Seasonal Occurrence of Jassid (an Insect Pest) on Bt. Cotton in Sourthern Punjab, Pakistan
Seasonal Occurrence of Jassid (an Insect Pest) on Bt. Cotton in Sourthern Punjab, Pakistan
Zeshan Siraj1*, Amjad Farooq1 and Zahid Mehmood2
1Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan; 2Director Central Cotton Research Institute, Multan, Pakistan.
Abstract | The current study was conducted to find out the population dynamics of Jassids in relation to climatic factors (air temperature, relative humidity and rainfall) on different cultivars of Bt. cotton from May-November 2011 -May-November 2013 at cotton research institute, Multan, Pakistan, Jassids appeared in 21st Standard Meteorological Week(SMW)and attain its peak in 36th SMW. Maximum and minimum mean population of jassids (4.19±0.16 and 0.03±0.13 per leaf) was observed in September and November 2011, respectively. Maximum and minimum population of jassids (2.43±0.11/leaf) and (0.00±014/ leaf) was recorded in 2012 September and November whereas maximum and minimum infestation of jassids 3.59 ±0.06 and 0.03±0.12 (per leaf) was observed in September and November 2013. Jassids population was recorded above the economic threshold level (ETL) in all three years. Jassids infestation was non-significantly correlated with temperature, relative humidity and average rainfall in all consecutive years 2011-2013. Data revealed that jassids population had a positive non-significant correlation with minimum temperature and minimum relative humidity in 2011 and 2013. A positive association of jassids with maximum temperature was observed in 2012-2013 whereas it was negatively correlated in 2011. Jassids infestation was negatively associated with average rain fall in 2011 and 2012 and had a positive relationship in 2013. A positive correlation of jassids with maximum humidity was observed in year 2011 and 2012 however it was negatively associtated in 2013. Peak infestation of average population of jassids was recorded in September .The present research concluded that infestation of sucking pest jassids is more frequent in Mid of September , therefore it is recommended for local farmers to do some control measures to avoid attack of jassids in this month priorly in order to reduce the economic loss,.It was concluded that mid of September was more suitable for incidence of jassids .
Received | April 26, 2018; Accepted | June 24, 2019; Published | August 26, 2019
*Correspondence | Zeshan Siraj, Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan; Email: zeshansiraj@hotmail.com
Citation | Siraj, Z., A. Farooq and Z. Mehmood. 2019. Seasonal occurrence of jassid (An insect pest) on Bt. cotton in sourthern Punjab, Pakistan. Sarhad Journal of Agriculture, 35(3): 856-863.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.3.856.863
Keywords | Genotypes, Correlation, Threshold, Level, Temperature, Rainfall, Humidity
Introduction
Jassid (Amarasca devastance) is the most important insect pest of cotton. It can cause severe damage to the plants by sucking the cell sap and reducing the area for photosynthesis (Parkash and Verma, 1986). Jassid nymph and adults both are harmful for the health of cotton plants as they inject their toxic saliva into the tissues of the leaves, and causes weakness in plant, leaf burning, drying and shedding of leaves and young ball. Heavy infestation may reduce the fruit making capacity of plant also reduce the fruiting part and arrests the growth of the plant (Bhat et al., 1986; Ratanoara et al., 1994). In order to reduce the damage caused by insects pesticides have been used for many years but it produces resistance in insects. Bacillus thuringiensis, commonly known as Bt. is a naturally occurring bacterium in soil. Its genes are capable to make a specific toxic protein which has been incorporated in the Bt. Cotton as insecticidal Bt genes (Ferry et al., 2006). It has been used as a biological pesticide for more than 50 years (Qaim and Zilberman, 2003). Bt cotton is very profitable for farmers as it has reduced the extensive use of pesticides and increase the crop production per hectare (Pray et al., 2002). It has decreased the chewing pest infestation but on the other hand it has increased the infestation of non-targeted insects, therefore farmers have increased the bio control activities of natural enemies in the fields (Lu et al., 2012).
