Prevalence of Mycoplasma gallisepticum in Poultry and Wild Life Birds Suspected of Chronic Respiratory Disease in Northern Pakistan
Prevalence of Mycoplasma gallisepticum in Poultry and Wild Life Birds Suspected of Chronic Respiratory Disease in Northern Pakistan
Nasir Abbas1, Muhammad Suleman1, Nazir Ahmad Khan2,*, Ijaz Ali3, Mubashir Rauf1,5 and Sadeeq ur Rahman4,*
1Department of Microbiology, Hazara University, Mansehra 21300, Khyber Pakhtunkhwa
2Department of Animal Nutrition, The University of Agriculture, Peshawar
3Department of Biosciences, Bioscience Block, Chak Shehzad Campus, Park Road, COMSATS Institute of Information Technology, Islamabad
4College of Veterinary Sciences and AH, Section Microbiology, Abdul Wali Khan University, Mardan, Khyber Pakhtunkhwa
5College of Veterinary Sciences, Bahauddin Zakariya University, Sub Campus Layyah
ABSTRACT
Seroprevalence and involvement of Mycoplasma gallisepticum (MG) in cases of chronic respiratory disease (CRD) in poultry and pheasantry birds of Northern Pakistan is underreported. In this study we report on the seroprevalence and isolation of MG from a total of 2341 poultry and 24 pheasantry birds. Overall, seropositivity of 46.56% (1090/2341) and 27.2% (6/24) against MG was observed among poultry- and pheasantry- birds, respectively. Broiler breeder and broiler showed 58.93% (264/448) and 37.23% (542/1456) seropositivity, respectively. A total of 17.1% (402/2341) samples collected from all these CRD-suspected birds were found positive on modified fray’s media indicating typical mycoplasma like colonies with appearance of fried egg or nipple like characteristic of 0.1 to 1 mm in diameter with a dense raised center in the middle. Interestingly, culture positivity for broiler samples was found higher (36.8%) as compared to breeders (33.5%) and layers (29.5%), respectively. Overall, of the cultured samples, 71.19% (215/302) were confirmed as M. gallisepticum by specie specific PCR. Furthermore, 82.43% of the confirmed isolates were recovered from Haripur, 82.35% recovered from Abbotabad and 60.53% were recovered from Mansehra. Finally, no significant difference (P<0.05) in PCR based positivity was found among all three types of bird population. Altogether, the observed high seropositivity and concomitant isolation of MG from CRD suspected birds investigated in the Northern region of Pakistan strongly suggest to initiate a comprehensive effective surveillance programme.
Article Information
Received 25 November 2017
Revised 01 January 2018
Accepted 18 January 2018
Available online 23 April 2018
Authors’ Contribution
NA, MS, SR and MR conceived and designed the work. NA and MS performed the experimental work. NA, SR and NAK analyzed the results and wrote the manuscript.
Key words
Mycoplasma gallisepticum, Chronic respiratory diseases, Seroprevalence, Postmortem lesion, Pheasantry, Poultry.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.3.1071.1077
* Corresponding authors: [email protected];
0030-9923/2018/0003-1071 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introduction
Mycoplasma gallisepticum (MG), causative agent of chronic respiratory diseases (CRD), is one of the most economically significant mycoplasma pathogens of poultry that has been reported from all over the world including Pakistan (Kleven and Levisohn, 1996). MG mainly localizes in the respiratory tract particularly in tracheal epithelium and air sacs. In line, the diseases is characterized by respiratory signs including conjustivitis with forthy ocular exudates, sneezing, coughing, nasal discharge, breathing difficulty and closed eyelid. Gross lesions of CRD include mild sinusitis, tracheitis and air sacculitis. Eyelids become swollen with ocular discharge and drainage from the nares can be seen. Postmortem examination reveals mucopurulent exudates in nasal cavities, trachea, and bronchi, fibrin purulent pericarditis and peri hepatitis (Bradbury, 2005; Levisohn and Kleven, 2000; Nascimento and Barreto, 2005). MG causes mitigation in poultry growth, feed conversion, egg production, egg hatchability and chick quality that lead to severe economic losses (Kleven, 2008). Transmission of MG infection has been reported by vertical via egg yolk through infected breeder flock as well as by horizontally through close physical contacts. .
