Physicochemical Properties of Dairy Creamer Stabilized by Hydrolyzed Goldband Snapper (Pristipomides Multidens) Visceral Oil
Research Article
Physicochemical Properties of Dairy Creamer Stabilized by Hydrolyzed Goldband Snapper (Pristipomides Multidens) Visceral Oil
Ria Dewi Andriani1, Lilik Eka Radiati1, Teti Estiasih2*
1Department of Animal Product Technology, Faculty of Animal Science Universitas Brawijaya, Jl. Veteran, Ketawanggede, Lowokwaru, Malang, East Java, Indonesia; 2Department of Food Science and Biotechnology, Faculty of Agricultural Technology, Universitas Brawijaya, Jl. Veteran, Ketawanggede, Lowokwaru, Malang, East Java, Indonesia.
Abstract | Dairy creamer is a liquid or powder milk product for milk substitution or cream in coffee, tea, chocolate, or cakes. The dairy creamer powder should be stable after reconstituting in water and have a good stability emulsion. Therefore, emulsifiers are important ingredients in dairy creamer preparation to prevent separation. Emulsifiers with good emulsifying and stabilizing properties will improve the stability and consistency of dairy creamer. Usually, emulsifiers used for dairy creamer are mono and diglycerides that produced from edible oils and fats such as sunflower, soybean, and palm oil. These common sources usually lack of health beneficial omega-3 fatty acids. This study evaluated the capability of hydrolyzed goldband snapper (Pristipomides multidens) visceral oil (HGSVO) as the omega-3 fatty acid containing emulsifier for dairy creamer produced by spray drying method. This HGSVO was obtained by hydrolyzing the goldband snapper oil with goldband snapper visceral lipase. The glycerides composition of HGSVO was monoglyceride, diglyceride, triglyceride, glycerol, and free fatty acids, and contained omega-3 fatty acids comprising of docosahexaenoic acid (DHA) of 17.62% and eicosapentaenoic acid (EPA) of 3.60%. HGSVO was formulated in dairy creamer at concentrations of 0.2%, 0.4%, and 0.6% (w/v). The results showed that the differences formulated of HGSVO in dairy creamer had a significant effect (p<0.05) on moisture content, emulsifying activity index (EAI), emulsion stability index (ESI), and solubility, which increased from HGSVO concentration of 0.20% to 0.40%, but decreased at 0.60%. Meanwhile, increasing HGSVO concentration decreased the particle size. This study did not include the sensory analysis and consumer acceptance as a new developed dairy creamer stabilized with HGSVO. Consumer acceptance is very important for the further study in comparison with commercial dairy creamer.
Keywords | Emulsifying activity, Emulsion stability, Hydrolyzed visceral oil, Particle size, Reconstituted dairy creamer
Received | June 20, 2024; Accepted | July 19, 2024; Published | August 12, 2024
*Correspondence | Teti Estiasih, Department of Food Science and Biotechnology, Faculty of Agricultural Technology, Universitas Brawijaya, Jl. Veteran, Ketawanggede, Lowokwaru, Malang, East Java, Indonesia.; Email: [email protected]
Citation | Andriani RD, Radiati LE, Estiasih T (2024). Physicochemical properties of dairy creamer stabilized by hydrolyzed goldband snapper (pristipomides multidens) visceral oil. Adv. Anim. Vet. Sci. 12(9): 1792-1801.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.9.1792.1801
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Dairy creamer also known as coffee creamer or simply creamer is a milk product in liquid or powdered form. Dairy creamers are the substitutes for milk or cream for coffee, tea, chocolate, or cake to lighten or sweeten the flavor. Dairy creamer is mostly used to enrich the creaminess of coffee, therefore increasing coffee consumption is accompanied by the increased demand of creamer. The world demand on coffee is projected to grow by 2.99% (2024-2029) (Statista, 2024). Coffee creamer is used to create a creamy texture, smoother and making it more palatable. The addition of creamers to coffee performs several functions, there are reduce bitter taste, whitening color, contribute to the coffee with a cream-like flavor and give body to the coffee (Euston, 2010).
It is important that creamers added to hot coffee solutions have good stability against de-oiling and sedimentation. Destabilization of creamer in coffee solution may be considered unappealing with white flakes or curds float on the surface or sediments forms at the bottom of coffee (Chung et al., 2017). Therefore, emulsifiers are an important ingredient in dairy creamer manufacture and help to prevent the separation of the creamer from its constituent parts, such as water and fat. Emulsifiers with good emulsifying and s
tabilizing properties can improve stability and consistency of dairy creamer. Rosida et al. (2016) reported that emulsifiers are substances that help blend two or more immiscible liquids, such as oil and water, into a stable mixture, maintain consistent texture and appearance, improve the creaminess and enhance sensory experience.
One of the emulsifiers used in dairy creamers is monoglyceride, which is generally recognized as safe (GRAS) by regulatory authorities when used in food products. Fredrick et al. (2013) stated that the utilization of unsaturated monoacylglycerol in dairy recombined cream can reduce the churning time, overrun, serum loss, and increase the firmness of whipped cream. In addition, the use of monoglyceride in mayonnaise production showed the highest stability and decreases with decreasing amount of monoglyceride used (Erdogan et al., 2019). (Loi et al. (2019) reported that unsaturated monoglyceride can be used to manipulate the droplet size distribution of the emulsion, providing emulsion stability and prevent creaming compared to saturated monoglyceride. Mono- and diglycerides are used concomitantly in dairy creamer and able to prevent feathering and destabilization of creamer emulsion. Creamer powder is added to hot coffee should have good stability against feathering, sedimentation, or de-oiling. The instability leads to unappealing with curd floating or white flake on the surface, or sediments in the bottom (Chung et al., 2017).
