Impact of Selected Insecticides on Apis mellifera L. (Hymenoptera: Apidae) under Controlled Conditions
Impact of Selected Insecticides on Apis mellifera L. (Hymenoptera: Apidae) under Controlled Conditions
Muhammad Aslam Farooqi*, Bakhtawar Irsa, Sajjad Ali, Asif Sajjad, Muhammad Waqar Hassan and Sohail Akhtar
Department of Entomology, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Pakistan.
ABSTRACT
The present study was conducted in conjunction with the efforts to study the contact toxicity of some insecticides i.e. acephate, lambda-cyhalothrin, diafenthiuron, profenofos and spirotetramat against adult workers of Apis mellifera L. These insecticides were frequently being used to control different insect pests of major crops in Punjab-Pakistan. The experimentation was performed under laboratory conditions (28±2°C and 65±5% R.H). Four different concentrations (i.e. 50 ppm, 100, 200 and 400 ppm) of each insecticide were evaluated against A. mellifera as derived from their recommended field doses. Mortality of A. mellifera was recorded at 0.5, 3, 6 and 24 hours after exposure to insecticides. To evaluate the toxic impact, median lethal concentration (LC50) of each tested insecticide was determined. The results obtained, revealed that all insecticides were highly toxic after 24 hours of exposure at maximum concentration. Acephate and lymda-cyhalothrin showed the maximum toxicity with their LC50 of 83.96 and 139.07 ppm, respectively while diafenthiuron and profenofos were moderate in toxicity with LC50 values of 150.53 and 169.42 ppm, respectively. Spirotetramat was the least toxic in this experiment with a LC50 of 181.51 ppm after 24 hours of exposure to tested bees at maximum concentration of 400 ppm. After adjusting these results of tested insecticides to their commercial formulated field dose applications, they were causing a potential threat to A. mellifera at their maximum recommended dose except spirotetramat.
Article Information
Received 29 August 2018
Revised 24 May 2019
Accepted 27 August 2019
Available online 22 October 2019
Authors’ Contribution
MAF, Conceived and designed the experiment and wrote the paper. BI, performed the experiment. SA, AS, WH and SA helped in statistical analysis.
Key words
Apis mellifera, Mortality, Insecticides, LC50
DOI: https://dx.doi.org/10.17582/journal.pjz/2020.52.1.193.198
* Corresponding author: [email protected]; [email protected]
0030-9923/2020/0193-0001 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Honeybees are regarded as the chief global pollinators owing to their important role in crop pollination and maintenance of wild plants communities (Ollerton et al., 2011) and contribute global food of worth153 billion € annually (Gallali et al., 2009). Honeybees mainly Apis mellifera L. and other bee species are the main source of pollination of many food crops. There are many sources of pollination, but honeybees play an important role by pollinating more than 75% of flowering plants across the world (Buchmann and Nabhan, 1996; Kremen et al., 2002) and almost from 100 valuable crops of the world, 70% are being pollinated by honeybees (Klein et al., 2007; Moritz et al., 2010).
According to estimation that 35% of food supply is entirely dependent on pollinators all across the world among which honeybees are main contributors (Klein et al., 2007). Many nutritionally rich and economically valuable crops of food such as fruits, vegetables and fodders, entirely depend upon bees for their pollination (Spivak et al., 2010). In many developed countries where agriculture is intense and mechanized, most of the agricultural crops totally rely on managed A. mellifera for pollination (UNEP, 2010).
From many years in the United States, 50 out of 250 crops are being pollinated from the colonies of managed honeybees (i.e A. mellifera) for the production of high quality commercial fruits and seeds (Atkins, 1992; Kremen et al., 2002; Spivak et al., 2010). In Pakistan, honeybees cause a significant increase in the quantity and quality of apples and sarson (Brasica compestris) crop (Parveen et al., 2000; Khan et al., 2004) and many other valuable crops.