At field, routine screening of MG infection in poultry is generally performed by a rapid slide plate agglutination test (SPAT) or enzyme-linked immunosorbent assay (ELISA) (Sarkar et al., 2005), and the presence of infection can be verified by detection of MG either by culture or DNA-based polymerase chain reaction (Islam et al., 2011; Muhammad et al., 2017). Increasing sequence data are made available recently contributing to improving mycoplasma DNA-based identification and detection. For example, a recent a rapid, new DNA microarray assay has been recently developed for parallel identification of almost 40 mollicutes of animal and human origin (Bottinelli et al., 2017). In principle, detection of MG can be by isolation of the organism and testing its pathogenicity using suitable host or detection of its DNA by PCR. MG-infected birds are advised to cull immediately to avoid further dissemination of infection.
In Pakistan, poultry industry is a vibrant sector and a major contributor of the economy. Since its first serological evidence of MG infection in Pakistan in 1984 (Shah, 1984), random reports from different parts of the country witnessed consistent presence of MG infection. During the year 1991-1995 an increased seroprevalence was recorded in Faisalabad division (Ahmad, 1998). During the last decade, consistent with the expansion of poultry industry in Pakistan, more frequent incidence of MG infection have been reported (Ahmad et al., 2008; Hanif and Najeeb, 2007; Mukhtar et al., 2012). The Northern region, mainly Abbotabad, Mansehra and Haripur, is central to poultry rearing and production due to its suitable climatic conditions. This region with approximately more than 30% of commercial and backyard poultry (more than 80 million poultry) population is known as hub of poultry rearing in Pakistan (Ayaz et al., 2010). Unfortunately, data regarding the prevalence of MG infection in poultry in Pakistan and particularly in the northern region the region is hardly available. In this report, we present a comprehensive report on the prevalence and circulation of MG in the northern region of Pakistan.
Materials and methods
Ethics approval and consent to participate
The current study was approved by the ethical committee of Hazara University, Mansehra, and all procedures including animal handling was performed according to local and national guidelines for animal ethics. Proper consent in written was obtained before sampling and postmortem examination of animals from the owners of the poultry farms and animals
Selection of area
Samples were collected from three different interconnected districts, Haripur, Abbottabad and Mansehra, of Khyber Pakhtunkhwa-Pakistan, located in the North of Pakistan. An estimated population of 2.5 million broiler, 4.0 million broiler breeder, 0.5 million commercial layer and 3.0 million rural poultry exist in this zone (Ayaz et al., 2010). Sample collection points included commercial poultry farms, Veterinary Research Center facility, live poultry markets and Pheasantries of Khyber Pakhtunkhwa. Of note, five different pheasantries that were sampled, only one is located at Mansehra district in the Northern region, while the rest of the four, Kotal Wildlife Division-Kohat, Lucky Breeding Center-Bannu, Peshawar Aviary-Peshawar and Kund Wildlife Park were located in other parts of the province.
Sample collection and types
This study was carried out during January 2009-March 2013. Nasal, tracheal and choanal cleft swabs were taken from live birds or fresh died CRD-suspected birds.. Additional swabs were taken from trachea, lungs and air sacs lesion and infected ovaries of postmortem-resected organs. Exudates were aspirated from the infraorbital sinuses and joint cavities if existed.
Sample size
This current study is a cross sectional epidemiological in nature. The sampling size was determined keeping in view the standards set by Office International des Epizootic (OIE) and considering estimated prevalence, desired level of confidence and acceptable error margin for achieving a minimum of 95 % sensitivity and specificity using the formulae:
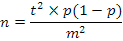
A total of 2341 birds were screened. Blood and swab samples for serology and culture, respectively, were obtained from all 2341 CRD suspected birds simultaneously. A total of 1609 swab samples obtained from tracheal/nasal/choanal region, while 732 swab sample were obtained from postmortem resected organs (Table I).
|
Area wise sample distribution |
Animal wise distribution |
|||||||
|
Total |
Mansehra |
Abbottabad |
Haripur |
Broiler |
Breeder |
Layer |
Total |
|
| Organs |
1609 |
609 |
500 |
500 |
1050 |
298 |
261 |
1609 |
| Swabs |
732 |
332 |
200 |
200 |
100 |
300 |
332 |
732 |
| Total |
2341 |
941 |
700 |
700 |
1150 |
598 |
593 |
2341 |
In the case of freshly dead birds, only those birds were sampled from which enough blood could be obtained. Furthermore, a total of 24 random blood and 24 tracheal/choanal cleft swab samples were taken from wild birds of pheasantries. Blood samples were processed for serum separation using standard methods (Tuck et al., 2009). All swab samples were transported as per standard procedures of OIE available at http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/AVIAN_MYCO.pdf and as described earlier (Ferguson-Noel et al., 2012; Zain and Bradbury, 1996).