The amphiphilic nature of monoglycerides and diglycerides, containing hydrophilic hydroxyl and hydrophobic long-chain alkyl groups, contributes to their emulsifying properties (Sudibyo et al., 2020; Liu et al., 2023), which allows it to interact with both oil and water function to obtain stable emulsion. These properties are further enhanced by the aliphatic lipophilic chain and hydroxyl groups in their chemical structure, making mono- and diglycerides highly valuable when added to the dairy creamer. The hydrophobic and hydrophilic properties of mono- and diglycerides are crucial in stabilizing emulsions, particularly in products with different densities between water and oil. Water phase and oil globule lead to ring formation on the surface of the emulsion known as creaming (Samios et al., 2013; McClements, 2015). The use of low molecular weight of emulsifier like mono- and diglyceride is found to affect emulsion stability due to the capability to displace milk protein from the oil water interface. Their ability to displace milk proteins from the oil-water interface influences emulsion stability, making them valuable components in formulations requiring stable emulsions (Pace et al., 2010; Radzi et al., 2022). Mono- and diglyceride production can be carried out by hydrolysis, esterification of glycerol with fatty acids, and glycerolysis using catalysts such as MgO and Ca (OH)2 requires a high reaction temperature of 220 to 250ºC.
Pure solely mono- and diglycerides are sometimes used in food products together. Both emulsifiers are produced by interesterification of glycerol with triglycerides, and then purified with molecular distillation (Chen, 2015). The sources of triglycerides for mono- and diglycerides synthesis are vegetable oils (Nitbani et al., 2020) that lack of main health beneficial omega-3 fatty acids of EPA and DHA. The use of EPA and DHA containing oils as the materials for mono- and diglycerides preparation will provide mono- and diglycerides containing EPA and/or DHA. So far, the conventional sources of EPA and DHA are fish body oils, and exploring unconventional fish oil sources is a challenge. Among fish oils, fish visceral oil is still limitedly used in many fish oil application. Fish visceral oil contains higher polyunsaturated fatty acids than that from muscle and head fish (Rohim et al., 2024). One of the aims to hydrolyze of fat or oils is to obtain mono- and diglycerides (Menalla et al., 2024). The advantage for this hydrolysis is producing the mixture mono- and diglycerides that contribute as emulsifiers and the hydrolysis of fish visceral oil will produce EPA and DHA containing emulsifier.
In this study, the emulsifier for dairy creamer was HGSVO, which was prepared from goldband snapper fish visceral oil. This oil was supposed to be rich in omege-3 fatty acids, mainly DHA and EPA. Putri et al. (2021) reported that fish oil from red snapper head have polyunsaturated fatty acids of 21.22%. Goldband snapper visceral oil was hydrolyzed using semi purified lipase extract from the viscera of goldband snapper. The ability of this hydrolyzed fish visceral oil to stabilize dairy creamer has not been evaluated yet. This study aimed to evaluate potential of HGSVO as an emulsifier and the effect of HGSVO concentration on the physicochemical properties of dairy creamer prepared by spray drying method.
MATERIALS AND METHODS
Research Materials
Goldband snapper viscera was obtained from a seafood processing industry, BeeJay Seafood, at Probolinggo, Esat Java, Indonesia. The ingredients for making creamer were maltodextrin DE 10-12, sodium caseinate, skim milk powder, anhydrous milk fat, and dipotassium hydrogen phosphate. All ingredients for dairy creamer are food grade. The chemicals used for emulsion stability index and emulsifying activity index analysis were analytical grade.
Goldband Snapper Visceral Oil Extraction
Goldband snapper oil was prepared by extraction of goldband snapper visceral using method proposed by Bonilla-Méndez and Hoyos-Concha (2018) with slight modification. The visceral was crushed using chopper. The crushed viscera than defatted using 1:30 (w/v) acetone, and stirring it in the plate stirrer for 45 minutes. The acetone than filtered using filter paper to remove the residual viscera. The defatting was continued until no yellowish color in acetone phase, marked all the visceral oil already extracted. The acetone phase than evaporated using rotary vacuum.
Hydrolyzed Goldband Snapper Oil Preparation
Hydrolyzed goldband snapper oil was prepared using a method described by Junior et al. (2018) by mixing goldband snapper oil and distilled water with the concentration of 1:4 (v/v). The mixture than homogenized using ultra turrax homogenizer. The pH emulsion than adjusted using phosphate buffer (pH 8.0) until it reaches pH 7.2, as the optimum pH of semi-purified lipase extract from goldband snapper viscera. After that, the 1% of SPLE was added to the mixture and homogenized by hand stirring. The hydrolysis was carried out in shaker water bath at 40°C and 100 rpm speed for 16 hours. After hydrolysis, the emulsion was separated using separation chamber to separate the upper phase (oil phase), middle phase (HGSVO phase) and lower phase (water or polar phase). This HGSVO contained of DHA 17.62% and EPA 3.60%. The composition of this HGSVO was 22.80% monoglyceride, 24.22% diglyceride, 21.46% triglyceride, 17.26% glycerol, and 14,27% free fatty acids.
Dairy Creamer Preparation
Dairy Creamer Emulsion Preparation: The dairy creamer emulsion was prepared using a method described by Hedayatnia and Mirhisseini (2018), initially, a mixture (A) comprising of sodium caseinate (2.5% w/w), dipotassium hydrogen phospate (2.5% w/w) and skim milk powder (7% w/w) prepared by dispersing them on 30 ml of 70°C distilled water and rotated in a hot plate stirrer for 5 min. A mixture (B) was prepared by dissolving 25% w/w of maltodextrin on 100 ml in a hot plate stirrer. Mixture (C) also prepared by mixing the anhydrous milk fat (8% w/w) with HGSVO with different concentrations (0.2%, 0.4% and 0.6% w/v). Subsequently, the mixture (A) was added to mixture (B) gradually, followed by the addition of mixture C and they were mixed using a hotplate stirrer for 5 minutes. All the mixture were homogenized using ultra turrax.
Dairy Creamer Spray Drying: The dairy creamer emulsion was fed into a lab scale spray dryer (Labfreez SD-18A). Before spraying, the emulsion was diluted with distilled water pH 7.0 until the viscosity reach below 4 mPa.s. The diluted emulsion than filtered through a filter cloth to eliminate the lump. Subsequently, the filtered emulsion was sprayed at the inlet temperature of 180°C, outlet temperature of 70°C, feed rate of 10% (4.5 ml/min), and 1 atm pressure, and concomitantly heated in a hotplate stirrer to keep the low viscosity.
Dairy Creamer Reconstitution: An emulsion preparation of reconstituted dairy creamer was conducted by dissolving the dairy creamer powder in water. Briefly, 1 g of powdered dairy creamer was dissolved in a 100 ml of water, then homogenized using a plate stirrer at room temperature.
