Optimum Salinity Level for Seed Production and Survival of Red Tilapia (Hybrid) in Concrete Tanks
Optimum Salinity Level for Seed Production and Survival of Red Tilapia (Hybrid) in Concrete Tanks
Abdul Malik1,4, Ghulam Abbas1*, Hameeda Kalhoro2, Illahi Bux Kalhoro3, Syed Sajjad A. Shah4 and Halima Kalhoro3
1Centre of Excellence in Marine Biology, University of Karachi, Karachi, Pakistan
2Department of Fresh Water Biology and Fisheries, University of Sindh, Jamshoro, Pakistan
3Department of Anatomy and Histology, Faculty of Animal Husbandry and Veterinary Science, Sindh Agriculture University, Tando Jam, Pakistan
4Directorate of Fisheries Sindh, Livestock and Fisheries Department, Govt. of Sindh, Pakistan
ABSTRACT
This study was conducted to examine the effects of varying salinity levels (0‰-30‰ with 5% increment) on breeding of red tilapia. Brood fish were stocked in hapa nets suspended in cemented rectangular tanks (11×7×4 feet) . Three females (mean weight 145 g) and one male (140 g) were kept per tank with 2 replications. They were fed with commercial floating pellets having 35% crude protein at 2% body weight twice a day for 60 days and eggs were collected weekly. Results showed that fertilized eggs were found to be greater in number at low salinity level as compared to higher salinity level. Survival of fry ranged from 1058 to 1100 at salinity level of 0‰ – 20‰ with 5% increment, after which fry number decreased significantly (P<0.05). The fertilized eggs did not differ for the fish at salinity of 0‰ –20‰, but decreased significantly when salinity levels were increased above 25‰. Water quality parameters remained as temperature (28.1±0.2°C), dissolved oxygen (6.10±0.1 ml/l), pH (6.6±0.1 ml/l) and ammonia (0.02±0.004 ml/l) throughout the study period. Taking into consideration the number of fertilized eggs and survival of fry data attained in the present study, a salinity level of 20% is suggested for red tilapia breeding in concrete tanks.
Article Information
Received 03 August 2016
Revised 12 December 2016
Accepted 27 January 2017
Available online 23 May 2017
Authors’ Contributions
AM was the main investigator of the study and GA supervised the whole work. HK and HK assisted in feed preparation. IBK proofread and edited the manuscript. SSAS assisted in fish juvenile stocking.
Key words
Breeding, Captivity, Red tilapia, Salinity, Survival.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.3.1049.1056
* Corresponding author: [email protected]
0030-9923/2017/0003-1049 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Aquaculture is increasingly emerging as one of the fastest growing and important economical agribusinesses throughout the world (FAO, 2014; Kevin et al., 2015). Demand for fin fish is expected to exceed all available supplies in the near future owing to the revolutionary changes taking place in the dietary habits of people all over the world and medical community has declared fishery products as healthy food (FAO, 2014). Fish and fishery products have recorded the highest increase in price, both in domestic and export market in recent years, compared to any other food item (Kevin et al., 2015). In order to control high prices, aquaculture technology development has become an urgent need to fulfil the shortage of animal protein for human beings.
Pakistan is situated in tropical belt and major of the country faces scarcity of rainfall. Due to this serious issue major portion of the land and underground water is affected with high levels of salinity (Mateen et al., 2004; Chaughtai et al., 2015). Due to this problem our commercial fish species are under stress and cannot perform well and thus fish culture industry has shown low growth and small productivity with less profitability of fish farming in Pakistan. These fishes breed fall under stress in brackish water as salinity affected ecological factors and natural food production (Chaughtai et al., 2015). Tilapia fry and fingerlings are being cultured on large scale in fresh water areas (Nandlal and Pickering, 2004).