Enzyme-linked immunosorbent assay (ELISA)
For seroprevalence ELISA was carried out according to the manufacturer’s instruction (FLOCKSCREEN™ Mg ELISA Kit). ELISA plate reader at 550 nm was used to record the absorbance according to the instructions. All broilers that were sampled were not vaccinated against MG, however, history of vaccination existed for few breeder and layers
Isolation of mycoplasma
Collected swab samples were used for inoculation into suitable media for isolation and identification of MG. All procedures, sample transportation, media preparation (Fry’s media), inoculation and growth were carried out according to the standard methods described in OIE manual of Diagnostic Tests and Vaccines for Terrestrial Animals (OIE, 2008 available at http://www.oie.int/fileadmin/Home). Inoculated tubes were daily checked for change in color from red to yellow and whirling movement. When color change was observed, isolates were then streaked onto agar plate media to observe colonial morphology under stereoscope. The identification of the Mycoplasma was made by the appearance of typical colonies i.e. fried egg or nipple live of 0.1 to 1 mm in diameter with a dense raised center in the middle (Shah et al., 2017a, b; Ferguson-Noel et al., 2012). Inoculum of samples with no color change was blindly subscultured on 7th day and observed for another two-to-four weeks. A sample was declared negative when no change in color or whirling movement was observed after 4 weeks of incubation.
Biochemical identification of Mycoplasma
The recovered Mycoplasma spp. were confirmed through a number of biochemical tests. These include glucose fermentation test, hydrolysis of L-arginin (Kleven and Levisohn, 1996) and digitonin sensitivity test (Power and Jordan, 1976).
Identification of M. gallisepticum by PCR
The isolates were subjected to identification through specie specific PCR. Primer pair MG-14F: 5’-GAGCTAATCTGTAAAGTTGGTC-3’ and MG-13R: 5’-GCTTCCTTGCGGTTAGCAAC-3’ was used in a PCR reaction as described earlier (Kleven and Bradbury, 2008; Lauerman, 1998). Genomic DNA was extracted by TIANamp Bacteria DNA Kit (TIANGEN, Beijing, China) according to the manufacturer’s instructions and as described earlier (Shah et al., 2017). PCR reaction was performed in a thermocylcer PCR machine (BIO RAD T100) in a total of 25 μl. The PCR reaction comprised of the first step of an initial denaturation at 95°C for 3 min followed by 34 cycles with denaturation at 94°C for 30 sec, annealing at 54°C for 30 sec and extension at 72°C for 90 sec and a final extension step of 5 min at 72°C. The expected amplified product was analyzed through agarose gel electrophoresis and visualized by Gel Doc™ EZ Gel Documentation System (Bio Rad, USA). Materials for isolation and growth of bacteria were purchased from Oxoid (Oxoid, UK). Primers were synthesized through Invitrogen. PCR master mix was purchased from Bioline (Bioline, London, UK).
Statistical analysis
Results obtained were subjected to statistical analysis using Chi-square test with 95% confidence intervals to assess statistical significance.
Results
Clinical features and postmortem findings
A total of 2341 CRD-suspected poultry birds from 246 open house poultry farms in three Districts -Mansehra, Abbottabad, and Haripur, of Khyber Pakhtunkhwa were examined. Clinical signs observed were diversified and included mainly coughing and sneezing. Respiratory dyspnea was evident as partial open beak respiration. Conjunctivitis, frothy nasal and ocular discharge were also observed in numerous infected birds (Supplementary Fig. S1). The disease was most evident in younger birds. Farm workers complained of low feed consumption, less weight gain and drop in egg production. In the case of pheasantry birds, conjunctivitis was the most common sign along with difficulty in respiration. We did not do any postmortem examination of the pheasantry birds. Postmortem of sick or freshly dead poultry birds revealed the presence of viscous frothy exudates in trachea with epithelial hemorrhages. Lungs were also affected indicated by dark reddish color with apparent hemorrhages and cloudy air sacs. Notably, in most of the layers typical pathomorphic form of cauliflower shaped ovary was noticeable in addition to hemorrhages in oviduct (Supplementary Fig. S1).