Bt cotton is specific for chewing pest as it has been incorporated a gene Bacillus thuringiensis commonly called Bt which make toxic protein Cry 1 Ac in cotton fiber which effect directly on target pest i.e chewing insects (Rashid et al., 2008).This protein has been reported very minute or nill in sap sucking insect pests of cotton through ELISA analysis (Lawo et al., 2009) therefore Bt cotton has been subjected to sucking pest more as compared to non-Bt (Naranjo, 2005), however scientist have introduced new Bt. cotton varieties which are not only effective against chewing insects but also effective for sucking insects specially Jassids (http://www.ccrim.org.pk/agronomy.html≠)It has been observed through ELISA there is less or absence of Bt toxic proteins in sap sucking insect pests of cotton, therefore Bt cotton has no effect on sucking insects (Lawo et al., 2009) therefore sucking insect infestation on Bt cotton is increased as compared to non Bt cotton (Naranjo, 2005), but new varieties of Bt cotton have shown resistance against jassids (http://www.ccrim.org.pk/agronomy.html≠).
The weather and climatic changes plays a key role to alter the status of insects. It may effect on their population dynamics, feeding behavior, and intensity of pest (Ayres and Schneider, 2009). Global alteration has an effect on anthropogenic and environmental changes. Intensity in climatic change has a crucial effect on insects and plants. It may also effect the immunity, growing rate, fecundity rate and physiological function (Yumamura et al., 2006; Ayres and Schneider, 2009; Zhang et al., 2014). Abiotic factors like thermal changes may affect the multiplication, emergence, dispersal ratio of insects (Yumamura et al., 2006; Zhang et al., 2014). Many abiotic factors such as temperature also effect on dispersal rate of insects. Thermal factors affect the insects by suppressing or stimulating its potential genetically, fecundity, and mortality rate and range of hosts (Finaly et al., 2012).
Humidity influences the growth of insects, it also changes the behavior of insects. It also increase the capability of insects to regulate loss of water. In some insects it may affect the development of insect. Humidity has negative relationship with many insects, many insects population decreases with increase in humidity, humid and cold environment is suitable for the development of jassids (Khan and Ullah, 1994; Shahid et al., 2012).
Rain fall is also an important climatic factor which may increase or decrease the insect pest infestation. Too much rain and extreme weather may cause mortality of insects. It may reduce the growth rate and feeding behavior of insects, were as it promotes the maggot propagation of some insects. Warm and humid conditions of environment are more suitable for growth of insects and its development (John et al., 2011). Present study was conducted to keep in view the importance of climatic factors with reference to inscet pest’s infestation especially jassids.
Materials and Methods
The experiment was conducted at Central Cotton Research Institute, Multan, Pakistan to study the Jassids infestation during years 2011-13 on different genotypes of Bt cotton cultivars VIP 333, Bt. 121, CIM 598 (Better to mention names) and their correlation with different climatic factors maximum and minimum temperature, humidity and average rainfall. The experiment was arranged on the basis of randomized complete block design (RCBD). There were 36 subplot having three replications. One variety in every subplot was sown on two and half feet bed, at 75cm distance between rows with plant to plant distance of 9cm. General agronomic practices were used for good results of yield. Jassids infestation on plants was recorded in morning through pest scounting souting (Makhdoom, 2006). Ten plants were randomly selected for data collection of jassids.1st leaf from upper portion of any plant randomly was observed from both side to count Jassids 2nd leaf from middle portion of another plant was observed for Jassids on both sides of the leaf and the 3rd leaf from lower portion of any other plant randomly was observed for Jassid count ,and so on (Sohail et al., 2003; Amjad and Aheer, 2007). Standard meteorological data i.e. temperature, relative humidity and average rainfall was collected from www.wunderground.com.
Statistical analysis
The data analysis was done by simple correlation between the population dynamics and various weather factors by using following formula.