Tilapias are considered as the world’s second most significant fish species for culture after the carps. They are native to Africa, but have been introduced in 140 countries of the world including Pakistan. Tilapia (Oreochromis sp.) are recognized as imperative food fishes for culturing and represent a major source of protein in USA, Asia, Africa and Caribbean (Chowdhury, 2011; Jaspe and Caipang, 2011; Daudpota et al., 2016). Tilapia are more resistant against diseases, breed easily in captivity, eat variety of foods and can grow easily in variety of the ecological conditions (Daudpota et al., 2016). Tilapias are rapidly becoming more acceptable worldwide by middle class and upscale producers. Culture of tilapia in saline waters areas is well documented built on several research studies done in the past (Cnaani and Hulata, 2011; Jaspe and Caipang, 2011; Ahmadi et al., 2015). Due to limited space for fresh water aquaculture and increasing load to provide food for increasing population, tilapia species are now being cultivated in brackish water ponds and also in sea cages.
The Nile tilapia can only tolerate brackish water with salinity up to 25 parts per thousand (ppt) while the Mozambique tilapia can tolerate salinity up to 40 ppt. Red Tilapia can survive in pure seawater up to 32 ppt (Jaspe and Caipang, 2011; PCAMRD, 1998). Due to this, tilapia species are the best option because they are omnivorous and can be easily adapted on artificial feed, survive at low oxygen levels, tolerate a wide range of salinity and can be cultured on low volume with high densities (Iqbal et al., 2012; Ronald et al., 2014). For sustainable aquaculture, availability of good quality fish seed in mass quantities is the basic requirement (Iqbal et al., 2012; Kevin et al., 2015). Nothing has so far been documented on the optimum requirement of salinity for breeding of red tilapia.
Therefore, this study was conducted to observe the influence of different salinity levels on breeding and survival of red tilapia for its seed production in captivity.
MATERIALS AND METHODS
Experimental setup
A 60-day experiment was conducted at the Sun-bright red Tilapia and Ornamental Hatchery Karachi, Pakistan Pvt. Ltd, about 8 km away from University of Karachi. Fourteen rectangular cemented tanks (11×7×4 feet) and fourteen nylon made hapa (10×6×3 feet) were used in the current study. Salinity was maintained with brine water (180 ppt) procured from salt pans to hatchery and its concentration in the experimental tanks was examined weekly by hand-refractometer (model S/Mill-E, Japan). Water depth was maintained at 3 ft in the tanks throughout the study period.
Brood-stock selection and stocking
Brood-stock of experimental fish (42 females and 14 males), ranging from 18.0 cm to 18.6 cm in length and 140 g to 145 g in weight, respectively. These brooders were selected from brood-stock pond of the hatchery and used for the breeding trials. After morphological examination for ripeness of brooders released into breeding nylon made hapa (10×6×3 feet) as following treatments: T1 (0‰), T2 (5‰), T3 (10‰), T4 (15‰), T5 (20‰), T6 (25‰) and T7 (30‰).
Experimental diet
Broodstock were fed the commercial floating pellets containing 40% crude protein (Oryza Organic ®) at 2 % of total biomass twice daily in all treatments during the breeding period.
Egg collection and incubation
After 7 days of stocking, all brooders were gathered at the corner of the hapa by means of bamboo and mouth of female’s tilapia were checked one by one to collect fertilized eggs. These eggs were collected from the mouth of incubating females weekly. After that, these eggs were cleaned and then stocked in incubatory jars separately for further development and hatching process (Ahmed et al., 2007; Valeta et al., 2013). The quantity, length and weight of these eggs were noted. Each incubator was stocked with different densities like 1980, 1961, 1930, 1910, 1890, 810 and 512 eggs per jar. Hatched yolk-sac fry were transferred into rectangular plastic tubs for further development and egg yolk absorption.