The use of agrochemicals put a key pressure on insect pollinators (Kuldna et al., 2009). Due to their indiscriminate use, colonies of honeybee are declining across the world. Agrochemicals can destabilize different types of pollinator communities before and after their application in the field crops (Potts et al., 2010). If the production of fruits and seeds are pollen limited then there will be certain effects on pollination due to low and variable pollinators which ultimately decreases the crop yield (Klein, 2009; Garibaldi et al., 2011).
Apis mellifera workers foraging in the field can be affected by getting in direct contact with crops treated with insecticides (Koch and Weisser, 1997), or they get toxic effect from fumes and dusts of insecticides during their flight (Prier et al., 2001). Insecticides also induce chronic toxicity in honeybees in terms of change in their foraging and learning behavior. Insecticides also affect their immune system and susceptibility to diseases (Desenex et al., 2007; Wu et al., 2011; Pettis et al., 2012). The decline of honeybee populations in the world is a serious problem. If this speed of their decline continues, the pollination of agricultural crops which depends upon bees will be badly affected (Fontaine et al., 2006; Potts et al., 2010). So it can cause the world food production at risk, because they are the main source of pollination for different crops, fruits and vegetables (Klein et al., 2007).
The current research was planned to evaluate the contact toxicity of some selected insecticides against A. mellifera, commonly being used on different fruits, vegetables and field crops to control different insect pests in Pakistan, so that awareness can be created among farmers to use only those insecticides which have minimum effects to honeybees.
MATERIALS AND METHODS
Insecticides formulations and solvents
Commercial formulations of five insecticides viz., acephate 75 SP (Commando), lambda- cyhalothrin 10 WG (Jumper), diafenthiuron 50 SC (Polo), profenofos 50 EC (Curacron) and spirotetramat 240 SC (Movento) were purchased from their respective manufacturing companies to check their residual contact toxicity against Apis mellifera L. under laboratory conditions (Table I). We prepared four concentrations (50, 100, 200 and 400 ppm) of each formulated insecticide as derived from their recommended field doses in acetone except spirotetramat whose concentration was made in ethyl acetate because it was not easily soluble in acetone.
Collection of A. mellifera
Newly emerged adult worker bees (A. mellifera) approximately 1-6 days of age were used in this experiment during second fortnight of March, 2017 and were identified from other bees on the basis of appearance of abundant light yellow setae on the dorsum of their thorax. During collection, these were kept in plastic cages to transport them in the laboratory. Bee hives were located in agriculture farm of University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur. No hive treatments to control diseases were conducted prior to these studies. Hives were exposed to smoke twice for 30–60 sec prior to collection. These bees were fed upon 50% sucrose solution in the laboratory. The bees were immobilized by keeping them in a refrigerator at – 4 0C for about 5 minutes. They were then allowed to recover from cold treatment and then kept under 28±2°C and 65±5% R.H in darkness for 20 minutes prior to insecticide exposure.
Insecticide stock solutions
Frist of all, stock solutions of 400 ppm for all the insecticides were prepared in solvents by adding calculated amount of each formulated insecticide with the help of micropipette. Flasks were shaked thoroughly until insecticides completely dissolved in solvents. The stock solution was then divided into two equal portions. One of them was reserved for treatment application and the volume of other portion was doubled by adding equal amount of solvent to make 200 ppm solution. In the same way, 200 ppm stock solution was divided into two equal portions. One of them was reserved for treatment application and the volume of other portion was doubled by adding equal amount of solvent to make 100 ppm solution. Above procedure was repeated with 100 ppm stock solution for making 50 ppm concentration of insecticides.