Characterization of Dairy Creamer Powder
Moisture Content: The moisture content of non-dairy creamer was measured according to the oven-drying method (AOAC, 1997). The sample was weighed in a crucible and dried to a constant weight in an oven at 105°C. Then the moisture content was calculated according to the mass loss of powder. The calculation for moisture content were calculated using following equation:
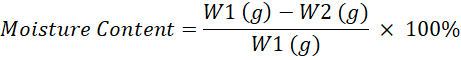
Particle Size: Particle size analysis was measured the method with some modifications explained by Kastner and Perrie, (2016), Sample was prepared by dilution of 0.1 g sample to 10 ml aquadest solution. 650 µL of samples were placed into omega cuvette and the particle size was read using Anton Paar Particle Size Analyzer Literizer by dynamic light scattering. The measuring range set from 0.3 nm to 10µm with the accuracy and repeatability is better than ±2% on NIST traceable standard.
Bulk Density: Bulk density of dairy creamer was measured using the method by Bae and Lee (2008), with minor modifications. Dairy creamer was placed to a measuring cylinder of known weight (W0) until it reaches known volume of the measuring cylinder (Vo). The cylinder was gently tapped to a flat surface to reach a constant volume until the sample have a flat surface. A measuring cylinder containing sample than weighed using analytical balance. The total weight recorded as (W1). The bulk density was calculated using following formula:
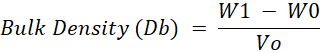
W1= Total weight
W0= Known weight
Vo= Volume of the measuring cylinder
Water Solubility: Determination of solubility referred to the (Pomeranz and Meloan, 2013) method by calculating the weight of the insoluble residue in a solution. Solubility was measured by dissolving 2 g of sample in 100 mL of distilled water and then filtering it with Whatman filter paper No. 42 which had previously been dried in an oven at 105℃ for 30 minutes and weighed (b). After filtration, the filter paper used was dried in an oven at 105℃ for 3 hours and then weighed (c) or until the weight was constant. Solubility is calculated as the percentage of the weight of the residue remaining on the filter paper to the weight of the sample. Solubility was calculated using the formula:
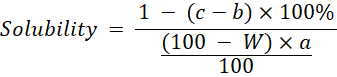
a= Weight of sample (g)
b= Weight of filter paper (g)
c= Weight of filter paper and residu (g)
W = Moisture content of sample (% wb)
Characterization of Reconstituted Dairy Creamer
Emulsifying Activity Index (EAI): Emulsifying activity index is measuring the surface area that stabilized by an emulsifier. The EAI was analyzed from the reconstituted dairy creamer emulsion. Emulsifying activity index refers to the method of (Zheng et al., 2014) by measuring the turbidity of the solution using UV-Vis spectrophotometry. 2 g of dairy creamer was dissolved in 20 ml of distilled water using magnetic stirrer at room temperature. A 15 ml of the sample was taken and 5 ml of oil was added then homogenized using a hand mixer for 1 min 0.1 ml of the mixture was taken with a micropipette and added with 0.1% SDS (sodium dodecyl sulfate) until the volume reached 10 ml then homogenized with a hand mixer for 1 minute. The absorbance of the solution was measured using UV-Vis spectrophotometry with a wavelength of 500 nm. The emulsifying activity index is calculated using the formula:
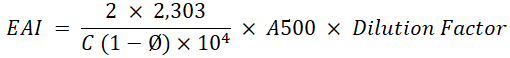
C= Emulsifier Concentration (g/mL)
Ø= Volume of oil fraction of the emulsion (v/v)
A500= Absorbance
Emulsion Stability Index (ESI): The emulsion stability indicates the ability of HGSVO to stabilize an emulsion. The ESI was analyzed from the reconstituted dairy creamer. Emulsion stability index determination referred to the method of El-Kheir et al. (2008) by measuring the turbidity of the solution using UV-Vis spectrophotometry. 2 gram of dairy creamer was dissolved in 20 ml of distilled water using a magnetic stirrer at room temperature. 15 ml of the sample was taken and 5 ml of oil was added then homogenized using a hand mixer for 1 minute. 0.1 ml of the mixture was taken with a micropipette and added with 0.1% SDS (sodium dodecyl sulfate) until the volume reached 10 ml then homogenized with a hand mixer for 1 minute. Solution was measured in UV-Vis spectrophotometry with a wavelength of 500 nm. The time first dissolved was calculated as T0 and the absorbance measured at that time was calculated as A0. Then the solution was left for 10 minutes at room temperature (T10) and the absorbance measured at that time was calculated as A10. The ESI is calculated as follows:
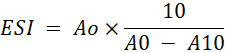
A0= Absorbance at T0
A10= Absorbance at T10
Statistical Analysis
This study used completely randomized design with three replications. The data was expressed as the average with deviation standard. The statistical analysis was conducted with one way analysis of variance (Anova) with a 95% interval using SPSS, and further analysis of significant treatment by Duncan test by using Minitab 17.
RESULTS AND DISCUSSION
Characteristics of Dairy Creamer Powder
Moisture Content: Increasing HGSVO concentration during creamer emulsion preparation did not always devcrease moisture content. The moisture content of HGSVO stabilized dairy creamer was ranging from 4.76 to 5.30%. These values were higher than that reported by Liu et al. (2023) that the non dairy creamer stabilized with diglycerides from coconut oil was 1.78%. The higher moisture content presumably was related to the composition of HGSVO that not only comprised of mon- and diglycerodes but also contained glycerol which is very polar.
Table 1: Characteristics of dairy creamer and its reconstituted emulsions at different concentration of hydrolyzed goldband snapper visceral oil (HGSVO).
|
Characteristic |
HGSVO Concentration |
||
|
0.20% |
0.40% |
0.60% |
|
|
Diary creamer powder |
|||
|
Moisture content (%) |
5.30 ± 0.07a |
4.76 b± 0.07b |
5.29± 0.04a |
|
Average particle size diameter (чm) |
0.90 ± 0.19a |
0.72 ± 0.05a |
0.46 ± 0.01b |
|
Bulk density (kg/m3) |
320 ± 20 |
360 ± 10 |
350 ± 5 |
|
Solubility (%) |
96.31 ± 0.27b |
97.81 ± 0.78a |
95.57 ± 0.59b |
|
Reconstituted dairy creamer |
|||
|
Emulsifying activity index (EAI, m2/g) |
2.35 ± 0.04b |
2.82 ± 0.04a |
2.38 ± 0.05b |
|
Emulsion stability index (ESI, min) |
93.45 ± 3.10a |
108.06± 0.64b |
94.53 ± 1.00a |
Different superscript in the same column showed significantly difference (p<0.05).