Seroprevalence of MG in poultry birds
ELISA indicated an overall prevalence of 46.56% (1090/2341). There was a significant difference (P<0.05) in seropositivity among layers (64%, 284/437), breeders (58%, 264/448) and broilers (37%, 542/1456). A total of 50.26% (385/766) poultry birds from District Abbotabad, 48.73% (555/1139) from District Mansehra and 34.40% (150/436) from Haripur were found seropositive against MG. A significant difference (P<0.05) in seropositivity of birds from Mansehra, Abbotabad and Haripur was identified. Few (n=11) of the breeders and layers were however vaccinated against MG at least 20 weeks prior sampling. However, these birds displayed typical clinical signs associated with CRD; and therefore, we assumed that the antibody titer was in fact due to infection. The prevalence data obtained through ELISA are mentioned in Table II.
Table II.- Seroprevalence of MG by ELISA in poultry birds of Northern Pakistan.
| Type of poultry |
Mansehra |
Abbotabad |
Haripur |
Total |
P Value |
|
Broiler |
|
|
|
|
0.002 |
| Tested |
570 |
510 |
376 |
1456 |
|
| Positive |
194 |
220 |
128 |
542 |
|
| Percentage |
34.04 |
43.14 |
34.04 |
37.23 |
|
| Breeder |
|
|
|
|
0.018 |
| Tested |
287 |
136 |
25 |
448 |
|
| Positive |
166 |
89 |
9 |
264 |
|
| Percentage |
57.84 |
65.44 |
36.00 |
58.93 |
|
| Layer |
|
|
|
|
0.000 |
| Tested |
282 |
120 |
35 |
437 |
|
| Positive |
195 |
76 |
13 |
284 |
|
| Percentage |
69.15 |
63.33 |
37.14 |
64.99 |
|
| Grand total |
0.000 |
||||
| Tested |
1139 |
766 |
436 |
2341 |
|
| Positive |
555 |
385 |
150 |
1090 |
|
| Percentage |
48.73 |
50.26 |
34.40 |
46.56 |
|
| P Value |
0.000 |
0.000 |
0.920 |
0.000 |
|
Identification of M. gallisepticum
Of the 2341 samples, 17.1 % (n=402) revealed mycoplasma growth identified based on biochemical profile and colonial morphology. There was a significant difference (P<0.05) in culture positivity among broiler (36.8%), breeder (33.5%) and layers (29.5%) (Table III; Fig. 1A).
PCR was performed to confirm the recovered isolates by targeting 16SrRNA gene of MG (Fig. 1B). Of the 402 isolates, 302 samples were randomly picked for PCR confirmation (Table IV). An overall 71.19% (215/302) of the tested isolates were confirmed MG by specie specific PCR. Of these confirmed isolates, 82.43% isolates were recovered from Haripur, 81.58% were recovered from Abbotabad and 60.53% were recovered from Mansehra.
Table III.- Culture positivity of swab samples.
| Type |
Tested |
Positive |
% |
P |
Frequency distribution of culture positive sample (n=402) |
|||||||
|
Broiler |
% |
Breeder |
% |
Layers |
% |
P |
||||||
|
Growth on specific media |
Organs |
1609 |
224 |
13.9 |
0.00 |
136 |
33 |
42 |
10.4 |
46 |
11 |
0.1 |
| Swabs |
732 |
178 |
24.3 |
12 |
2.9 |
93 |
23.1 |
73 |
18 |
0.0 |
||
| Grand total |
2341 |
402 |
17.1 |
|
148 |
36 |
135 |
33.5 |
119 |
29 |
0.0 |
|
Furthermore, a total of 37.2% (542/1456) cultured samples from broiler, 58.9% (264/448) from layers and 64.9% (284/437) from breeders were identified as MG by PCR. Statistical analysis showed that breeders were carrying significantly higher (P<0.05) number of isolates than broiler.