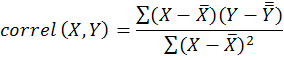
Where;
Correl (X, Y) is the simple correlation coefficient; x is first variable i.e. abiotic component; Y is the second variable i.e. population of insect-pest; is the mean of first variable and is the mean of second variable
Results and Discussion
Population dynamics of jassids
Jassids infestation was initially observed in 21st SMW of June during a research period of 2011-2013 and was recorded above the economic threshold level in all cultivated months.
In 2011 maximum average number of jassids 4.19/leaf was recorded in 36th SMW in September and negligible average population of 0.15/leaf was observed in 41th SMW in 1st week of November to onwards (Figure 1). Maximum average counts of jassids 2.43/leaf in 2012 was recorded in 36th SMW in September and negligible counts of 0.13/leaf was observed in 41th SMW in November to onwards (Figure 2). In 2013 jassids population started appearing in 21st SMW and maximum number 3.59/leaf was observed in 36th SMW in September, started decreasing further and became negligible 0.18/leaf from 42th SMW in November to onwards (Figure 3). Average jassids count was above the economic threshold level in all years.
Sharma et al. (2004) recorded the presence of the jassids in 23rd SMW in June and observed peak population in 32nd SMW in August. Agarwal et al. (2007) reported peak population of jassids in 32nd and 33rd SMW in August and September. Dhaka and Pareek (2008) observed a gradual increase of jassids from last week of August 32nd SMW to September. 33rd SMW. Bhute et al. (2012) recorded maximum population of jassids in 40th SMW in last week of October. Shahid et al. (2012) observed the maximum population of jassids in October. Peak population of jassids was recorded in August (Lanjar et al., 2014; Latif et al., 2014). Nagendra (2015) recorded maximum population of jassids in 3rd week of September. Soni and Dakhad (2016) reported highest population of jassids in September. The results of present study are in agreement of studies conducted by (Agarwal et al., 2007; Dhaka and Pareek 2008; Nagendra, 2015; Soni and Dakhad, 2016).
Table 1: Population dynamics of Jassids in relation to weather factors during 2011.
| Dates | Standard Meteorological week(SMW) | Temperature (0C) | Relative Humidity (%) | Average RF(mm) | Mean Jassids Population (per leaf) | ||||
| Tmax | Tmin | Average | Max | Min | Average | ||||
| 06June-12 June | 21 | 45 | 31 | 38 | 44 | 17 | 24 | 0.8 | 0.71 |
| 13 June-19June | 22 | 44 | 31 | 38 | 54 | 20 | 36 | 1.2 | 0.78 |
| 20 June-26June | 23 | 42 | 31 | 36 | 66 | 28 | 48 | 1.6 | 0.65 |
| 27 June-03 July | 24 | 40 | 33 | 36 | 67 | 29 | 48 | 1.9 | 0.50 |
| 04July-10July | 25 | 41 | 30 | 36 | 66 | 23 | 42 | 1.9 | 0.78 |
| 11July-17July | 26 | 40 | 32 | 36 | 70 | 37 | 53 | 1.7 | 1.09 |
| 18July-24July | 27 | 40 | 31 | 36 | 75 | 42 | 54 | 1.4 | 1.05 |
