Sero-Epidemiology of Q Fever (Coxiellosis) in Small Ruminants Kept at Government Livestock Farms of Punjab, Pakistan
Sero-Epidemiology of Q Fever (Coxiellosis) in Small Ruminants Kept at Government Livestock Farms of Punjab, Pakistan
Qudrat Ullah1,*, Huma Jamil1, Zafar Iqbal Qureshi1, Muhammad Saqib2 and Heinrich Neubauer3
1Department of Theriogenology, University of Agriculture, Faisalabad, Pakistan
2Department of Clinical Medicine and Surgery, University of Agriculture, Faisalabad, Pakistan
3Friedrich-Loeffler-Institut, Naumburger Street 96A, 07743 Jena, Germany
ABSTRACT
Coxiellosis, a zoonotic disease caused by Coxiella burnetii, acts as a major trade barricade and adversely affects the productive and reproductive capabilities of animals. This study was planned to investigate the sero-prevalence of Coxiellosis and its relationship with some important risk factors in small ruminants kept at nine government livestock farms of Punjab, Pakistan. A total of 1000 blood samples (500 each from goats and sheep) were collected from animals kept at the respective livestock farms. An indirect-Enzyme Linked Immunosorbent Assay (iELISA) was used to detect anti-C. burnetii antibodies in the serum. Serological analysis revealed overall flock-level sero-prevalence of 92.3% which varied from 88.88% in sheep and 100% in goat, while individual level sero-prevalence was 15.6% in sheep and 15.0% in goats, the difference being non-significant. A significant (P<0.05) association was found between seropositivity against C. burnetii and variables like livestock farm, type of farming, region (locality of farm), presence of ticks, animal health status and season of sampling. In conclusion, the study indicates the occurrence of antibodies to C. burnetii at the government livestock farms included in the study.
Article Information
Received 10 August 2018
Revised 17 September 2018
Accepted 19 September 2018
Available online 22 November 2018
Authors’ Contribution
QU analyzed the samples and data, and executed the experiments. HJ, ZIQ and MS reviewed the article and designed the experiments. HN critically reviewed the experimental design and research data and also provided diagnostic support.
Key words
Coxiellosis, Pakistan, Small ruminants, Government livestock farms, Indirect ELISA.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.1.135.140
* Corresponding author: [email protected]
0030-9923/2019/0001-0135 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Coxiellosis is a worldwide zoonosis caused by a Gram-negative, obligate intracellular organism known as Coxiella burnetii. Although, the organism has wide range of hosts including domestic animals, rodents, reptiles, pets, birds, wild animals and arthropods, but ruminants, especially sheep and goats, are the main animal reservoirs and source of infection for humans (Van der Brom et al., 2015; Saglam and Sahin, 2016). The disease usually occurs as subclinical infection in domestic animals, however, infected ruminants may show fever, arthritis, conjunctivitis, mastitis, and genital problems that are usually not differentiated with other infections having similar clinical signs (Saglam and Sahin, 2016). The disease is mainly transmitted through aerosol. Birth products like aborted fetus, fetal membranes and vaginal secretion, and milk, urine and feces contaminated with C. burnetii can dry and mixed with air leading to human infection. Ticks may play a role in transmission of disease in animals but still it is dubious in humans (Anonymous, 2016).
The organism exists in two different antigenic forms based upon lipopolysaccharide (LPS) structure i.e., Phase-I and Phase-II. Phase-I is virulent form, having full length LPS and is responsible for causing disease in both animals and humans, while Phase-II is avirulent form with truncated non-reversible LPS molecule. Virulent phase-I form undergo changes to transform into avirulent Phase-II form upon serial passages in cell culture or repeated inoculations in embryonated eggs. Phase-II bacteria are incapable to multiply within immune competent cells (Mori et al., 2013; Shah et al., 2015).