Table IV.- Specie specific PCR of cultured-mycoplasma isolates.
| Area |
Tested |
PCR positive |
% |
P Value |
| Mansehra |
0.000 |
|||
| Broiler |
60 |
45 |
75.00 |
|
| Breeder |
52 |
49 |
94.23 |
|
| Layer |
40 |
30 |
75.00 |
|
| Total |
152 |
92 |
60.53 |
|
| Abbottabad | ||||
| Broiler |
41 |
32 |
78.05 |
|
| Breeder |
18 |
16 |
88.89 |
|
| Layer |
17 |
14 |
82.35 |
|
| Total |
76 |
62 |
81.58 |
|
| Haripur | ||||
| Broiler |
50 |
39 |
78.00 |
|
| Breeder |
10 |
10 |
100.00 |
|
| Layer |
14 |
12 |
85.71 |
|
| Total |
74 |
61 |
82.43 |
|
| Grand total |
302 |
215 |
71.19 |
|
Seropositivity of MG in pheasantry birds
A total of 24 sera samples from pheasantry birds were also screened through ELISA indicated that 6 (27.27%) were found positive (Table V). Of the 24 tracheal/nasal swab samples obtained from these CRD suspected pheasantry birds, 4 were found positive for mycoplasma, of which, only 1 sample (Dhodial Pheasantry, District Mansehra) was confirmed as MG by PCR (Table V).
Discussion
Chronic respiratory disease (CRD) remains a consistent challenge for the expanding poultry industry of Pakistan. Despite its huge economic impact on the poultry industry, unfortunately, an overall country-wide systemic surveillance programme of monitoring CRD is lacking. Consequently, despite random available reports on the incidence of CRD from different regions of the country, overall epidemiological information is missing. The Northern Pakistan, particularly, Districts of Abbotabad, Mansehra and Haripur due to its climatic conditions and accessibility are considered as hub of the poultry industry of Pakistan, where most of the local or controlled poultry sheds of broiler, broiler breeders and layers are located. In a time span of over 4 years during the current comprehensive surveillance programme that covered most of the poultry sheds operating in three different districts of Northern Pakistan, 2341 each of blood (for seroprevalence) and swabs samples (for isolation) were analysed. Our results indicated an overall 46.56% (1090/2341) seropositivity with significantly higher seropositivity of 64.99% (284/437) in layer as compared to broiler and breeder. Of all these CRD-suspected birds, 17.1% (402/2341) were positive on modified fray’s media indicating typical mycoplasma like colonies.
Table V.- Screening of suspected wild life birds for CRD.
|
Location of Phesantary |
Serum |
ELISA* + (%) |
Culture positivity |
PCR positivity |
|
Kotal Wildlife Division Kohat |
4 |
1 (25) |
0 |
Not done |
| Lucky Breeding Center, Bannu |
3 |
0 (0) |
1 |
0 |
| Peshawar Aviary |
3 |
0 (0) |
0 |
Not done |
| Kund Wildlife Park |
3 |
1 (50) |
1 |
0 |
| Dhodial Pheasantry Manshera |
11 |
4 (36.36) |
2 |
1 |
| Total |
24 |
6 (27.27) |
4 |
1 |
*Serum samples positive against MG using ELISA.
Clinical signs and symptoms were diversified, but mainly respiratory system in all birds and additionally reproductive system in layers and breeders were badly affected. This was also reflected in the postmortem examination revealed by the accumulation of viscous frothy exudates in trachea with epithelia hemorrhages in all affected birds, while typical pathomorphic form of cauliflower shaped ovary was noticeable in addition to hemorrhages in oviduct of layers and breeders as reported earlier (Osman et al., 2009; Gondal et al., 2015). In most of the cases, mycoplasmosis gets complicated as a result of involvement of secondary infection thereby complicating the clinical picture. Mycoplasma, however, multiplies mainly in the lungs and trachea causes chronic macroscopic lesions such as catarrhal exudates in nasal and paranasal passages and trachea (Gondal et al., 2015).