| 25 July-31Aug | 28 | 39 | 30 | 34 | 79 | 34 | 55 | 1.2 | 1.23 |
| 01Aug-07Aug | 29 | 40 | 31 | 36 | 70 | 32 | 48 | 1.0 | 2.90 |
| 08 Aug-14Aug | 30 | 33 | 26 | 30 | 84 | 58 | 77 | 0.9 | 3.06 |
| 15 Aug-21Aug | 31 | 37 | 27 | 32 | 79 | 41 | 61 | 0.7 | 3.04 |
| 22 Aug-28Aug | 32 | 40 | 30 | 35 | 84 | 36 | 52 | 0.5 | 3.42 |
| 29Aug-04 Sep | 33 | 36 | 27 | 32 | 89 | 56 | 74 | 0.4 | 3.91 |
| 05 Sep-11Sep | 34 | 30 | 26 | 28 | 100 | 84 | 93 | 0.3 | 4.12 |
| 12 Sep-18Sep | 35 | 34 | 27 | 30 | 94 | 58 | 79 | 0.2 | 4.02 |
| 19 Sep-25Sep | 36 | 36 | 28 | 32 | 79 | 42 | 58 | 0.1 | 4.19 |
| 26 Sep-02Oct | 37 | 35 | 24 | 30 | 89 | 48 | 66 | 0.1 | 0.37 |
| 03 Oct-09Oct | 38 | 34 | 24 | 29 | 79 | 48 | 65 | 0.1 | 0.60 |
| 10 Oct-16Oct | 39 | 35 | 23 | 29 | 78 | 25 | 49 | 0.1 | 0.30 |
| 17 Oct-23Oct | 40 | 35 | 19 | 27 | 78 | 23 | 45 | 0.1 | 0.40 |
| 26 Oct-01Nov | 41 | 32 | 18 | 25 | 75 | 18 | 40 | 0.1 | 0.15 |
| 02Nov- 08Nov- | 42 | 32 | 17 | 24 | 82 | 31 | 62 | 0.1 | 0.07 |
| 09Nov-15Nov | 43 | 26 | 17 | 22 | 83 | 41 | 61 | 0.1 | 0.05 |
| 16Nov-22Nov | 44 | 29 | 18 | 24 | 88 | 42 | 65 | 0.2 | 0.03 |
Relationship of population dynamics of jassids with weather variables
Population of jassids was observed high during suitable climatic conditions (Table 1). During 2011 peak average population of jassids 4.19 / leaf was observed in 36th SMW when maximum and minimum temperature and relative humidity was 36oC and 28oC, and 79% and 42%, respectively. with average rain fall of 0.1mm. It became negligible (0.15/leaf) in last week of October when maximum and minimum temperature and relative humidity was 32 oC and 18 oC, 75% and 18%, respectively and average rainfall was 0.1mm.
Highest infestation of jassids 2.43/leaf was recorded in 2012 (Table 2) in 36th SMW at maximum and minimum temperature, maximum relative humidity was 34 oC and 26 oC 89% and 44% respectively with average rain fall of 0.2mm.Jassids count was negligible 0.13/leaf in 41 th SMW and further when maximum and minimum temperature and relative humidity was 29 oC, 18 oC,77% and 26% respectively and average rainfall was 0.1mm maximum population of jassids in 2013 was 3.59/leaf in 36th SMW (Table 3) at maximum and minimum temperature and Maximum and minimum relative humidity was 38 oC and 28 oC and , 34% and 35% respectively withaverage rain fall was 0.2mm.
A negligible average population 0.21/leaf from 41th SMW to onwards was recorded when maximum and minimum temperature and relative humidity was 37 oC and 21 oC and 78% and 30% respectively with average rainfall was 0.1mm. The present study is strongly agreed with study conducted by (Agarwal et al. 2007; Dhaka and Pareek, 2008; Nagendra, 2015; Soni and Dakhad, 2016).
Correlation of jassids population with weather factors
During the year 2011, correlation between jassids
Table 2: Population dynamics of Jassids in relation to weather factors during 2012.