The moisture content decreased at HGSVO concentration of 0.20 to 0.40% but further increased in concentration also increased the moisture content (Table 1). HGSVO comprised mainly of mono- and diglycerides which are amphiphilic in nature, thus possess a polar head group that could interact with water. The interactions between monoglycerides and water possibly make a formation of mesomorphic phases (Vereecken et al., 2010). Mono- and diglycerides had a polar head group with a hydrophobic tail so that this type of molecule can easily form a lamellar lyotropic liquid crystalline phase and can assemble with each other by itself in an oil-water mixture. Monoglycerides form a multilamellar crystal shell around the oil droplets so that it can be stable in the water matrix (Marangoni et al., 2008). Water in small quantities might be the present in the monoglycerides (Vereecken et al., 2010). The HGSVO concentration of 0.20% has the highest moisture content. At this concentration, HGSVO to some extent had the ability to bind free water. These lipids swell and rearrange into the aqueous-organic interface and enhance their surfactant properties (Tawfik et al., 2019). At HGSVO concentration of 0.40%, the moisture content is the lowest. It was supposed that the concentration of 0.40 produces the most stable emulsion indicating by the highest EAI and ESI. The HGSVO efficiently stabilized and covered anhydrous milk fat thus lowered their interaction with water. However, increasing HGSVO concentration to 0.60% also increased the moisture content. HGSVO also contained glycerol which is very polar and able to bind water (Scheppers and Muzenda, 2015). At high concentration, the concentration of glycerol also increased thus increasing moisture content.
Moisture content can also be influenced by the type of drying used, in this study spray drying was used. The high amount of maltodextrin used will also affect the moisture content because of its tendency to bind with free moisture or its high hygroscopic properties (Hedayatnia and Mirhosseini, 2018). If the moisture content exceeded the
concentration which can cause the lamellar phase swelling, then a lamellar dispersion can form. This phase has a structure like liposomes consisting of concentric bimolecular shells of monoglycerides and diglycerides alternating with water shells (de Walle et al., 2008).
Average Particle Size Diameter: Increasing HGSVO concentration decreased average particle diameter of dairy creamer powder particle significantly (p-value >0.05) (Table 1). It meant that higher HGSVO concentration produced smaller particle size. The particle size affected the solubility rate during dissolution in water (Awuchi et al., 2019). The smallest particle size was on HGSVO 0.6% (0.46 μm), followed by HGSVO 0.4% (0.72 μm). The largest was HGSVO 0.2% (0.90 μm). The particle size is directly influenced by the surface area, which is affected by the lipid sites at the oil-water interface (Liu et al., 2021). Unsaturated monoglycerides resulted in larger sizes particles and diameters (Méndez-Velasco et al., 2012). At low HGSVO concentration, the emulsifier of mono and diglycerides had a limitation to cover interfacial region in the emulsion. Méndez-Velasco et al. (2012) stated that there is a coalescence process occurs when emulsion droplets is uncovered properly leads to collide resulting a larger size formation. This can increase the average size over time (Awuchi et al., 2019). Emulsifiers like unsaturated monoglycerides can manipulate droplet size distribution in emulsions, providing stability and preventing creaming compared to saturated monoglycerides (Chung et al., 2017). In this study, HGSVO comprised mainly by unsaturated fatty acid including EPA and DHA, perhaps produced more stable emulsion and better dairy creamer particle.
The HGSVO concentration played a significant role in dairy creamer particle size. The small and uniform particle size produced a smooth and creamy product (Chaves et al., 2024). HGSVO is supposed to maintain the particle size stability of dairy creamer. Proper covered anhydrous milk fat by emulsifier and other amphiphilic ingredients in the creamer, such as caseinate, will protect the expose of the lipid phase into the particle surface. The uncovered anhydrous milk fat in dairy creamer will make the lower solubility in water during dairy creamer reconstitution. It is also let exposes of the fat to the environment that can lead to fat oxidation.
Hedayatnia and Mirhosseini (2018) reported particle size of 101.45 μm in reduced fat dairy coffee. Gassi et al. (2008) reported the cream particle size of 0.45 to 200 μm with 3 peaks with the median about 0.5 μm, 4 μm and 45 μm. The particle size of skim and whole milk powder was 83.87 and 128.76 μm (Pugliese et al., 2017). It can be concluded that the particle size of dairy creamer from this sudy is smaller than other dairy products, indicating the HGSVO was usitable to stabilized dairy creamer and produced tiny particles.
Bulk Density: Bulk density of HGSVO stabilized dairy creamer was not affected by HGSVO concentration (p-value >0.05) (Table 1). Bulk density is a fundamental parameter in materials science and crucial for characterizing solid materials. Bulk density is volumetric density or apparent density which is defined as the mass of many objects such as particles in a material divided by the total volume. The total volume includes the particle volume, internal pore volume and interparticle cavity volume (Awuchi et al., 2019). The food industry wants high bulk density because it can reduce packaging volume and reduce shipping costs. The higher the bulk density of the powder, the smaller the volume packaging or storage space required (Putri et al., 2016). Bulk density also depends on several factors such as geometry, size, solid density, and surface properties (Iwe et al., 2016), density, the amount of air trapped in the particles (occluded air) and the amount of air between the particles (interstitial air). Besides those factors, the drying process affects the quality, high or low, bulk density of the product, including inlet temperature and outlet temperature of spray dryer (Putri et al., 2016).
Data in Table 1 shows that bulk density tends to increase from HGSVO concentration of 0.20 to 0.40%, and further increasing concentration decreased the bulk density. Bulk density is an indicator of the powder compactness. Higher bulk density indicated that the powder was more compact. In this study, the bulk density may be affected by the moisture content. The highest bulk density was 360 kg/m3 at HGSVO concentration of 0.40%, which exhibited the lowest moisture content.