Water quality parameters
Water temperature of the tanks were monitored at 08:00 AM and 04:00 PM at 10 cm and 30 cm depths in each hapa daily with mercury thermometer. Dissolved oxygen (DO) was determined at the same time with a portable test kit (Merck KGaA, 64271, Germany). The pH was found with waterproof pH meter (EzDO 6011, Taiwan) and ammonia was determined using portable test kits (Merck KGaA, 64271, Germany) on weekly basis.
Statistical analysis and calculation
Data on fecundity, fertilization of eggs, fry hatchability and its survival were evaluated by analysis of variance (ANOVA) using Minitab 17.0 version statistical software. Fertilization, hatchability, unfertility, survival and eggs per gram body weight were calculated by using the following formulae (Brian, 2015):
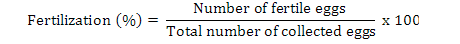
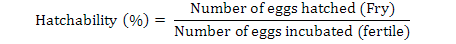
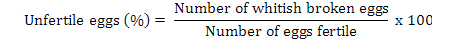
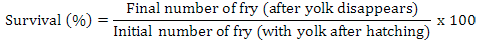
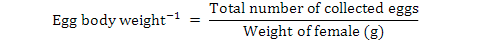
Results
Water quality
Water quality parameters are given in Table I. Water temperature did not vary more than one degree among replicates throughout the study period; ranging from 27.5°C to 28.6°C; mean 28.1°C. Salinity of the tank water ranged from 0% to 30%. No statistically significant difference (P>0.05) was observed in DO concentration (6.0 ml/l to 6.2 ml/l, mean 6.1±0.10 ml/l) of seawater in each experimental tank. There was no significant effect of introduced feed on pH of seawater in each tank. The pH values was found to be 6.5 to 6.7 with a mean of 6.6±0.1. Ammonia remained as 0.02±0.004 ml/l throughout the study period.
Fecundity, fertility, hatching and survival
Highest fecundity (number of eggs; Table II) was observed in T1, T2, T3, T4, and T5 (2065±4.2, 2030±4.2, 2010±4.5, 1995±4.5 and 1988±3.8, respectively) which was significantly different from T6 and T7 (995±21.0 and 700±21.0, respectively). Significant fertility of eggs were shown in T1 (1980±3.2), T2 (1961±3.1), T3 (1930±5.8), T4 (1910±5.5) and T5 (1900±11.0) and decreased in T6 and T7 (810±11.0, 512±4.7), respectively, as shown in Table II and Figure 1. Similar condition was found for the hatchlings in the treatments. Maximum survival of fry was achieved in low salinity groups (Table I, Fig. 1). Fertilization, hatchability and survival rates were significantly higher in T1, T2, T3, T4, and T5 than those of T6 and T7 groups (Table II). The quantity of fertilized eggs hatched in female’s mouth were not significantly different among groups at 0%-20% salinity level and above this level they were found to be inversely proportional to 30% salinity level (Table II). Number of eggs in a gram of fish body weight were recorded in all treatments; highest eggs were obtained per female, i.e. T1 = 4.50, T2 = 4.50, T3 = 4.40, T4 = 4.40 and T5 = 4.0 (Table III).
Development stages
Embryonic development was divided up to six periods i.e., from zygote to cleavage (Fig. 2A), blastula (Fig. 2B), gastrula (Fig. 2C), pharyngula (Fig. 2D), hatching (Fig. 2E, F), larval (Fig. 2G, H) and juvenile (Fig. 2I, J). The zygote post fertilization (dpf) characterized through cytoplasmic continues growth to make the blastodisc at animal
Table I.- Water quality parameters recorded throughout the study period.