|
Dates
|
Standard Meteorological week(SMW) | Temperature (0C) | Relative Humidity (%) | Average RF(mm) | Mean Jassids Population (per leaf) | ||||
| Tmax | Tmin | Average | Max | Min | Average | ||||
| 06June-12 June | 21 | 36 | 27 | 32 | 61 | 26 | 44 | 0.7 | 1.27 |
| 13 June-19June | 22 | 41 | 27 | 34 | 51 | 09 | 29 | 1.1 | 0.61 |
| 20 June-26June | 23 | 47 | 31 | 37 | 66 | 15 | 38 | 1.5 | 0.63 |
| 27 June-03 July | 24 | 39 | 30 | 34 | 58 | 32 | 47 | 1.9 | 0.41 |
| 04July-10July | 25 | 44 | 32 | 38 | 52 | 17 | 30 | 1.9 | 0.33 |
| 11July-17July | 26 | 47 | 32 | 37 | 66 | 35 | 48 | 1.7 | 0.31 |
| 18July-24July | 27 | 40 | 31 | 36 | 70 | 35 | 52 | 1.5 | 0.22 |
| 25 July-31Aug | 28 | 38 | 31 | 34 | 70 | 42 | 56 | 1.2 | 0.22 |
| 01Aug-07Aug | 29 | 38 | 30 | 34 | 70 | 36 | 53 | 1.1 | 1.26 |
| 08 Aug-14Aug | 30 | 40 | 32 | 36 | 66 | 33 | 46 | 0.9 | 1.93 |
| 15 Aug-21Aug | 31 | 37 | 28 | 32 | 82 | 44 | 63 | 0.7 | 1.99 |
| 22 Aug-28Aug | 32 | 37 | 30 | 34 | 65 | 43 | 53 | 0.6 | 2.14 |
| 29Aug-04 Sep | 33 | 37 | 30 | 34 | 74 | 39 | 58 | 0.4 | 2.02 |
| 05 Sep-11Sep | 34 | 36 | 28 | 32 | 89 | 50 | 60 | 0.3 | 2.21 |
| 12 Sep-18Sep | 35 | 36 | 29 | 30 | 89 | 55 | 69 | 0.2 | 2.33 |
| 19 Sep-25Sep | 36 | 34 | 26 | 30 | 89 | 44 | 65 | 0.2 | 2.43 |
| 26 Sep-02Oct | 37 | 35 | 24 | 30 | 78 | 36 | 51 | 0.1 | 0.38 |
| 03 Oct-09Oct | 38 | 37 | 23 | 30 | 73 | 24 | 48 | 0.1 | 0.40 |
| 10 Oct-16Oct | 39 | 34 | 22 | 28 | 69 | 26 | 42 | 0.1 | 0.50 |
| 17 Oct-23Oct | 40 | 31 | 18 | 24 | 88 | 4 | 37 | 0.1 | 0.23 |
| 26 Oct-01Nov | 41 | 29 | 18 | 24 | 77 | 26 | 49 | 0.1 | 0.13 |
| 02Nov- 08Nov | 42 | 31 | 18 | 24 | 77 | 26 | 52 | 0.1 | 0.05 |
| 09Nov-15Nov | 43 | 28 | 15 | 22 | 88 | 36 | 61 | 0.1 | 0.03 |
| 16Nov-22Nov | 44 | 28 | 16 | 22 | 88 | 39 | 61 | 0.2 | 0.00 |
population and various weather factors, maximum and minimum temperature, maximum and minimum humidity and average rainfall was found non-significant (Table 4). It was negatively associated with maximum temperature and average rainfall and its relationship was low respectively. Jassids infestation was positively correlated with minimum temperature, maximum and minimum relative humidity and its relationship is medium and high respectively.
During year 2012, The correlation between jassids population and all weather factors, maximum and minimum temperature, maximum and minimum humidity and average rainfall was non-significant. It was positively associated with maximum and minimum temperature maximum and minimum humidity but has a low relationship with them where as it has a high association with minimum humidity. and its relationship was low and high respectively whereas it had a negative and small correlation with average rainfall (Table 4).
In 2013, The association between jassids population and abiotic factors, maximum and minimum temperature, maximum and minimum humidity and average rainfall was non-significant. Its relationship was medium and positive with maximum and minimum temperature, minimum humidity and has positively but small relationship with average rainfall. Its correlation was negative and medium with maximum relative humidity (Table 4). Present results are in agreement of the studies conducted by (Gogi, 2006; Bhute et al., 2012) who observed a positive association of jassids with minimum and maximum temperature and relative humidity. Present findings are also agreed with (Seif, 1980; Shahid et al., 2012) who reported a negative association of jassids with maximum temperature and Soni and Dhakad, 2016 who recorded a negative relation of jassids with average rain fall.