The bulk density of dairy creamer in this study was lower than other similar powder. Rosida et al. (2016) reported the bulk density of non-dairy creamer ranging from 550 to 650 kg/m3, depending on protein sources and sodium caseinate concentration. Ding et al. (2020) reported the commercial milk powder medium bulk density ranged from 409 to 494 kg/m3. Lower bulk density in this study possibly is related to the composition of the dairy creamer. The dairy creamer used maltodextrin as a filler in a considerable amount thus affected the bulk density significantly. Maltodextrin has a lower bulk density than milk protein, milk fat, and lactose as the major components in milk powder thus producing overall lower bulk density. The bulk density of maltodextrin was 330-490 kg/m3 (Takeiti et al., 2010), meanwhile, the bulk density of milk powder was 407–666 kg/m3. The increasing HGSVO concentration tended to increased bulk density, that meant more compact powder was produced. Smaller particle had less void among particles thus resulted in higher bulk density. According to Mohammed et al. (2019), bulk density may be affected by the particle size and also the moisture content of the materials.
Solubility:Solubility in water is a key parameter for the quality of dairy creamer. High solubility of dairy creamer is expected to ease the mixing of dairy creamer with hot coffee. The HGSVO concentration affected the solubility significantly (Table 1). Similar to moisture content and bulk density, the solubility increased at HGSVO concentration from 0.20 to 0.40%, and further in HGSVO concentration (0.60%) decreased the solubility. The highest solubility was 97.81% at HGSVO concentration of 0.60%, and the lowest was 95.57% at HGSVO concentration of 0.60%. HGSVO acted as emulsifier that facilitate the joining of anhydrous milk fat with water. In the dairy creamer particles, milk fat was covered by low molecular emulsifier HGSVO and caseinate. Bota were able to be a bridge between milk fat with water. Increasing HGSVO concentration to 0.40% increase the ability of the dairy creamer particle to interact with water, hydrated, and then dissolved, thus increased the solubility. However, at the higher HGSVO concentration, the solubility decreased due to the more non polar compounds from HGSVO were present such as free fatty acids and residual triglycerides. The presence of non polar lipids can reduce the water absorption capacity of a food which can lead reduce solubility (Awuchi et al., 2019).
High solubility in a food can effect on the food of high digestibility so that it is easy to digest and indicates excellent use as a formula in food (David et al., 2015). The solubility of dairy creamer in this study ranged from 95.57 to 97.81%. Rosida et al. (2016) reported the solubility of non dairy creamer was 32.33 – 42.05% depending of the leguminosae protein sources. This study revealed better solubility indicating HGSVO is suitable to stabilize dairy creamer.
Characterisctics of Reconstituted Dairy Creamer Emulsion
Dairy creamer is used by mixing with hot water and then pour into the hot beverages such as coffee or tea. It is also used by mixing the dairy creamer powder directly into hot drinks. This process produced and emulsion drink which is desirable to be stable during serving. Therefore, the analysis of dairy creamer emulsion after mixing with water is very important.
Emulsifying activity index (EAI): EAI is an indicator of the capability of HGSVO to produce emulsion that representing by the extent of interfacial area with the unit of m2/g. Table 1 showed that the HGSVO concentration affected the EAI significantly. Th increasing HGSVO concentration from 0.20 to 0.40% increased EAI, and then decreased at the concentration of 0.60%. HGSVO was able to stabilize emulsion and created interfacial area due to the presence of the amphiphilic nature of mono- and diglycerides. It is similar to milk, the presence of milk protein as one of dairy creamer ingredients also assisted in stabilizing the emulsion (Braun et al., 2019). At the higher low molecular surfactant concentration, the milk proteins were displaced from the interfacial region, which led to destabilization (Rouimi et al., 2005). This phenomenon occurred in reconstituted dairy creamer, which at high HGSVO concentration of 0.60%, the EAI decreased due to destabilization. The displacement of caseinate at the milkfat globule interface might take place. This displacement led to uncovered interfacial area which was susceptible to flocculation and then coalescence. This destabilization resulted in the interfacial area to decrease.
The highest EAI was observed at HGSVO concentration at 0.40% (2.82 m2/g) and the lowest at concentration of 0.20% (2.35 m2/g). Ay low HGSVO concentration of 0.20%, it seemed that the amount of HGSVO was not sufficient to stabilize fat globule interfacial are. HGSVO was not purified to have only mono- and diglycerides but also other fish visceral oil hydrolyzed produicts comprising of free fatty acids and glycerol, besides also contained unhydrolyzed triglycerides. Those compounds had no contribution for emulsion stabilization due to lack of amphipillic properties. The caseinate as the amphiphilic protein also contributed to stabilization of fat globules of reconstituted creamer. However, big molecular protein is less flexible than surfactants to migrate and self-arrange at the interfacial area of emulsions (Cabra et al., 2008; Zhang et al., 2022). Therefore, the contribution of HGSVO as an emulsifier was more significant.
The EAI of reconstituted dairy creamer was ranging from 2.35 to 2.82 m2/g. Lajnaf et al. (2020) reported EAI of camel sweet whey was 2 m2/g, and 4.33 and 4.87 m²/g for whey protein isolate and sodium caseinate. The high EAI was obtained from soybean hydrolysate stabilized fish oil emulsion of 13.17 m2/g (Padial-Domínguez et al., 2020). The untreated milk phospholipids emulsion had EAI of with 32.2 m2/g and the EAI of milk protein concentrate stabilized emulsion was 28.1 m2/g. After ultrasonic treatment, EAI increased to 55.3 m2/g and 53.4 m2/g respectively. The soybean oil emulsion stabilized with saturated distilled monoglyceride had the EAI value of 12.62 m2/g (Liu et al., 2020). This study revealed that HGSVO is able to stabilize reconstituted dairy creamer emulsion and exhibited low EAI due to low purity of emulsifier in the HGSVO.