|
Salinity (‰) |
Temp. (°C) |
Dissolved oxygen (ml/L) |
p.H |
Ammonia(ml/L) |
| 0 |
28.0 ± 0.7 |
6.2 ± 0.09 |
6.6 ± 0.03 |
0.02 ± 0.001 |
| 5 |
28.0 ± 0.5 |
6.2 ± 0.05 |
6.6 ± 0.04 |
0.02 ± 0.001 |
| 10 |
28.2 ± 0.6 |
6.1 ± 0.06 |
6.8 ± 0.06 |
0.02 ± 0.002 |
| 15 |
28.0 ± 0.7 |
6.2 ± 0.05 |
6.6 ± 0.02 |
0.02 ± 0.001 |
| 20 |
28.3 ± 0.6 |
6.0 ± 0.09 |
6.7 ± 0.04 |
0.03 ± 0.002 |
| 25 |
28.4 ± 0.7 |
6.0 ± 0.08 |
6.9 ± 0.04 |
0.03 ± 0.001 |
| 30 |
28.0 ± 0.5 |
6.2 ± 0.04 |
6.6 ± 0.05 |
0.02 ± 0.002 |
Values are mean ± standard error of two replicates.
Table II.- Morphometric and breeding performance of red tilapia (Hybrid) on different salinity during 60 days.
|
Salinity |
|||||||
|
T1 (0‰) |
T2 (5‰) |
T3 (10‰) |
T4 (15‰) |
T5 (20‰) |
T6 (25‰) |
T7 (30‰) |
|
| Morphometric parameters | |||||||
| Weight (g)/ female |
145.7±2.16a |
145.0±2.65b |
145.5±2.20a |
145.0± 2.60b |
145.2± 2.60b |
145.1 ±2.60b |
145.4 ±2.60b |
| TL (cm)/ female |
18.5±0.75a |
18.0±0.60b |
18.6±0.43a |
18.1± 0.29b |
18.1± 0.30b |
18.0 ±0.55b |
18.3 ±0.65b |
| SL (cm)/ female |
14.6±0.35a |
14.0±0.37b |
14.5±0.34a |
14.0± 0.36b |
14.6± 0.34a |
14.4 ±0.33b |
14.6 ±0.37a |
| Body depth (cm) |
7.6±0.15a |
7.1±0.14b |
7.5±0.16a |
7.2± 0.14b |
7.3± 0.14b |
7.2 ±0.12b |
7.5 ±0.14a |
| Breeding parameters | |||||||
| Total eggs |
2065±4.2a |
2030±4.2a |
2010±4.5a |
1995± 4.5a |
1988± 3.8a |
995± 21.0b |
700 ±21.0b |
| Total fertile eggs |
1980±3.2a |
1961±3.1a |
1930±5.8a |
1910± 5.5a |
1900± 11.0a |
810± 11.0 a |
512 ±4.7b |
| Total unfertile eggs |
85±4.4c |
69±4.4c |
80±9.6b |
85± 4.4c |
88± 4.1c |
185± 4.4c |
188 ±7.3a |
| Total hatchlings |
1584±13.1a |
1568±13.2a |
1540±8.3a |
1528± 12.5a |
1512 ±5.0a |
380± 12.4a |
142 ±5.0b |
| Total fry |
1108±5.1a |
1100±5.3a |
1090±9.6a |
1070± 10.6a |
1058 ±7.7a |
185± 7.4a |
75 ±3.2b |
| Fertilization % |
95.9±0.0a |
96.6±0.0a |
96.0±0.0a |
95.7± 0.0a |
95.6 ±0.0b |
81.4± 0.0a |
73 ±0.0c |
| Hatchability % |
80.0±0.0a |
80.0±0.0a |
80.0±0.0a |
80.0± 0.0a |
80± 0.0a |
47.0± 0.0a |
27.7 ±0.0c |
| Survival % |
70.0±0.0b |
70.1±0.0a |
70±0.0a |
70.8± 0.0a |
70.0± 0.0b |
48.7± 0.0a |
52.8 ±0.0c |
Different superscripts in the same row are significantly different (P< 0.05). Values are mean ± standard error of two replicates. TL, total length; SL, standard length.