Table 3: Population dynamics of Jassids in relation to weather factors during 2013.
| Dates | Standard Meteorological week(SMW) | Temperature (0C) | Relative Humidity (%) | Total RF (mm) | Mean jassid Population (per leaf) | ||||
| Tmax | Tmin | Average | Max | Min | Average | ||||
| 06June-12 June | 21 | 47 | 30 | 38 | 58 | 08 | 28 | 1.1 | 2.47 |
| 13 June-19June | 22 | 38 | 28 | 33 | 84 | 49 | 57 | 1.1 | 2.30 |
| 20 June-26June | 23 | 43 | 31 | 37 | 58 | 18 | 36 | 1.5 | 2.18 |
| 27 June-03 July | 24 | 39 | 31 | 35 | 59 | 37 | 48 | 1.9 | 2.01 |
| 04July-10July | 25 | 42 | 32 | 37 | 58 | 22 | 37 | 1.9 | 0.28 |
| 11July-17July | 26 | 40 | 31 | 36 | 77 | 31 | 54 | 1.7 | 0.32 |
| 18July-24July | 27 | 41 | 32 | 56 | 58 | 33 | 46 | 1.5 | 0.38 |
| 25 July-31Aug | 28 | 36 | 26 | 31 | 94 | 49 | 72 | 1.2 | 0.40 |
| 01Aug-07Aug | 29 | 38 | 30 | 34 | 79 | 44 | 59 | 1.1 | 2.73 |
| 08 Aug-14Aug | 30 | 38 | 29 | 34 | 79 | 44 | 58 | 0.9 | 2.86 |
| 15 Aug-21Aug | 31 | 33 | 27 | 30 | 89 | 53 | 70 | 0.7 | 2.97 |
| 22 Aug-28Aug | 32 | 38 | 27 | 32 | 89 | 41 | 60 | 0.6 | 2.99 |
| 29Aug-04 Sep | 33 | 36 | 28 | 32 | 79 | 43 | 60 | 0.4 | 3.49 |
| 05 Sep-11Sep | 34 | 36 | 27 | 32 | 84 | 46 | 64 | 0.3 | 3.52 |
| 12 Sep-18Sep | 35 | 39 | 27 | 33 | 61 | 25 | 44 | 0.2 | 3.57 |
| 19 Sep-25Sep | 36 | 38 | 28 | 33 | 34 | 35 | 52 | 0.2 | 3.59 |
| 26 Sep-02Oct | 37 | 36 | 28 | 32 | 73 | 37 | 52 | 0.1 | 0.65 |
| 03 Oct-09Oct | 38 | 35 | 28 | 32 | 68 | 39 | 50 | 0.1 | 0.56 |
| 10 Oct-16Oct | 39 | 37 | 29 | 33 | 95 | 38 | 52 | 0.1 | 0.55 |
| 17 Oct-23Oct | 40 | 34 | 24 | 29 | 78 | 36 | 58 | 0.1 | 0.49 |
| 26 Oct-01Nov | 41 | 37 | 21 | 27 | 78 | 30 | 50 | 0.1 | 0.21 |
| 02Nov- 08Nov- | 42 | 30 | 16 | 23 | 82 | 23 | 49 | 0.1 | 0.18 |
| 09Nov-15Nov | 43 | 24 | 13 | 18 | 88 | 35 | 62 | 0.1 | 0.05 |
| 16Nov-22Nov | 44 | 27 | 13 | 20 | 82 | 30 | 56 | 0.2 | 0.02 |
Table 4: Correlation of jassids population with weather factors.
| Year | Insect-pest | Max. temperature (0C) | Min. temperature (0C) | Max. humidity (%) | Min. humidity (%) | Average RF (mm) |
| 2011 | Jassids | -0.069ns | 0.242 ns | 0.537ns | 0.679 ns | -0.231ns |
| 2012 | Jassids | 0.115 ns | 0.45 ns | 0.206ns | 0.569ns | -0.156ns |
| 2013 | Jassids | 0.340 ns | 0.39 ns | -0.223 ns | 0.231ns | 0.014ns |
Ns: nonsignificant.