Emulsion Stability Index (ESI): ESI is an indicator of emulsion stability due to destabilization process such as flocculation, coalescence, and creaming. Table 1 showed that the ESI significantly increased from HGSVO concentration from 0.20 to 0.40% and then decreased in further increased in HGSVO concentration. The most stable emulsion was obtained at HGSVO concentration of 0.40% (108.06 min) and least stable was observed at HGSVO concentration of 0.20% (93.45 min). The phenomenon of ESI was similar to ESI, which at HGSVO concentration of 0.20%, HGSVO was not sufficient to cover fat globule interface thus resulted in destabilization. At HGSVO concentration of 0.60%, the casein displacement from fat globule interface might to occur and produced uncovered interfacial area resulting in destabilization and decreasing ESI. One molecule of protein has some binding sites at fat globule interface in a polymeric form, thus covered wider regions than one molecule of surfactant (Relkin et al., 2002). The displacement of protein by the presence of high concentration of surfactant, such as HGSVO, creates to uncovered fat globule surface which is susceptible to flocculation.
In this study, the HGSVO seemed to be able to stabilize reconstituted dairy creamer emulsion. This property is very important to assure that there is no destabilization during serving the drinks. The ESI in this study was ranging from 93.45 to 108.06 min. This value quite high compared to other previous studies on dairy emulsions. Sun et al. (2022) reported ESI of milk protein concentrate and milk phospholipid stabilized emulsions were ranging from 30-60 min. Liu et al. (2020) reported the ESI value of 265.54 min for soybean oil stabilized succinyl monoglyceride emulsion. This study exhibited high ESI of reconstituted dairy creamer emulsions, an important quality feature of the creamer. The good emulsion stability is needed to prevent the sedimentation, feathering, or de-oiling of the creamer during serving.
The presence of abundant casein contributed to stabilize reconstituted creamer emulsion with HGSVO. The advantage of protein emulsifier is the ability to adsorb on the surface of oil droplets and prevent aggregation through repulsive forces (Sun et al., 2022). This can suppress the effect of protein in stabilizing the cream by creating a barrier layer around the oil droplets which is called the protein-lipid interaction effect (Nylander et al., 2019).
CONCLUSIONS AND RECOMMENDATIONS
Hydrolyzed goldband snapper visceral oil was suitable as an emulsifier for dairy creamer. The increasing concentration of HGSVO decreased the particle size of dairy creamer powder, however the reconstituted dairy creamer emulsion stability decreased at HGSVO more than 0.40%. The concentration 0.40% also exhibited the highest emulsifying activity and emulsion stability and produced the best reconstituted dairy creamer emulsion.
This study proved that the hydrolyzed fats or oils without purification could be applied as an emulsifier in the dairy creamer. The advantage of using fish visceral oil is contributed by the presence of EPA and DHA, thus providing health beneficial emulsifier and dairy creamer. Further studies are important to compare the characteristics of HGSVO stabilized dairy creamer with the commercial one. It is also important to evaluate the sensory properties and consumer acceptance for this product, besides the oxidative and storage stability of the dairy creamer powder.
ACKNOWLEDGEMENTS
The authors would like to thank Ministry of Education, Culture, Research and Technology for the Research Grant (Penelitian Disertasi Doktor) Year 2023 and financial support for this research project.
NOVELTY STATEMENT
The novelty in this study is the hydrolyzed goldband snapper fish visceral oil without purification is suitable to use as an emulsifier in dairy creamer with the advantage containing EPA and DHA as the health beneficial fatty acids.
AUTHORS’S CONTRIBUTION
Ria Dewi Andriani: Contributed to the data collection, data analysis and manuscript preparation.
Teti Estiasih: Had the role of designing and generating the concept, supervising, monitoring and controlling the research, data analysis and interpretation, and manuscript preparation.
Lilik Eka Radiati: Contributed to supervise the research.
Conflict of Interest
The authors declare there is no conflict of interest.
REFERENCES
AOAC (1997). Association of official analytical chemists international official methods of analysis. 16th Edition, AOAC, Arlington.
Awuchi CG, Igwe VS, Echeta CK (2019). The functional properties of foods and flours. International J. Adv. Acad. Res. Sci. Technol. Eng., 5(11): 139-160.
Bae EK, Lee SJ (2008). Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul., 25(8): 549-560. https://doi.org/10.1080/02652040802075682
Bonilla-Méndez JR, Hoyos-Concha JL (2018). Methods of extraction, refining and concentration of fish oil as a source of omega-3 fatty acids. Corpoica. Ciencia y Tecnología Agropecuaria. 19(3): https://doi.org/10.21930/rcta.vol19_num2_art:684
Braun K, Hanewald A, Vilgis TA (2019). Milk emulsions: structure and stability. Foods, 8(10): 483. https://doi.org/10.3390/foods8100483
Cabra V, Arreguín R, Farres (2008). A emulsifying properties of proteins. Bol. Soc. Quím. Méx., 2(2): 80-89.
Chaves KF, de Castro GLP, Boemer JPR, da Silva VM, da Cunha RL, Luccas V, Ribeiro APB (2024). Lipid phase characterization and reformulation of chocolate spreads to reduce saturated fatty acids. Braz. J. Food Technol., 27: 1-18. https://doi.org/10.1590/1981-6723.08523
Chen L (2015). Emulsifiers as food texture modifiers. In: Modifying Food Texture, Woodhead Publishing Series in Food Science, Technology and Nutrition, Sawston, United Kingdom, Volume 1: Novel ingredients and processing techniques. https://doi.org/10.1016/B978-1-78242-333-1.00002-4
Chung C, Sher A, Rousset P, McClements DJ (2017). Use of natural emulsifiers in model coffee creamers: physical properties of quillaja saponin-stabilized emulsions. Food Hydrocolloids, 67: 111-119. https://doi.org/10.1016/j.foodhyd.2017.01.008
David O, Kwadwo SO, Badu E, Sakyi P (2015). Proximate composition and some functional properties of soft wheat flour. Int. J. Innovative Res. Sci. Engi. Technol., 753-758.
de Walle DV, Goossens P, Dewettinck K (2008). Influence of the polarity of the water phase on the mesomorphic behaviour and the α-gel stability of a commercial distilled monoglyceride, 41(10): 1020-1025. https://doi.org/10.1016/j.foodres.2008.07.007
Ding H, Li B, Boiarkina I, Wilson, DI, Yu W, Young BR (2020). Effects of Morphology on the Bulk Density of Instant Whole Milk Powder. Foods, 9(1024): 1-19. https://doi.org/10.3390/foods9081024
El-Kheir MKS, Yagoub AEGA, Baker AAA (2008). Emulsion-stabilizing effect of gum from Acasia senegal (L) wild. the role of quality and grade of gum, oil type, temperature, stirring time and concentration. Pak. J. Nutr., 7(3): 395-399. https://doi.org/10.3923/pjn.2008.395.399
Erdogan MNK, Ketenoglu O, Tekin A (2019). Effect of monoglyceride content on emulsion stability and rheology of mayonnaise. J. Food Sci. Technol., 56(1): 443-450. https://doi.org/10.1007/s13197-018-3506-2
Euston SR (2010). Emulsifiers in dairy products and dairy substitutes. Springer Science+Business Media, New York.