Table III.- Fertilized eggs of red tilapia on different salinity levels.
|
Salinity (‰) |
Total length (cm) |
Total weight (g) |
Fertilized eggs/Female |
Fertilized eggs/ (g)⃰ |
| 0 |
18.5 ± 0.75 |
145.7±2.16 |
660 ± 7.0 |
4.50 ± 0.14 |
| 5 |
18.0 ± 0.60 |
145.0±2.65 |
654 ± 3.1 |
4.50 ± 0.13 |
| 10 |
18.6 ± 0.43 |
145.5 ± 2.20 |
643 ± 3.0 |
4.40 ± 0.13 |
| 15 |
18.1 ± 0.29 |
145.0 ± 2.60 |
637 ± 1.4 |
4.40 ± 0.12 |
| 20 |
18.1 ± 0.30 |
145.2 ± 2.60 |
630 ± 1.4 |
4.30 ± 0.12 |
| 25 |
18.0 ± 0.55 |
145.1 ± 2.60 |
270 ± 1.4 |
1.9 ± 0.1 |
| 30 |
18.3 ± 0.65 |
145.4 ± 2.60 |
171 ± 1.3 |
1.2 ± 0.1 |
Different letters in the same row represent significant difference (P< 0.05). The aforementioned values are mean ± standard error. ⃰ Number of eggs per gram=total number of eggs/weight of female (g).
– cleavage period (0–1-4 h post fertilization (hpf), 1 day pole and the arrival of a different peri vitelline space and cleavage stage categorized through a series of mitotic partitions, caused in several blasto-meres. Blastula stage started from fourth hour to twentieth hour after post fertilization, categorized with two separate layers of the blastoderm, an external enveloping coating and a supplementary yolk syncytial layer. Gastrula stage took place from 20th h up to 40th h after post fertilization (2dpf) which is described as germ ring presence surrounding the margin of blastoderm and embryonic cover, that metamorphic from germ ring to animal pole to forming neural duct. Pharyngula stage starts after 40th h till 88th h after post fertilization (2-4 dpf) characterized with rudiment pharyngeal arches, which were present, but difficult to differentiate independently at earlier period. Hatching stage started on 88th h till 116th h after post fertilization (4-5 dpf) which is described as morphogenesis in pharyngeal skeleton. The development of larval stage starts after the hatching till the yolk sac absorption. Development of lava
Table IV.- Developmental stages of red tilapia during the study period.
| Developmental stages |
Hours post- fertilization |
Days post- fertilization |
|
| Embryo | Cleavage (2–32 cells) |
2-4 |
1 |
| Blastula |
4–20 |
1 |
|
| Gastrula |
20-40 |
2 |
|
| Pharyngula |
40-88 |
2-4 |
|
| Hatching |
88–116 |
4-5 |
|
| Larva | Early larva |
116–140 |
5-6 |
| Late larva |
208–274 |
9-12 |
|
| Juvenile | Early juvenile |
306-352 |
13-15 |
| Late juvenile |
552- 672 |
23-28 |
slowly and gradually started to move its jaws, pectoral fins and opercula flaps. It categorized with expansion of swim-bladder and pharyngeal boney structure prior to starting external feeding this stage started from 116th till to 274th h after post fertilization (5-12 dpf). Juvenile development began after larval stage to develop all body parts and look like parents until the first maturation of gametes and started from 306th to 672nd hours after post fertilization (13-28 dpf) (Table IV).