Bioassay
Surface residual bioassay method (Radwan and Taha, 2012; Farooqi et al., 2016) was used for testing contact toxicity of insecticides to A. mellifera in 1.5 liter plastic jars. These jars were washed out to remove all contaminations and were air dried before making insecticide coating. 5 ml of each concentration (400, 200, 100 and 50 ppm) were taken in micropipettes and were applied into each jar, while in control treatment only 5 ml of acetone was applied. Then jars were shaken thoroughly so that concentration might reach every corner of the jars and were kept for drying for about 10 minutes. A group of 10 bees were released in each jar after it was completely air dried and were covered tightly with muslin cloths. These jars were placed on smooth and clean surface under 28±2°C and 65±5% R.H.
Collection of data
Data was recorded at 0.5, 3, 6, and 24 hours after treatment application. Mortality of bees was examined under sterioscope. All bees in a jar were put on the Petri dishes. Those which were moving actively returned into jars. A needle was inserted into immobile body of tested bees. Those which did not show any movement were considered as dead. Total number of dead bees were counted and mentioned in a data sheet.
Table I. List of insecticides with trade names, common names, chemical group and toxicity.
|
Trade name |
Common name |
Chemical group |
Toxicity level |
|
Jumper 10WG |
lymbda-cyhalothrin |
Pyrethroids |
II/WHO |
|
Curacron 50EC |
profenofos |
Organophosphate |
II/WHO |
|
Commando 75SP |
acephate |
Organophosphate |
II/WHO |
|
Movento 240SC |
spirotetramat |
New Chemistry |
IV/WHO |
|
Polo 50SC |
diafenthiuron |
Benzoyl phenylureas |
IV/WHO |
Table II. Contact toxicity of different insecticides against A. mellifera after different time intervals using surface residual method.
|
Insecticides |
Observations in hours |
LC50 |
Slope ±SE |
X2 |
Fiducial (C.I) |
P-value |
|
Lymda-cyhalothrin |
0.5 03 06 24 |
382.63 367.54 246.30 139.07 |
0.00272±0.00087 0.00300±0.00078 0.00326±0.00077 0.00462±0.00085 |
4.35452 7.9399 14.1996 18.1935 |
289.4-995.6 276.8-596.71 180.64-359.2 119.3-221.33 |
<0.001 <0.001 <0.001 <0.001 |
|
Profenofos |
0.5 03 06 24 |
392.44 354.62 318.05 169.42 |
0.00281±0.00083 0.00262±0.00082 0.00275±0.00077 0.00540±0.00092 |
4.21154 8.44877 12.3767 15.0166 |
298.16-976.4 212.60-768.4 232.1-534.4 96.51-183.3 |
<0.001 <0.001 <0.001 <0.001 |
|
Acephate |
0.5 03 06 24 |
396.76 343.33 187.77 83.96 |
0.00286±0.00092 0.00281±0.00074 0.00432±0.00081 0.00606±0.00105 |
4.41542 6.9330 13.9330 27.1856 |
314.17-948.6 192.76-452.4 101.88-161.4 39.07-121.6 |
<0.001 <0.001 <0.001 <0.001 |
|
Spirotetramat |
0.5 03 06 24 |
390.02 364.31 342.4 181.51 |
0.00279±0.00088 0.00259±0.00085 0.00294±0.00078 0.00446±0.00083 |
4.2972 7.2503 9.1621 13.2279 |
309.68-922.4 268.34-684.2 255.99-555.5 132.8-240.3 |
<0.001 <0.001 <0.001 <0.001 |
|
Diafenthiuron |
0.5 03 06 24 |
389.83 361.77 333.94 150.53 |
0.00277±0.00087 0.00268±0.00083 0.00297±0.00078 0.00430±0.00084 |
4.86562 7.12724 9.6822 18.0082 |
293.87-777.7 264.7-695.34 242.87-596.8 98.20-205.9 |
<0.001 <0.001 <0.001 <0.001 |
LC50s are in ppm of different solutions of insecticide used; C.I: confidence interval 95 %; observations are showing different time periods and P<0.001 mean that results are highly significant.