Fredrick E, Heyman B, Moens K, Fischer S, Verwijlen T, Moldenaers P, der Meeren PV, Dewettinck K (2013). Monoacylglycerols in dairy recombined cream: II. the effect on partial coalescence and whipping properties. Food Res. Int., 51: 936-945. https://doi.org/10.1016/j.foodres.2013.02.006
Gassi JY, Famelart MH, Lopez C (2008). Heat treatment of cream affects the physicochemical properties of sweet buttermilk. Dairy Sci. Technol., 88: 369-385. https://doi.org/10.1051/dst:2008006
Hedayatnia S, Mirhosseini H (2018). Quality of reduced-fat dairy coffee creamer: affected by different fat replacer and drying methods. Descriptive Food Sci., 6: 115-136. https://doi.org/10.5772/intechopen.76367
Iwe MO, Onyeukwu U, Agiriga AN (2016). Proximate, functional and pasting properties of FARO 44 rice, African yam bean and brown cowpea seeds composite flour. Cogent Food Agric., 2(1): 1-10. https://doi.org/10.1080/23311932.2016.1142409
Junior SJH, Juliana NRR, Luiz AG, Vitolo M (2018). Conversion of triolein into mono-and diacylglycerols by immobilized lipase. Arab. J. Sci Eng., 43: 2247-2255. https://doi.org/10.1007/s13369-017-2635-7
Kastner E, Perrie Y (2016). Particle size analysis of micro and nanoparticles. Analytical Techniques in the Pharm. Sci., 677-699. https://doi.org/10.1007/978-1-4939-4029-5_21
Lajnaf R, Trigui I, Samet-Bali O, Attia H, Ayadi MA (2020). Comparative study on emulsifying and physico-chemical properties of bovine and camel acid and sweet wheys. J. Food Eng., 268: 1 – 32. https://doi.org/10.1016/j.jfoodeng.2019.109741
Liu F, Li C, Liu C, Zheng J, Bian K, Bai H, Zhang Y, Cao Y, Xie Y, Xiao X (2023). Improvement of hydrophobicity and gas permeability of the polyvinyl alcohol film utilizing monoglyceride coating and diatomaceous earth filling and its application to fresh-cut mango. Sustainable Chem. Eng., 11(29): 10938-10949. https://doi.org/10.1021/acssuschemeng.3c03149
Liu H, Zhang J, Wang H, Chen Q, Kong B (2021). High-intensity ultrasound improves the physical stability of myofibrillar protein emulsion at low ionic strength by destroying and suppressing myosin molecular assembly. Ultrason. Sonochem., 74: 105554. https://doi.org/10.1016/j.ultsonch.2021.105554
Liu L, Pan Y, Zhang X, Zhang Y, Li X (2021). Effect of particle size and interface composition on the lipid digestion of droplets covered with membrane phospholipids. J. Agric. Food Chem., 69: 159-169. https://doi.org/10.1021/acs.jafc.0c04945
Liu X, Xu W, Wang W, Luo R, Yang B, Lan D, Wang Y (2023). Physicochemical properties and feasibility of coconut oil-based diacylglycerol as an alternative fat for healthy non-dairy creamer. Food Chem., X. 19(100749). https://doi.org/10.1016/j.fochx.2023.100749
Liu Y, Wei ZC, Deng YY, Dong H, Zhang Y, Tang XJ, Li P, Liu G, Zhang MW (2020). Comparison of the effects of different food-grade emulsifiers on the properties and stability of a casein-maltodextrin-soybean oil compound emulsion. Molecules, 25(3): 1-16. https://doi.org/10.3390/molecules25030458
Loi CC, Eyres GT, Birch EJ (2019). Effect of mono-and diglycerides on physical properties and stability of a protein-stabilised oil-in water emulsion. J. Food Eng., 240: 56-64. https://doi.org/10.1016/j.jfoodeng.2018.07.016
Marangoni AG, Idziak H, Rush JWE (2008). Controlled Release of Food Lipids Using Monoglyceride Gel Phases Regulates Lipid and Insulin Metabolism in Humans. Food Biophys., 3: 241-246. https://doi.org/10.1007/s11483-008-9054-y
McClements DJ (2015). Food emulsion principles, practies, and technique. Third Ed, CRC Press, Boca Raton.
Menalla E, Serna JG, Cantero D, Cocero MJ (2024). Hydrothermal hydrolysis of triglycerides: Tunable and intensified production of diglycerides, monoglycerides, and fatty acids. Chem. Eng. J., 493: 152391. https://doi.org/10.1016/j.cej.2024.152391
Méndez-Velasco C, Goff HD (2012). Fat structures as affected by unsaturated or saturated monoglyceride and the ir effect on ice cream structure, texture and stability. Int. Dairy J., 24(1): 33-39.