Discussion
The present research provides information about ability to produce maximum eggs of red tilapia on different salinity levels in captivity. Maximum fertilization of eggs (94.6-96.6%) was obtained on salinity level of 0% to 20%. These results were more or less similar to each other and were greater than the results of Brian (2015). He acquired 82%-85% fertilized eggs from Oreochromis niloticus. Rodriguez et al. (2015) found 66.7%, 71.8% and 65% fertilized eggs on different salinity levels i.e. 0%, 5% and 15% in red tilapia, which are lower than those of the present study. Rehman et al. (2015) reported 67%–81% fertilization rate with HCG+HMG and HCG + Ovaprim artificial stimulating hormones on snakehead fish (Channa marulius) which is lower than the first five treatments (0% - 20%) and in contrast with the treatments 6 and 7 (25% - 30%) of this study. A similar association among salinity and egg production was advocated by Akinwande et al. (2012) while studying fish breeding stages in well-ordered hatchery environments. They achieved 80% fertilization of Clarias species (intraspecific hybrid). These results are also lower from treatment 1-5 of the present study. Some other observations are also on record on the influence of different salinity concentration on fish breeding. For instance, Martins et al. (2015) while studying the effect of salinity on artificial reproduction of silver catfish (Rhamdia quelen) found 85% – 93% fertilization. A similar conclusions were also reached by Hakim et al. (2008) who documented 90% – 97% fertilization in common carp.
In the present study, hatching rate in treatments 1-5 remained 80% which was significantly higher than that of the treatments 6 and 7 ranging from 47% to 27.7%. These findings are in conformity with those of Akinwande et al. (2012) who found hatchability rate 79.1%–83.3% in Clarias spp. al low salinity levels. The trend of hatching rate for Nile tilapia agree with those of Almeida et al. (2013). In the study of Martins et al. (2015), highest hatching rate (83.3%) were found at zero salinity for silver catfish, Rhamdia quelen; although this is in contrast with the present results of treatment 1, 2, 3, 4 and 5 while and were higher than treatment 6 and 7. Young-Sulem et al. (2008) obtained maximum hatchability (65.3%) at various turbidity levels for Clarias gariepinus at salinity of 0%-20%.
Survival rate of fry were 70%–70.8% among 1-5 treatments while 48.7%-52.8% were obtained on treatment 6-7. These results contradicted with the observations of Hakim et al. (2008). They studied survival rate of common carp fry on different concentration of urea + NaCl. According to Brian (2015), higher survival rate (71.4 %) was obtained in Nile tilapia fry with red background color. Olufeagba and Okomoda (2015) while studying genetic improvement of Heterobranchus longifilis through intraspecific hybridization of different strains from Nigeria, indicated that survival remained 10.47% - 90.4% on parental and experimental crosses in H. longifilis similar to the findings of this study.
The number of eggs per gram body weight were obtained as 1.2 – 4.5 in the present study which is in between with the pervious results of Ahmed et al. (2007). They obtained 1-5 eggs per gram body weight in tilapia Niloticus. Fujimura and Okada (2007) mentioned that embryonic development took 552-672 h post fertilization (hpf) period and 23-28 days post fertilization (dpf) period for the same species. However, some variations in the results of the present study might have been due to climatically and geographical changes or might be due to environmental factors. Water quality factors such as temperature (28.1±0.2°C), dissolved oxygen (6.10±0.1 ml/l), pH (6.6±0.1) and ammonia (0.02±0.004 ml/l) were similar to the recommended values throughout the study period (Ahmed et al., 2007; Valeta et al., 2013; Khalfalla et al., 2008; Nandlal and Pickering, 2004; Hussain, 2004; Tahoun, 2007; Daudpota et al., 2016).
Conclusion
In this study, it can be suggested that red tilapia breed successfully up to 20% salinity and give maximum survival rate of fry. Due to climate change and sea intrusion our agricultural land particularly near the coast of Sindh become saline, due to which agriculture production may be effected as well. These areas may be utilized for fish farming to overcome the protein deficiency especially animal origin and will be the source of income for the peoples of these areas. In this way, our aquaculture sector will be promoted.
Acknowledgements
The senior author is grateful to the Higher Education Commission, Islamabad for providing fellowship to complete this work as a part of his Ph.D. research. He is very much thankful to Mr. Muhammad Hanif Soomro, owner of Sunbright red Tilapia and Ornamental Hatchery for giving hatchery facilities to conduct this study smoothly.
