |
68.27 |
| Confidor |
51.51B ± 0.59 |
69.65B ± 0.51 |
48.45B ± 0.51 |
75.77B ± 1.15 |
46.17B ± 1.61 |
74.15B ± 0.81 |
48.71 |
73.19 |
| Jozer |
50.12B ± 0.62 |
68.41B ± 0.54 |
47.66B ± 0.57 |
74.82BC ±1.26 |
45.76B ± 1.67 |
73.85B ± 0.85 |
47.84 |
72.36 |
| Pouch |
32.45E ± 0.80 |
50.61E ± 0.75 |
29.22E ± 0.80 |
56.59E ± 1.58 |
27.12D ± 1.86 |
55.71E ± 0.56 |
29.59 |
54.30 |
| Senator |
34.00E ± 0.76 |
52.12E ± 0.71 |
31.29E ± 0.74 |
58.37E ± 1.49 |
29.08CD± 1.83 |
57.18E ± 0.63 |
31.46 |
55.89 |
| Control |
59.03A ± 0.53 |
76.36A ± 0.47 |
55.70A ± 0.46 |
80.70A ± 1.09 |
56.19A ± 1.57 |
80.36A ± 0.78 |
56.97 |
79.14 |
Values are mean of three independent replications. Different letters in superscript following values indicate statistical significance (at P ≤ 0.05) among treatments within a column.
The second most effective insecticide against targeted insect pests was Jozer 20%SL (imidacloprid + acetamiprid). This formulation caused a significant reduction in the population of aphids (51 and 85%), jassids (42 and 72%), whiteflies (37 and 64%), thrips (42 and 69%), red cotton bugs (42 and 57%) and dusky cotton bugs (41 and 65%). Along with Confidor, Jozer was also the most effective formulation against aphids and jassids. These findings are in accordance with the results of Ali et al. (2005), Iqbal et al. (2013), Afzal et al. (2014) and Simon-Delso et al. (2015). The least effective insecticides were Pouch 35%SC (pyriproxyfen + etofenprox) and Senator 41.6% EC (imidacloprid + pyriproxyfen), while Blaster 72.5%WP (imidacloprid + acephate) and Bugatti 50%SC (imidacloprid + bifenthrin) gave an intermediate response against all targeted sucking insect pests (Tables II, III, IV, V, VI, VII).
Regarding the effect of insecticides on non-target insect species (predators and parasitoids; Tables VIII, IX, X, XI, XII), most of the insecticide formulations tested caused more than 50% reduction of all beneficial insect fauna. This is not in agreement with the results of a recent study done by Sarwar and Sattar (2016) which reported no significant effect of endosulfan 35% EC and monocrotophos 36% SL on cotton insect pests and natural enemies complex. However, in our study it was observed that parasitism of whitefly individuals by Encarsia spp. was reduced from 50 to 76% on an average basis. Pouch 35%SC (pyriproxyfen + etofenprox) remained at the top with least reduction of the population of ants (30 and 38%), parasitic wasps (20 and 26%), coccinellid beetles (32 and 34%), green lacewings (28 and 55%) and Encarsia parasitism (29 and 54%), respectively for 3DPT and 7DPT (Tables VIII, IX, X, XI, XII). Although Etofenprox is a broad spectrum pyrethroid with detrimental effects on natural enemies of arthropods (Vanaclocha et al., 2013), its combination with pyriproxyfen resulted in attenuating its harmfulness to ants, wasps, lacewings and predatory coccinellids. Many new chemistry insect growth regulator such as pyriproxyfen and buprofezin have been demonstrated as very target specific and with least residual effects on non-target species of insect predators and regulators (Naranjo et al., 2004; Naveed et al., 2008; Messelink et al., 2014).