Statistical analysis
Statistical analysis was performed using Probit Procedure (Finny, 1971) to determine LC50, 95% Confidence interval for LC50 and Chi- square goodness- of- fit test for each insecticide tested. Each LC50 determination was based on the different concentrations of insecticides. The percent (%) mortality was calculated and corrected using (Abbott’s, 1925) formula as follows:
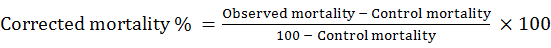
RESULTS AND DISCUSSION
The results showed that all the insecticides tested in this study were proved to be toxic against adult workers of A. mellifera at different exposure of time intervals and different concentrations (Table II). The contact toxicity of insecticides differed significantly at different concentrations and after different post-exposure intervals. The mortality of A. mellifera increased with the increase in concentrations of insecticides and exposure periods. After 24 hours of exposure, the LC50 value was 139.07 ppm for lymda-cyhalothrin, 169.42 for profenofos, 83.96 for acephate, 181.51 for spirotetramat and 150.53 ppm in case of diafenthiuron with maximum concentration (400 ppm). However, there was a great variation in mortality and LC50s of A. mellifera at 0.5, 3 and 12 hours after treatment. LC50s are used to determine or represent toxicity of a specific insecticide. The current bioassays were performed according to the requirements and criteria set by EPPO (1992), with the honeybees’ mortality rate less than 10% in control treatments.
The order of toxicity observed for these insecticides in this experiment against A. mellifera was; acephate >lymda-cyhalothrin>difenthiuron>profenofos>spirotetramat. The results for comparison of % corrected mortality of A. mellifera with different concentrations of insecticides at different time intervals are presented in Figure 1.
The negative impacts of pesticides has resulted decline in honeybee populations is well known across the world (Alaux et al., 2010; Neumann and Carrek, 2010; Henry et al., 2012; Pettis et al., 2012). Previously, different reports from different world researchers have been documented about contact toxicity of agricultural insecticides against honeybees. According to Raghunandan and Basavarajappa (2013) the population of honeybees is decreasing drastically in different countries throughout the world due to use of insecticides. The previous results of Thomas and Phadke, (1994) are in accordance to our current findings. They reported that chlorpyrifos induced 100% mortality in three species of honeybees after 6 hours of its exposure at high concentration (0.06%) under laboratory conditions. Abrol and Rajinder (2003) also confirmed the toxic impact of chlorpyrifos to honeybees (A. mellifera) by contact method of its exposure with a LC50 of 0.0354 ug/bee however, they used topical application procedure with different concentrations. The results of Sharma and Dharam (2005) are inconsistent with these studies as they reported that organophosphates are highly toxic to honeybees and caused 100% mortality by contact toxicity with high cocentrations of 0.05 and 0.09% of active ingridients under laboratory conditions. Similarly, Letelier et al. (2012) also reported that chlorpyrifos is highly toxic to A. mellifera under laboratory conditions on the basis of its LD50 value.
The acute toxicity of acephate against adult workers of A. mellifera has also been reported by Muranjan et al. (2006). The findings of Farooqi et al. (2015) are also in accordance with the current studies where they stated that profenofos is highly toxic against A. mellifera under laboratory conditions by surface residual contact method after 24 hrs of its exposure with a LC50 of 20.6 ppm at its maximum concentration. The findings of Bailey et al. (2005) showed less toxicity of lambda-cyhalothrin where it was considered moderate in toxicity; however, their method of its toxicity determination was different against A. mellifera. But, the findings of Akca et al. (2009) are in full agreement to the results of our studies, where they reported the acute contact residual toxicity of lambda-cyhalothrin after 1, 8, 16 and 24 hrs after its exposure to A. mellifera where it proved to be highly toxic against bees under controlled conditions. Similarly, the previous findings of Melisie et al. (2015) supports the results of these studies where they showed the highly toxic impact of lambda-cyhalothrin and profenofos to A. mellifera after 24 hrs of its exposure.