ELISA results of the current study indicated that almost half of the birds were seropositive against indicating quite higher seropositivity as compared to previously reported by 10% by Ahmad et al. (2008), however, our findings are in agreement with a higher seropositivity (49.1) reported from poultry birds in Faisalabad-Pakistan (Mukhtar et al., 2012). Although, in the current study we also included a few vaccinated breeders and layers (n=11), however, we only sampled those vaccinated birds with a history of vaccination older than 20 weeks. None of the broiler birds was vaccinated. The lower frequency of (37%) of seropositivity of broilers as compared to layers (64%) and breeders (58%) is possibly due to shorter exposure as broilers are marketed generally after 4 weeks of age. Nevertheless, isolation frequency of M. gallisepticum was not significantly different between all types of birds suggesting that the higher seropositivity in layers and breeders was either due to presence of antibodies as a result of vaccination or previous exposure to pathogen. We assume that the prevalence of MG in the area is higher as isolation of mycoplasma is quite cumbersome. Particularly, it is hard to culture MG from flock which is concurrently infected with one or more nonpathogenic Mycoplasma sp. that would overtake the pathogenic and more slow-growing MG in vitro. Possibly, therefore, despite half of the poultry samples were seropositive for MG; however, around 17% samples were successfully cultured. A direct PCR reaction on samples in such cases for laboratories is therefore vital to optimize for timely detection of MG.
Conclusion
We report on the high the high seropositivity (46%) among different poultry birds in the Northern region of Pakistan. This recorded high seropositivity with concomitant high frequency (17.1%) of MG isolation suggests its involvement in CRD infection among poultry. A countrywide comprehensive effective surveillance program should therefore be initiated aiming controlling CRD among poultry.
Acknowledgements
The current study was funded by Higher Education Commission of Pakistan provided under indigenous PhD fellowship programme granted to Nasir Abbas. The authors acknowledge support and help provided during sample collection, transportation and analysis of the Veterinary Research Institute, Abbotabad, Khyber Pakhtunkhwa.
There is supplementary material associated with this article. Access the material online at: http://dx.doi.org/10.17582/journal.pjz/2018.50.3.1071.1077
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Ahmad, A., Rabbani, M., Yaqoob, T., Shabbir, M. and Akhtar, F., 2008. Status of IgG antibodies against Mycoplasma gallisepticum in non-vaccinated commercial poultry breeder flocks. Int. J. Poult. Sci., 18: 61-63.
Ahmad, I., 1998. Diagnosis and control of Mycoplasma gallisepticum infection in poultry. Final Technical Report, Poultry Research Institute, Rawalpindi, Pakistan.
Ayaz, M., Sajid, M., Khan, S., Qureshi, M., Rehman, A. and Khawaja, N., 2010. Prevalence of Avian Influenza and its economic impact on poultry population of Hazara region Pakistan. Sarhad J. Agric.., 26: 629-633.
Bottinelli, M., Passamonti, F., Rampacci, E., Stefanetti, V., Pochiero, L., Coletti, M., Fabrizio, R., Doreene, RH. and Christiane, S., 2017. DNA microarray assay and real-time PCR as useful tools for studying the respiratory tract Mycoplasma populations in young dairy calves. J. med. Microbiol., 66: 1342-1349. https://doi.org/10.1099/jmm.0.000571
Bradbury, J., 2005. Poultry mycoplasmas: Sophisticated pathogens in simple guise. Br. Poult. Sci., 46: 125-136. https://doi.org/10.1080/00071660500066282
Ferguson-Noel, N., Laibinis, V.A. and Farrar, M., 2012. Influence of swab material on the detection of Mycoplasma gallisepticum and Mycoplasma synoviae by Real-Time PCR. Avian Dis., 56: 310-314. https://doi.org/10.1637/9972-102411-Reg.1
Gondal, M., Rabbani, M., Muhammad, K., Yaqub, T., Babar, M. and Sheikh, A., 2015. Characterization of Mycoplasma gallisepticum isolated from commercial poultry flocks. J. Anim. Pl. Sci., 25: 108-113.
Hanif, A. and Najeeb, M., 2007. Comparison of conventional bacterial isolation, rapid slide agglutination and polymerase chain reaction for detection of Mycoplasma gallisepticum in breeder flocks. Pak. J. Life Soc. Sci., 5: 1-5.
Islam, A., Aslam, A., Chaudhry, Z.I., Ahmed, M.U.D., Rehman, H.U. and Saeed, K., 2011. Pathology of Mycoplasma gallisepticum in naturally infected broilers and its diagnosis through PCR. Prevalence, 48: 46.