Similarly, the interaction between the insecticides and post-treatment observation intervals demonstrated ≤ 50% reduction in the population of Encaria species (24–48% reduction). Average parasitism at 3DPT and 7DPT for all insecticides indicated that Confidor and Jozer had parasitism values very close to that recorded in control plots at both post-treatment intervals. These two insecticides were found comparatively safe for Encarsia wasps with least effect on whitefly parasitism. However, these findings are in contrast to a study done by Van de Veire and Tirry (2003) who demonstrated that most of neonicotinoid insecticides such as imidacloprid and acetamiprid were harmful and toxic for many Encarsia parasitic wasps.
In brief, the insecticidal formulations Confidor 20%SL (imidacloprid) and Jozer 20%SL (imidacloprid + acetamiprid) were the most toxic and effective against the sucking insect pest complex infesting Bt-cotton, while the insecticidal formulations Pouch 35%SC (pyriproxyfen + etofenprox) and Senator 41.6%EC (imidacloprid + pyriproxyfen) remained the least effective. However, Pouch 35%SC caused minimum reduction of predatory or parasitic insects. Moreover, cotton plots treated with Confidor and Jozer also exhibited whitefly parasitism very close to that recorded in controls for both post-treatment observations. Therefore, based on these findings, Confidor and Jozer are recommended to be further integrated in the management strategies against sucking pests’ complex.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Abbas, M.A., 2001. General agriculture. Publ. Emporium, 2nd (ed.), Pakistan, pp. 352.
Abro, G.H., Syed, T.S., Tunio G.M. and Khuhro, M.A., 2004. Performance of transgenic Bt cotton against insect pest infestation. Biotechnology, 3: 75-81. https://doi.org/10.3923/biotech.2004.75.81
Afzal, M., Rana, S.M., Baber, M.H., Haq, I.U., Iqbal Z. and Saleem, H.M., 2014. Comparative efficacy of new insecticides against whitefly, Bemisia tabaci (Genn.) and jassid, Amrasca devastans (Dist.) on Cotton, Bt-121. Biologia, 60: 117–121.
Ahmad, Z., 1999. Pest problems of cotton, a regional perspective. Proc. Regional consultation, insecticides resistance management in cotton. Pakistan Central Cotton Committee, pp. 5-21.
Ali, A. and Aheer, G.M., 2007. Varietal resistance against sucking insect pests of cotton under Bahawalpur ecological conditions. J. agric. Res., 45: 1-5.
Ali, M.A., Rehman, R., Tatla, Y.H. and Ali, Z., 2005. Evaluation of different insecticides for the control of whitefly on cotton crop in Karor district Layyah. Pak. Entomol., 27: 5-8.
Asi, M.R., Afzal, M., Anwar, S.A. and Bashir, M.H., 2008. Comparative efficacy of insecticides against sucking insect pests of cotton. Pak. J. Life Sci., 6: 140-142.
Asif, M.U., Muhammad, R., Akbar, W. and Tofique, M., 2016. Relative efficacy of some insecticides against the sucking insect pest complex of cotton. Nucleus, 53: 140-146.
Aslam, M., Khan, A.H., Rasheed, T. and Khan. I.H., 2001. Monitoring whitefly, Bemisia tabaci (Genn.) on cotton. Pakistan J. Zool., 33: 261-264.
Aslam, M., Razzaq, M., Rana, S. and Faheem, M., 2004. Efficacy of different insecticides against sucking insect-pests on cotton. Pak. Entomol., 25: 155-159.
Bo, B.Y., 1992. IPM on cotton in Asia and Pacific regions. Pak. Cottons, 36: 87-100.
Hossain, S.M.A., Baque, M.A. and Amin, M.R., 2013. Comparative effectiveness of seed treating and foliar insecticides against sucking pests of cotton and impact on their natural enemies. Bangladesh J. agric. Res., 38: 61-70.
Iqbal, J., Nadeem, M., Assi, M.S., Fiaz, M.M. and Hassan, M.W.U., 2013. Comparative efficacy of some insecticides against sucking insect pests on mungbean, Vigna radiata (L) Wilczek. Gomal Univ. J. Res., 29: 31-37.