According to a previous experimentation of Stanely (2010), diafenthiuron also showed the highly toxic effect against honey bees on the basis of its LD50 and LC50. In another study of Stanely et al. (2016), diafenthiuron proved to be highly toxic to A. mellifera after 48 hrs of its exposure where it caused 80.6% mortality of bees. We could not find any published work regarding contact toxicity of spirotetramat, therefore, present results are difficult to compare and discuss with previous findings of researchers.
CONCLUSION
The present investigations on contact toxicity of insecticides against honeybees (A. mellifera) showed that all the tested insecticides proved to be toxic when bees were exposed with different concentrations using surface residual method under laboratory conditions. This emphasizes an urgent need of their limited use during blooming periods of flowers. So the current findings suggest that there is need to conduct consistent reviews of different insecticides which are bring used on different field crops to ensure sustainable development and management of beekeeping for better pollination of valuable crops.
ACKNOWLEDGEMENTS
The author is grateful to Higher Education Commission (HEC) of Pakistan for providing financial assistance under SRGP No. 21-1075/HEC/2016. The author also pays his special thanks to Prof. Dr. Anjum Suhail (Late), who made me (author) able to carry entomological research studies at National and International level.
Statement of conflict of Interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
REFERENCES
Abbott, W.S., 1925. A method of computing the effectiveness of an insecticide. J. econ. Ent., 18: 265-267. https://doi.org/10.1093/jee/18.2.265a
Abrol, D.P. and Rajinder, S.A., 2003. Relative toxicity of some insecticides to Apis mellifera L. J. Asia-Pacific Ent., 6: 235-237. https://doi.org/10.1016/S1226-8615(08)60192-2
Akca, I., Tuncer, C., Güler, A. and Saruhan, I., 2009. Residual toxicity of 8 different insecticides on honey bee (Apis mellifera Hymenoptera: Apidae). J. Anim. Vet. Advan., 8: 436-440.
Alaux, C., Brunet, J.L., Dussaubat, C.F., Mondet, Tchamitchan, S., Cousin, M., Brillard, J., Baldy, A., Belzunces L.P. and Conte, L.E., 2010. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microb., 12: 774-782. https://doi.org/10.1111/j.1462-2920.2009.02123.x
Atkins, E.L., 1992. Injury to honey bee by poisoning. In: The hive and the honey bee (ed. J.E. Graham). Dadant and Sons, Hamilton, pp. 1153-1208.
Bailey, J., Scott-Dupree, C., Harris, R., Tolman, J. and Harris, B., 2005. Contact and oral toxicity to honey bees (Apis mellifera) of agents registered for use for sweet corn insect control in Ontario, Canada. Apidologie, 36: 623-633. https://doi.org/10.1051/apido:2005048
Buchmann, S.L. and Nabhan, G.P., 1996. The forgotten pollinators. Island Press, Washington, D.C.
Desneux, N., Decourtye, A. and Delpuech, J.M., 2007. The sub-lethal effects of pesticides on beneficial arthropods. Annu. Rev. Ent., 52: 81-106. https://doi.org/10.1146/annurev.ento.52.110405.091440
EPPO, 1992. Guideline on test methods for evaluating the side effects of plant protection products on honeybees. OEPP/EPPO Bull, 22: 203-215. https://doi.org/10.1111/j.1365-2338.1992.tb00483.x
Farooqi, M.A., Hassan, M., Sabri, M.A. and Javed, N., 2015. Toxicity of different insecticides against Apis Mellifera L. and Apis dorsata F. and determination of their residues in raw honey. PhD, Thesis, U.A.F.
Farooqi, M.A., Hasan, M. and Arshad, M., 2016. Toxicity of three commonly used nicotinoids and spinosad to Apis mellifera L. (Hymenoptera: Apidae) using surface residual bioassays. Pakistan J. Zool., 48: 1983-1983.
Finny, D.J., 1971. Probit analysis. Cambridge University Press, Cambridge, UK.