Conclusions and Recommendations
Present study concluded that Jassids started to appear in June and peak population was recorded in September and became decline at the end of October according to climate. Last week of September (36th SMW) was observed more suitable for its peak infestation and a decline and negligible population of jassids was recorded from 1st week of November (41st SMW) to onwards. Present findings of 2011- 2012 concluded that jassids have a significant positive association with minimum and maximum temperature and minimum and maximum humidity whereas jassids population had a negative relationship with maximum temperature in 2011. Jassids showed a non-significant and positive relationship with maximum humidity, minimum and maximum humidity and minimum humidity in years 2011, 2012 and 2013 respectively.
There was a negative association of jassids with average rain fall in years 2011-2012 whereas it had a positive relationship in 2013. Maximum relative humidity was negatively associated with jassids in 2012-2013. The present findings could be used by researchers for further studies in order to find out the association of abiotic factors in relation to jassid in order to control its infestation. It is recommended that farmers should be careful about insect attack with these weather parameters as low temperature, rain fall and humidity are in favour of jassids infestation.
Author’s Contribution
Zeshan Siraj: Conducted the research and wrote the article.
Amjad Farooq: Supervised the research and helped in writing the article.
Zahid Mehmood: Supervised the experimental work, collected the data and compiled the research work.
Novelty Statement
This study highlights the importance of weather parameters which favour or not for the jassids infestation so that farmers can adopt control measures to avoid the growth of its popu-lation prior the time.
References
Aggarwal, N., D.S. Brar and G.S. Buttar. 2007. Evaluation of Bt and non-Bt version of two cotton hybrids under different spacing against sucking insects-pests and natural enemies. J. Cotton. Res. Dev. 21: 106-110.
Amjad, A. and G.M. Aheer. 2007. Varietal resistance against sucking insect pests of cotton under Bahawalpur ecological conditions. J. Agric. Res. 45: 205–208.
Ayres, J.S. and D.S. Schneider. 2009. The role of anorexia in resistance and tolerance to infections in Drosophila. Plos. Biol. 7: 1000-1005. https://doi.org/10.1371/journal.pbio.1000150
Bhute, N.K., B.B. Bhosle, B.V. Bhede, D.G. More. 2012. Population dynamics of major sucking pests of Bt cotton. Ind. J. Entomol. 74 (3): 246- 252.
Dhaka, S.R. and B.L. Pareek. 2008. Weather factors influencing population dynamics of major insect pests of cotton under semi arid agro-ecosystem. Ind. J. Entomol. 70: 157-163.
Ferry, N., J. Edwards, T. Gatehouse, P. Capell, Christou and A.M.R. Gatehouse. 2006. Transgenic plants for insect control: a forward looking scientific perspective. Tran. Res. 15: 13-19. https://doi.org/10.1007/s11248-005-4803-x
Finaly-Doney, M. and G.H. Walter. 2012. Behavioral responses to specific prey and host plant species by a generalist predatory coccinellid (Cryptolaemus montrouzieri Mulsant). Bio. Control. 63: 270-278. https://doi.org/10.1016/j.biocontrol.2012.09.004
John, C., Palumbo., A.C. Yuma and A.Z. Yuma. 2011. Weather and Insects. UA. Veg. IPM. Update. 2: 6-7.
Khan, S.M. and Z. Ullah. 1994. Population dynamics of sucking pests of cotton in Dera Ismail Khan. SJA. 10:285-290.
Latif, Z., S. Ahmed, Sohail, K., L. Khan and M. Ishfaq. 2015. Population density of Jassids (Amarasca biguyyula biguttula) and thrips (Thrips tabaci). J. Bio. Environ. Sci.7: 272-280.