Sero-diagnostic techniques are preferably used for diagnosis of this infection, with ELISA being ideal for detection of antibodies against C. burnetii (Ezatkhah et al., 2015; Hadush et al., 2016; Ahmad et al., 2017). In Pakistan, the disease was first reported in camels in 1955. To the best of our knowledge only five epidemiological studies (1955-2016) have reported coxiellosis in Pakistan. In the current study, sero-prevalence of C. burnetii is investigated in small ruminants kept at nine government livestock farms of Punjab, Pakistan. We selected these livestock farms as they reflects the major livestock population of Punjab and there prevails a higher annual disease incidence of human and animal origin (Directorates of Animal and Human Health, Punjab).
Materials and methods
Sample collection
For this study, blood samples were collected from nine government livestock farms including LES (Livestock Experimental Station) Alladad Jahnia, LES Khushab, LES Fazilpur, LES Jogaitpur, LES Rakh Kharewala, LPRI (Livestock Production and Research Institute) Bahadarnagar, LES Rakh Ghulaman, GLF (Government Livestock Farm) Kallurkot and Fine wool sheep farm (FWSF)-205TDA located in Punjab province (205,344 km2, 70.00° E and 30.00° N in the semi-arid lowlands zone). The average annual rainfall ranges between 46 cm in the plains and 96 cm in sub-mountainous regions and the highest rainfall is during July-September. The average annual temperature of Punjab varies between -2 to 45°C (Shabbir et al., 2016; Anonymous, 2018). Blood samples from goats could be collected from only four farms, while sheep blood samples were collected from all nine farms. Using Thrusfield (2007) formula, minimum of 384 samples from sheep and goat farms were required. Thus for the current study, 500 blood samples from sheep and 500 from goat were collected from all these farms.
Approximately 10 ml of blood was collected from the jugular vein of each animal using disposable needles and evacuated blood collection tubes (Improvacuter, Shanghai International Hamburg Holding, gmbH, Germany), and centrifuged at 4500 rpm for 10 min to separate serum. Serum obtained was shifted to disposable screw caped cryovials (Cryo STM; Greiner Bio-one, GmbH Frickenhausen, Germany) and stored in a deep freezer at -20°C till its use for serological analysis.
Ethics statement
Blood samples were collected from small ruminants as per guidelines of the International Animal Care and Use Committee (IACUC) and after obtaining the consent of Secretary Livestock & Dairy Development department, Punjab, Pakistan (wide letter No.SO(I&C)/L7DD/2-6/2016). The samples were processed based on the approval of the Ethical Research Board at the University of Agriculture Faisalabad, Pakistan (vide letter No. ORIC/3253, dated November 16, 2013).
Sero-diagnosis
Serum samples were analyzed using Q fever Indirect ELIZA (CHEKIT* Q fever Antibody ELISA Test Kit, IDEXX, Liebefeld, Switzerland). For interpretation of results, ELISA reader was used to read the plates at optical density (OD) value of 450 nm (Anthos 2020, Wals, Austria) as per manufacturer’s recommendation. The OD values of samples were then analyzed in relation to the positive and negative controls using following formula:
Controls
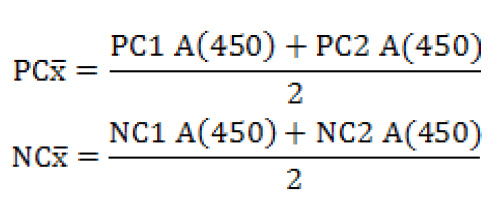
Validity criteria
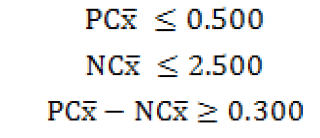
Samples

Serum samples having OD value of 40% or more were considered ELISA-positive, suspected if the values were between 30-40% and negative if OD values were less than 30%.
Statistical analysis
WinPepi version 11.15 was used for calculation of prevalence percentage and respective 95% confidence interval, while Statistix version 8.1 software was used for calculation of Chi-square values (χ2), df (degree of freedom) and P-value for different variables. Chi-square test was applied for calculation of significance of association (P< 0.05) between seropositivity against C. burnetii and various farm associated variables.