Fishel, F.M., 2010. The EPA conventional reduced risk pesticide program. UF/IFAS EDIS Document PI-224. Available at: http://edis.ifas.ufl.edu/pi224.
Fontaine, C., Dajoz, I., Meriguet, J. and Loreau, M., 2006. Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS. Biol., 4: e1. https://doi.org/10.1371/journal.pbio.0040001
Gallai, N., Salles, J.M., Settele, J. and Vaissière, B.E., 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ., 68: 810-821. https://doi.org/10.1016/j.ecolecon.2008.06.014
Garibaldi, L.A., Aizen, M.A., Klein, A.M., Cunningham, S.A. and Harder, L.D., 2011. Global growth and stability in agricultural yield decrease with dependence on pollinator services. Proc. natl. Acad. Sci. USA, 108: 5909-5914. https://doi.org/10.1073/pnas.1012431108
Henry, M., Beguine, M. and Require, F., 2012. A common pesticide decreases foraging success and survival in honeybees. Science Express, March 29, 2012. 4 PP.
Khan, M.R. and Khan, M.R., 2004. The role of honey bees Apis mellifera L. (Hymenoptera: Apidae) in pollination of apple. Pak. J. biol. Sci., 7: 359-362. https://doi.org/10.3923/pjbs.2004.359.362
Klein, A.M., 2009. Nearby rainforest promotes coffee pollination by increasing spatio temporal stability in bee species richness. Forest. Ecol. Manage., 258: 1838-1845. https://doi.org/10.1016/j.foreco.2009.05.005
Klein, A.M., Vaissiere, B.E., Cane, J.H., Dewenter, I.S., Cunningham, S.A., Kremen, C. and Tscharntke, T., 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. B. Biol. Sci., 274: 303-313. https://doi.org/10.1098/rspb.2006.3721
Koch, H. and Weisser, P., 1997. Exposure of honey bee during pesticide application under field conditions. Apidologie, 28: 439-447. https://doi.org/10.1051/apido:19970610
Kremen, C., Williams, N.M. and Thorp, R.W., 2002. Crop pollination from native bees at risk from agricultural intensification. Proc. natl. Acad. Sci. U.S.A., 99: 16812-16816. https://doi.org/10.1073/pnas.262413599
Kuldna, P., Peterson, K., Poltimae, H. and Luig, J., 2009. An application of DPSIR framework to identify issues of pollinator loss. Ecol. Econ., 69: 32-42. https://doi.org/10.1016/j.ecolecon.2009.01.005
Letelier, L.C., Spina, Y.M. and Branchiccela, B.M., 2012. Acute contact toxicity test of insecticides (cipermethrin 25, lorsban 48E, thionex 35) on honeybees in the southwestern zone of Uruguay. Chemosphere, 88: 439-444. https://doi.org/10.1016/j.chemosphere.2012.02.062
Melisie, D., Damte, T. and Thakur, A.K., 2015. Effects of some insecticidal chemicals under laboratory conditions on honey bees (Apis melifera L. Hymenoptera: Apidae) that forage on onion flowers. Afri. J. agri. Res., 10: 1295-1300.
Moritz, R.F., Miranda, J.D., Fries, I., Conte, Y.L., Neumann and Paxton, R.J., 2010. Research strategies to improve honeybee health in Europe. Apidologie, 41: 227-242. https://doi.org/10.1051/apido/2010010
Muranjan, P., Gandhale, C.S., Chaudhari, B.D., Patil, P., Pokharkar, D.S. and Naik, R.L., 2006. Toxicity of ready-mix formulations of pyrethroids and ad-mixed insecticides on forager honey bee, Apis cerana indica Fabricius. Annls Pl. Prot. Sci., 14: 90-93.