Langar, A.G., B.K. Solangi, S.A. Khuhro and A. W. Solangi., 2014. Insect infestation on Bt. and non-Bt. cotton cultivars. Sci. Qual. Manag. 27: 55-58.
Lawo, N.C., F.L. Wackers and R. Jorg. 2009. Indian Bt cotton varieties do not affect the performance of cotton aphids. Plos. One. 4: 4804. https://doi.org/10.1371/journal.pone.0004804
Lu, Y.H., K.M. Wu, Y.Y. Jiang, N. Guo and Desneux. 2012. Widespread adoption of Bt. cotton and insecticide decrease promotes biocontrol services. Nat. 487: 362-365. https://doi.org/10.1038/nature11153
Makhdoom, M.M. 2006. Cultivation and care of cotton, central cotton research institute. Publ. No. 61: 12-40.
Nagendra, S. 2015. Studies on population dynamics of key pests of cotton. Int. Jr. Agric. Tech. 11(5): 1161-1176. http://www.ccrim.org.pk/agronomy.html≠; www.wunderground.com.
Parkash, O. and N.N. Verma. 1986. Effect of different plant extract applied by different method against jassid (Amrasca biguttula biguttlua) (Dist) and white fly (Bemisia tabaci) on brinjal during Pre fruiting crop. Ind. J. Ent. 47: 66-77.
Seif, A.A. 1980. Seasonal fluctuation of adult population of whitefly, Bemisia tabaci on cotton and its relationship with weather parameter. J. Cott. Res. Develop. 5: 181-189.
Shahid, M.R., J. Farooq, A. Mehmood, F.M. Ilahi, M. Riaz, A. Shakeel, V.P. Mag and A. Farooq. 2012. Seasonal occurrence of sucking insect pest in cotton ecosystem of Punjab, Pakistan. AAB BiofluxX. 4: 26.
Sohail, A.N., N. Shahid, R. Zia-Ur and B. Mohsin. 2003. Comparitive incidence of and insect pest complex on cotton varieties subjected to organic and synthetic fertilizers. Int. J. Agric. Biol. 5:236-238.
Pray, C.E., J. Huang and R. Hu. and S. Rozelle. 2002. Five years of Bt. cotton in China the benefits continue. Plant. J. 31: 423-430. https://doi.org/10.1046/j.1365-313X.2002.01401.x
Qaim, M. and Z. David. 2003. Yield effects of genetically modified crops in developing countries. Sci. 299: 900–902. https://doi.org/10.1126/science.1080609
Rashid, B., Z. Saleem., T. Husnain and S. Riazuddun. 2008. Transformation and inheritance of Bt. genes in Gossypium hirsutum. J. Pl. Bio. 51: 248-254. https://doi.org/10.1007/BF03036123
Ratanoara, A., M. Sheikh., J.R. Patel and N.M. Patel. 1994. Effect of weather parameter on brinjal jassid (Amrasca biguttula biguttlua). Ishida. Gujarat Agric. Univ. Res. J. 19: 39-43.
Soni, R. and N.K. Dhakad. 2016. Seasonal incidence of cotton Jassids, Amrasca Biguttula (Ishida) on Transgenic Bt cotton and their correlation with weather parameters. IJAIR: 4 (6) 2319-10473.
Yumamura, K., M. Yokazawa, M. Nishimori, Y. Ueda and T. Yokosuka. 2006. How to analyse long-term insect population dynamics under climate change: 50 year data of three insect pests in paddy fields. Poplulation Ecol. 48: 38-48. https://doi.org/10.1007/s10144-005-0239-7
Zhang, S., Z. Cao, Q. Wang, F. Zhang and T.X. Liu. 2014. Exposing eggs to high temperatures affects the development, survival and reproduction of Harmonia axyridis. J. Therm. Biol. 39: 40-44. https://doi.org/10.1016/j.jtherbio.2013.11.007
To share on other social networks, click on any share button. What are these?