Conflict of interest statement
We declare that we have no conflict of interest.
References
Ahmadi, N., Baroiller J.F.D., Cotta, H. and Morillon, R., 2015. Adaptation to salinity. In: Climate change and agriculture Worldwide (ed. E. Torquebiau), Springer, pp. 45-58.
Ahmed, A.M., Abdalla, S. and George, T.T., 2007. Egg enumeration, incubation, hatching and development of the “Miracle Fish” Oreochromis niloticus in the Sudan. Aquacult. Can., AAC Spec. Publ., 12: 60-64.
Akinwande, A.A., Fagbenro, O.A. and Adebayo, O.T., 2012. Fertilization, hatchability, survival and larval biometry in interspecific and intergeneric hybridization in heterobranchus longifilis, clarias gariepinus and clarias anguillaris under controlled hatchery conditions, Elixir Aquacult., 43: 6696-6700.
Almeida, D.B., Da Costa, M.A.P., Bassini, L.N., Calabuig, C.L.P., Moreira, C.G.A., Rodrigues, M.D.N., Pe´rez, H.J., Tavares, R.A., Varela, Jr, A.S. and Moreira, H.L.M., 2013. Reproductive performance in female strains of Nile tilapia, Oreochromis niloticus. Aquacult. Int., 21: 1291-1300. https://doi.org/10.1007/s10499-013-9630-0
Brian, O., 2015. Effect of tank background colour on the hatchability of Orochromis niloticus eggs and survival of fry. Int. J. Fish. aquat. Stud., 2: 81-86.
Chaughtai, M.I., Mehmood, K. and Awan, R., 2015. Growth performance of carp species fed on salt-tolerant roughages and formulated feed in brackish water under polyculture system. Pakistan J. Zool., 47: 775-781.
Chowdhury, D.K., 2011. Optimal feeding rate for Nile tilapia (Oreochromis niloticus), M.Sc. thesis, Department of Animal and Aquacultural Sciences, Norwegian University of Life Sciences, pp. 76.
Cnaani, A. and Hulata, G., 2011. Improving salinity tolerance in tilapias: past experience and future prospects. Isr. J. Aquacult. Bamidgeh, 63: 1-21.
Daudpota, A.M., Abbas, G., Kalhoro, I.B., Shah, S.S.A., Kalhoro, H., Rehman, M.H. and Ghaffar, A., 2016. Effect of feeding frequency on growth performance, feed utilization and body composition of Nile tilapia, Oreochromis niloticus cultured in low salinity water. Pakistan J. Zool., 48: 171-177.
Daudpota, A.M., Siddiqui, P.J.A., Abbas, G., Narejo, N.T., Shah, S.S.A., Khan, N. and Dastagir, G., 2014. Effect of dietary protein level on growth performance, protein utilization and body composition of Nile tilapia cultured in low salinity water. Int. J. Int. Mult. Stud., 2: 135-147
FAO, 2014. The state of world fisheries and aquaculture 2014, Rome, pp. 223.
Fujimura, K. and Okada, N., 2007. Development of the embryo, larva and juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae), Developmental staging system. Develop. Growth Different, 49: 301-324. https://doi.org/10.1111/j.1440-169X.2007.00926.x
Hakim, A.E., Gamal, E. and Zeinab, A. and Greisy, E., 2008. Effect of removal of egg adhesiveness on hatchability and effect of different levels of salinity on survival and larval development in common carp, Cyprinus carpio. J. appl. Sci. Res., 4: 1935-1945.
Hussain, M.G., 2004. Farming of tilapia: Breeding plans, mass seed production and aquaculture techniques, pp. 149.
Iqbal, K.J., Qureshi, I.A., Ashraf, M., Rehman, M.H.U., Khan, N., Javid, A., Abbas, F., Mushtaq, M.M.H., Rasool, F. and Majeed, H., 2012. Effect of different salinity levels on growth and survival of Nile tilapia (Oreochromis niloticus). J. Anim. Pl. Sci., 22: 919-922.