Neumann, P. and Carreck, N.L., 2010. Honey bee colony losses. J. Apic. Res., 49: 1-6. https://doi.org/10.3896/IBRA.1.49.1.01
Ollerton, J., Winfree, R. and Tarrant, S., 2011. How many flowering plants are pollinated by animals? Oikos, 120: 321-326. https://doi.org/10.1111/j.1600-0706.2010.18644.x
Perveen, N., Alhariri, M., Ahmad, M. and Suhail, A., 2000. Insecticidal mortality, foraging behavior and pollination role of honeybee (Apis mellifera L.) on sarson (Brassica campestris L.) crop. Int. J. Agric. Biol., 4: 332–333.
Pettis, J.S., Van Engeldsorp, D., Johnson, J. and Dively, G., 2012. Pesticides exposure in honeybee results in increased levels of the gut pathogen noseema. Naturwissenschaften, 99: 153-158. https://doi.org/10.1007/s00114-011-0881-1
Potts, S.G., Biesmeijer, J.C., Kremen, C., Neumann, P., Schweiger, O. and Kunin, W.E., 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol., 25: 345–353. https://doi.org/10.1016/j.tree.2010.01.007
Prier, K.R.S., Lighthart, B. and Bromenshenk, J.J., 2001. Adsorption model of aerosolized bacterial spores (Bacillus subtilis variety niger) onto free-flying honey bees (Hymenoptera: Apidae) and its validation. Environ. Ent., 30: 1188-1194. https://doi.org/10.1603/0046-225X-30.6.1188
Radwan, E.M.M. and Taha, H.S., 2012. Toxic and biochemical effects of different insecticides on the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Egypt. Acad. J. biol. Sci., 4 (1): 1- 10. https://doi.org/10.21608/eajbsf.2012.17272
Raghunandan, K.S. and Basavarajappa, S., 2013. Analysis of multi-floral honey of the giant honeybee, Apis Dorsata F., for pesticide residues in Southern Karnataka, India. Eur. J. zool. Res., 2:22-28.
Sharma, D. and Dharam, P.A., 2005. Contact toxicity of some insecticides to honeybee Apis mellifera (L.) and Apis cerana (F.) J. Asia. Pacif. Ent., 8: 113-115. https://doi.org/10.1016/S1226-8615(08)60079-5
Spivak, M., Mader, E., Vaughan, M. and Euliss, N.H., 2010. The plight of the bees. Environ. Sci. Tec., 45: 34-38. https://doi.org/10.1021/es101468w
Stanley, J., Chandrasekaran, S., Preetha, G., Kuttalam, S. and Jasmine, R.S., 2016. Selective toxicity of diafenthiuron to non-target organisms: honey bees, coccinellids, chelonus, earthworms, silkworms and fish. J. Pl. Protec. Res., 56: DOI: 10.1515. https://doi.org/10.1515/jppr-2016-0001
Stanley, J., Chandrasekaran, S., Preetha, G. and Kuttalam, S., 2010. Toxicity of diafenthiuron to honey bees in laboratory, semi-field and field conditions. Pest. Manage. Sci., 66: 505-510. https://doi.org/10.1002/ps.1900
Thomas, J. and Phadke, K.G., 1994. Relative toxicity of oxydemeton-methyle, chloropyrifos and quinolphos to honey bees (Apis cerana indica). Ind. J. agric. Sci., 64: 207-209.
UNEP, 2010. UNEP emerging issues: Global honey bee colony disorder and other threats to insect pollinators. United Nations Environment Programme.
WHO, 2000. Recommended classification of pesticides by hazard and guide lines to classification Geneva, World Health Organization (document reference WHO/PCS/01.4).
Wu, J.Y., Anelli, C.M and Sheppared, W.S., 2011. Sublethal effects of pesticides residues in brood comb on worker honeybees (Apis mellifera L.) development and longativity. PLoS One, 6: 11-17. https://doi.org/10.1371/journal.pone.0014720
To share on other social networks, click on any share button. What are these?