Kleven, S., 2008. Control of avian mycoplasma infections in commercial poultry. Avian Dis., 52: 367-374. https://doi.org/10.1637/8323-041808-Review.1
Kleven, S. and Bradbury, J., 2008. Avian mycoplasmosis (Mycoplasma gallisepticum, M. synoviae). OIE manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees). Office International des Epizooties, Paris, pp. 482-496.
Kleven, S.H. and Levisohn, S., 1996. Mycoplasma infections of poultry. Mol. Diagn. Proced. Mycoplasmol., 2: 283-292. https://doi.org/10.1016/B978-012583806-1/50142-5
Lauerman, L., 1998. Mycoplasma PCR assays. In: Nucleic acid amplification assays for diagnosis of animal diseases (ed. L.H. Lauerman). American Association of Veterinary Laboratory Diagnosticians, Turlock, CA, pp. 41-42.
Levisohn, S. and Kleven, S., 2000. Avian mycoplasmosis (Mycoplasma gallisepticum). Rev. Scientif. Tech., 19: 425-442. https://doi.org/10.20506/rst.19.2.1232
Mukhtar, M., Awais, M.M., Anwar, M.I., Hussain, Z., Bhatti, N. and Ali, S., 2012. Seroprevalence of Mycoplasma gallisepticum among commercial layers in Faisalabad, Pakistan. J. Basic appl. Sci., 8: 1.
Muhammad, F., Fareed, S.K., Zafar, U., Khan, T.A., and Ahmad, A., 2017. Development and evaluation of culture-enhanced Tetra-PCR for differential diagnosis of Mycoplasma gallisepticum and M. synoviae. Pakistan J. Zoo., 49: 2133-2140. http://dx.doi.org/10.17582/journal.pjz/2017.49.6.2133.2140
Nascimento, E.R., Pereira, V., Nascimento, M. and Barreto, M., 2005. Avian mycoplasmosis update. Rev. Brasil. Ciênc. Avícola, 7: 1-9.
Osman, K., Aly, M., Amin, Z. and Hasan, B., 2009. Mycoplasma gallisepticum: An emerging challenge to the poultry industry in Egypt. Rev. Scientif. Tech., 28: 1015-1023. https://doi.org/10.20506/rst.28.3.1940
Power, J. and Jordan, F., 1976. Unilateral enlargement of the eye in chicks infected with a strain of M. gallisepticum. Vet. Rec., 99: 102-103. https://doi.org/10.1136/vr.99.6.102
Sarkar, S., Rahman, M., Rahman, M., Amin, K., Khan, M. and Rahman, M.M., 2005. Sero-prevalence of Mycoplasma gallisepticum infection of chickens in model breeder poultry farms of Bangladesh. Int. J. Poult. Sci., 4: 32-35. https://doi.org/10.3923/ijps.2005.32.35
Shah, A., 1984. Occurrence of chronic respiratory disease of domestic fowls in West Pakistan. M. Sc. thesis, Department of Veterinary Microbiology, University of Agriculture, Faisalabad, Pakistan.
Shah, M., Sadique, U., Ahmad, S., Qureshi, S., Rahman, S. and Hassan, Z., 2017. Molecular identification and comparative antimycoplasmalactivity of three indigenous medicinal plants against mycoplasmaputrefaciens isolated from sheep. J. Anim. Pl. Sci., 27: 5.
Shah, M.K., Saddique, U., Ahmad, S., Hayat, Y., Rahman, S. and Hassan, M.F., 2017. Isolation rate and antimicrobial susceptibility profiles of Mycoplasma mycoides subspecies capri field isolates from sheep and goats in Pakistan. Small Rumin. Res., 153: 188-192. https://doi.org/10.1016/j.smallrumres.2017.06.002
Tuck, M.K., Chan, D.W., Chia, D., Godwin, A.K., Grizzle, W.E. and Krueger, K.E., 2009. Standard operating procedures for serum and plasma collection: Early detection research network consensus statement standard operating procedure integration working group. J. Proteome Res., 8: 113-117. https://doi.org/10.1021/pr800545q
Zain, Z.M. and Bradbury, J.M., 1996. Optimising the conditions for isolation of Mycoplasma gallisepticum collected on applicator swabs. Vet. Microbiol., 49: 45-57. https://doi.org/10.1016/0378-1135(95)00177-8
To share on other social networks, click on any share button. What are these?