El-Naggar, J.B. and Zidan, N.E.H.A., 2013. Field evaluation of imidacloprid and thiamethoxam against sucking insects and their side effects on soil fauna. J. Pl. Protec. Res., 53: 375-387. https://doi.org/10.2478/jppr-2013-0056
Luttrell, R.G., Teague, T.G. and Brewer, M.J., 2015. Cotton insect pest management. In: Cotton, 2nd ed., Agronomy Monograph 57. American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc., Madison, WI, pp. 509–546. https://doi.org/10.2134/agronmonogr57.2014.0072
Kannan, M., Uthamasamy, S. and Mohan, S., 2004. Impact of insecticides on sucking pests and natural enemy complex of transgenic cotton. Curr. Sci., 86: 726-729.
Khan, I., 2010. Efficacy of different insecticides against army worm, Spodoptera litura from different districts of Punjab. M.Sc. thesis, Agriculture College, Gomal University, Dera Ismail Khan, Pakistan, pp. 176.
Majeed, M.Z., Javed, M., Riaz, M.A. and Afzal, M., 2016. Population dynamics of sucking pest complex on some advanced genotypes of cotton under unsprayed conditions. Pakistan J. Zool., 48: 475-475.
Messelink, G.J., Bennison, J., Alomar, O., Ingegno, B., Tavella, L., Shipp, L., Palevsky, E. and Wäckers, F., 2014. Approaches to conserving natural enemy populations in greenhouse crops: current methods and future prospects. BioControl, 59: 377-393. https://doi.org/10.1007/s10526-014-9579-6
Naranjo, S.E., Ellsworth, P.C. and Hagler, J.R., 2004. Conservation of natural enemies in cotton: Role of insect growth regulators for management of Bemisia tabaci. Biol. Contr., 30: 52-72. https://doi.org/10.1016/j.biocontrol.2003.09.010
Naveed, M., Salam, A., Saleem, M.A. and Sayyed, A.H., 2008. Effect of foliar applications of some insecticides on Bemisia tabaci, predators and parasitoids: implications in its management in Pakistan. Phytoparasitica, 36: 377-387. https://doi.org/10.1007/BF02980817
Sarwar, M. and Sattar, M., 2016. An analysis of comparative efficacies of various insecticides on the densities of important insect pests and the natural enemies of cotton, Gossypium hirsutum L. Pakistan J. Zool., 48: 131-136.
Shah, S.I.A, Malik, T.H., Khan, I.R. and Hussain, Z., 2017. Screening of USDA cotton accessions against sucking insect pests complex and cotton leaf curl virus (CLCuV) disease with major emphasis on abiotic factors. Pakistan J. Zool., 49: 1159-1173.
Shivanna, B.K., Naik, B.G., Nagaraja, R., Basavaraja, M.K., Swamy C.M.K. and Karegowda, C., 2011. Bio efficacy of new insecticides against sucking insect pests of transgenic cotton. Int. J. Sci. Nature, 2: 79-83.
Simon-Delso, N., Amaral-Rogers, V., Belzunces, L.P., Bonmatin, J.M., Chagnon, M., Downs, C. and Goulson, D., 2015. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res., 22: 5-34. https://doi.org/10.1007/s11356-014-3470-y
Tayyib, M., Sohail, A., Shazia, A.M. and Jamil, F.F., 2005. Efficacy of some new chemistry insecticides for controlling the sucking insect pests and mites on cotton. Pak. Entomol., 27: 63-67.
Vanaclocha, P., Vidal-Quist, C., Oheix, S., Montón, H., Planes, L., Catalán, J., Tena, A., Verdú, M.J. and Urbaneja, A., 2013. Acute toxicity in laboratory tests of fresh and aged residues of pesticides used in citrus on the parasitoid Aphytis melinus. J. Pest Sci., 86: 329-336. https://doi.org/10.1007/s10340-012-0448-8
Van de Veire, M. and Tirry, L., 2003. Side effects of pesticides on four species of beneficials used in IPM in glasshouse vegetable crops: “worst case” laboratory tests. Int. Org. biol. Integr. Contr. Bull., 26: 41-50.
To share on other social networks, click on any share button. What are these?