Mohammed NK, Tan CP, Manap MYA, Muhialdin BJ, Hussin ASM (2019). Production of functional non-dairy creamer using Nigella sativa oil via fluidized bed coating technology. Food and Bioprocess Technol., 12: 1352-1365. https://doi.org/10.1007/s11947-019-02294-y
Nitbani FO, Tjitda JTP, Nurohmah BA, Wogo HE (2020). Preparation of fatty acid and monoglyceride from vegetable oil. J. Oleo Sci., 69(4): 277-295. https://doi.org/10.5650/jos.ess19168
Nylander T, Arnebrant T, Cárdenas M, Bos M, Wilde P (2019). Protein/emulsifier interactions. In food emulsifiers and their applications, 3rd ed by Hasenhuettl GL, Hartel RW. Springer International Publishing: Cham, Switzerland. https://doi.org/10.1007/978-3-030-29187-7_5
Pace S, Gonzalez P, Devoisselle JM, Milhiet PE, Brunela D, Cunin F (2010). Grafting of monoglyceride molecules for the design of hydrophilic and stable porous silicon surfaces. New J. Chem., 34: 29-33. https://doi.org/10.1039/B9NJ00469F
Padial-Domínguez M, Espejo-Carpio FJ, Pérez-Gálvez R, Guadix A, Guadix EM (2020). Optimization of the emulsifying properties of food protein hydrolysates for the production of fish oil-in-water emulsions. Foods, 9(5): 1-16. https://doi.org/10.3390/foods9050636
Pomeranz Y, Meloan CE. (2013). Food analysis: theory and practice. Springer US, Swiss.
Pugliese A, Cabassi G, Chiavaro E, Paciulli M, Carini E, Mucchett G (2017). Physical characterization of whole and skim dried milk powders. J. Food Sci Technol., 54(11): 3433-3442. https://doi.org/10.1007/s13197-017-2795-1
Putri DN, Hanif AM, Teguh S, Noor H (2021). Physicochemical properties and fatty acid profiles of fish oil from red snapper (Lutjanus malabaricus) refined from various NaOH concentrations. Agrointek, 15(1): 1026-2037. https://doi.org/10.21107/agrointek.v15i4.11098
Putri HLR, Hidayati A, Widyaningsih TD, Wijayanti N, Maligan JM (2016). Quality control of non dairy creamer to drying process condition on spray dryer at PT. Kievit Indonesia, Salatiga: a review. J. Pangan dan Agroindustri, 4(1): 443-448.
Radzi MRM, Zulqarnain, Yusoff MHM, Azmi N, Anuar MR (2022). Esterification of glycerol derived from biodiesel with fatty acids to monoglycerides – malaysian perspective. Chem. Biol. Eng. Rev., 10(1): 22-36. https://doi.org/10.1002/cben.202200013
Relkin P, Fosseux PY, Aubry V (2002). Composition of fat protein layer in complex food emulsions at various weight ratios of casein-to-whey proteins. Dairy Sci. Technol., 82(5): 567-578. https://doi.org/10.1051/lait:2002033
Rohim A, Estiasih T, Susilo B, Nisa FC (2024). Extraction of healthy oils from fish viscera by conventional and advanced technologies. Grasas y Aceites, 75(2). https://doi.org/10.3989/gya.0751231.1999
Rosida DF, Mulyani T, Septalia LR (2016). A comparative study of non-dairy cream based on the type of leguminosae protein in terms of physico chemical properties and organoleptic. Agric. Agric. Sci. Procedia, 9: 431-439. https://doi.org/10.1016/j.aaspro.2016.02.160
Rouimi S, Schorsch C, Valentini C, Vaslin S (2005). Foam stability and interfacial properties of milk protein–surfactant systems. Food Hydrocolloids, 19(3): 467-478. https://doi.org/10.1016/j.foodhyd.2004.10.032
Samios D, Nicolau A, Roza MB (2013). Polymers from natural products. Moni. Polymerization React., 427-450. https://doi.org/10.1002/9781118733813.ch22
Scheepers JJ, Muzenda (2015). Glycerol—a viable solvent for absorption of highly polar solutes i: behaviour of molecular interactions. J. Clean Energy Technol., 3(4): 282-286. https://doi.org/10.7763/JOCET.2015.V3.209
Statista (2024). Coffee – worldwide. https://www.statista.com/outlook/cmo/hot-drinks/coffee/worldwide#revenue. Accessed on July 10, 2024.
Sudibyo H, Setyaningrum F, Rochmadi, Fahrurrozi M. (2020). A catalyst reusability study in palm fatty acid distillate and glycerol esterification using multi-criteria decision analysis and reaction kinetics approach. ASEAN J. Sci. Technol. Dev., 37(2): 37-43. https://doi.org/10.29037/ajstd.612
Sun Y, Chen H, Chen W, Zhong Q, Shen Y, Zhang M (2022). Effect of ultrasound on pH-shift to improve thermal stability of coconut milk by modifying physicochemical properties of coconut milk protein. LWT Food Sci. Technol., 167(113861): 1-10. https://doi.org/10.1016/j.lwt.2022.113861
Sun Y, Yu X, Hussain M, Li X, Liu L, Liu Y, Ma S, Kouame KJE, Li C, Leng Y, Shilong J (2022). Influence of milk fat globule membrane and milk protein concentrate treated by ultrasound on the structural and emulsifying stability of mimicking human fat emulsions. Ultrason. Sonochem., 82(105881): 1-10. https://doi.org/10.1016/j.ultsonch.2021.105881
Takeiti CY, Kieckbusch TG, Queiroz FPC (2010). Morphological and physicochemical characterization of commercial maltodextrins with different degrees of dextrose-equivalent. Int. J. Food Prop., 13(2): 411-425. https://doi.org/10.1080/10942910802181024
Tawfik MS, Abdel-Ghaffar KA, Gamal AY, El-Demerdash FH, Gad HA (2019). Lycopene solid lipid microparticles with enhanced effect on gingival crevicular fluid protein carbonyl as a biomarker of oxidative stress in patients with chronic periodontitis. J. Liposome Res., 1-34. https://doi.org/10.1080/08982104.2019.1566243
Vereecken J, Meeussen W, Lesaffer A, Dewettinck K (2010). Effect of water and monoglyceride concentration on the behaviour of monoglyceride containing fat systems. Food Res. Int., 43: 872-881. https://doi.org/10.1016/j.foodres.2009.12.008
Zhang H, Zhao X, Chen X, Xu X (2022). Thoroughly review the recent progresses in improving O/W interfacial properties of proteins through various strategies. Front. Nutr., 9(1043809). https://doi.org/10.3389/fnut.2022.1043809
Zheng M, Jia ZB, Jiang JX (2014). Emulsifying and foaming properties of soy protein isolates with covalent modification by (-) epigallocatechin 3-gallate. Adv. J. Food Sci. Technol., 6(2): 238-240. https://doi.org/10.19026/ajfst.6.17
To share on other social networks, click on any share button. What are these?