Jaspe, C.J.C. and Caipang, M.A., 2011. Small-scale hatchery and larval rearing techniques for local strains of saline tolerant tilapia, Oreochromis spp. ABAH Bioflux, 3: 71-77.
Kevin, F., Janjua, R.S.N. and Ashraf, M., 2015. Aquaculture handbook, fish farming and nutrition in Pakistan, pp. 441.
Khalfalla, M.M., Hammouda, Y.A., Tahoun, A.M. and Abo-State, H.A.M., 2008. Effect of broodstock sex ratio on growth and reproductive performance of blue tilapia Oreochromis aureus (Steindachner) reared in hapas. Proceedings of 8th International Symposium on Tilapia in Aquaculture, pp. 115-124.
Martins, G.B., Sergio, R.N.P., Juvencio, L.F.P., Denise C.B. and Ricardo, B.R., 2015. Salinity on artificial reproduction of silver catfish (Rhamdia quelen), Ciência Rural San Maria, 45: 458-463.
Mateen, A., Afzal, M., Ahmad, I. and Hafeez-ur-Rehman, M., 2004. Salinity tolerance of Rohu (Labeo rohita) and its hybrid under different temperature regimes. Int. J. agric. Biol., 6: 1030-1032.
Nandlal, S. and Pickering, T., 2004. Tilapia fish farming in Pacific Island countries, Volume 2, Tilapia grow-out in ponds. Secretariat of the Pacific Community, Noumea, New Caledonia, pp. 49.
Olufeagba, O. and Okomoda, V.T., 2015. Preliminary report on genetic improvement of Heterobranchus longifilis through Intraspecific Hybridization of different strains from Nigeria. J. Aquacult. Eng. Fish. Res., 1: 45-48. https://doi.org/10.3153/JAEFR15004
PCAMRD (Philippine Council for Aquatic and Marine Research and Development), 1998. Tilapia culture. Currents, 3: 13.
Rehman, H.R., Ashraf, M., Abbas, F., Iqbal, K.J., Qureshi, I.A. and Andleeb, S., 2015. Effect of different synthetic hormones and/or their analogues on induced spawning in Channa marulius. Pakistan J. Zool., 47: 745–752.
Rodriguez-Montes De Oca, G.A., Román-Reyes, J.C., Alaniz-Gonzalez, A., Serna-Delval, C.O., Muñoz-Cordova, G. and Rodríguez-González, H., 2015. Effect of salinity on three tilapia (Oreochromis sp.) strains: hatching rate, length and yolk sac size. Int. J. aquat. Sci., 6: 96-106.
Ronald, N., Gladys, B. and Gasper, E., 2014. The effects of stocking density on the growth and survival of Nile Tilapia (Oreochromis niloticus) fry at Son Fish Farm, Uganda. J. Aquacult. Res. Dev., 5: 222. https://doi.org/10.4172/2155-9546.1000222
Tahoun, A.M.A., 2007. Studies on some factors affecting the production and reproduction of Nile tilapia. PhD. Dissertation. Fac. of Agriculture, Kafr El-Sheikh University, Egypt, pp. 223.
Valeta, J.S., Likongwe, J.S., Kassam, D. and Maluwa, A.O., 2013. Temperature-dependent egg development rates, hatchability and fry survival rate of Lake Malawi Tilapia (Chambo), Oreochromis karongae (Pisces: Cichlidae). Int. J. Fish. Aquacult., 5: 55-59.
Yong-Sulem, S., Brummett, R.E. and Tchoumboué, J., 2008. Hatchability of African catfish, Clarias gariepinus eggs in hapas and in basins: a diagnostic study of frequent inhibition by rainfall and water stagnation. Tropicultura, 26: 39-42.
To share on other social networks, click on any share button. What are these?