Results
Univariable analysis of data was performed to determine relationship of farm-associated variables with seropositivity against C. burnetii in small ruminants (Tables I, II). Results revealed an overall sero-prevalence of 15.3% at the studied farms. Prevalence of the disease varied significantly (P<0.01) among various livestock farms with highest prevalence at 205-TDA farm (65.9%), while lowest at Rakh Ghulaman farm (4.8%). At flock-level, higher sero-prevalence was recorded in goat flocks (100%), while lower in sheep flocks (88.8%), the difference being non-significant (p>0.05) between the two flocks. As far as type of farming was concerned, sero-prevalence was significantly higher (p<0.01) in farms where sheep and goats were kept together (17.2%) compared to farms where only sheep or goats were kept (5.98%). Regarding region-wise prevalence of the disease, highest sero-prevalence was recorded in Western region (21.2%) followed by Southern (7.9%) and Central (7.7%) regions of Punjab, the difference being significant (p<0.01). Similarly, a significantly (P<0.01) higher sero-prevalence was recorded in animals infested by ticks (60.1%) compared to those without ticks infestation (6.6%). Weak/emaciated animals (43.0%) showed significantly (P<0.01) higher seropositivity for C. burnetii compared to healthy ones (9.5%). When season of sampling was considered, significantly (p<0.01) higher prevalence of anti-C. burnetii antibodies was recorded in samples collected during wet season (21.1%) compared to those collected during dry season (13.4%).
Table I.- Sero-prevalence of Coxiellosis at nine government livestock farms of Punjab, Pakistan. The samples were analyzed using Indirect ELISA.
|
Livestock farm |
Positive / screened |
Prev. (%) |
95% CI |
χ2 value |
|
FWSF-205TDA |
27/41 |
65.9 |
49.4-79.9 |
χ2=86.82** |
|
Rakh Kharewala |
74/283 |
26.2 |
21.1-31.7 |
|
|
Kallurkot |
3/22 |
13.6 |
2.9-34.9 |
|
|
Khushab |
6/45 |
13.3 |
5.4-26.8 |
|
|
Bahadarnagar |
15/132 |
11.4 |
6.5-18.0 |
|
|
Jogaitpur |
4/37 |
10.8 |
3-25.4 |
|
|
Fazilpur |
3/52 |
5.8 |
3.2-9.5 |
|
|
Alladad Jahania |
14/243 |
5.8 |
1.2-15.9 |
|
|
Rakh Ghulaman |
7/145 |
4.8 |
2.0-9.7 |
|
|
Overall |
153/1000 |
15.3 |
12.3-18.7 |
*, means significant (P<0.05); **, means highly significant (P<0.01).
Discussion
Although Coxiellosis has been known to the scientist since 1935, however more attention was given to this disease after its largest outbreak in Dutch’s human population during 2007-2010 (Hadush et al., 2016). Pakistan is an agro-economic based country where livestock plays an integral role in livelihood of rural poor. Approximately 8 million Pakistani families are linked with livestock raising obtaining more than 35% earnings from livestock production activities (Anonymous, 2017). Since, the infection has zoonotic potential, this close animal-human relationship and lack of awareness among farmer’s society could possibly risk zoonosis and threaten “One Health” approach. To the best of our information, this is the first epidemiological survey investigating the sero-prevalence of Coxiellosis in small ruminants maintained at various government livestock farms of Punjab, Pakistan.
In the current study, seropositivity against C. burnetii antibodies varied significantly (P<0.05) among various livestock farms of Punjab with highest prevalence (65.9%) at 205-TDA farm and lowest (4.8%) at Rakh Ghulaman farm. Zahid et al. (2016) also reported a very high herd level sero-prevalence (73.1%) of C. burnetii infection in small ruminants of Punjab ranging from 58.8% to 94.4%, while a very low flock level sero-prevalence was recorded by Lambton et al. (2016) in sheep (3%) and goats (10.2%) in Great Britain. Farm-level factors e.g. locality of farm, flock density, distance to the nearby livestock farm and the number of visiting peoples, and professional farm workers
Table II.- Univariate analysis of farm-related variables associated with prevalence of antibodies to Q fever in small ruminants kept at nine government livestock farms of Punjab, Pakistan.
|
Variable |
Level |
Pos./screened |
Prev. (%) |
95% CI |
p-value |
|
Flock-level prevalence |
Goat |
4/4 |
100 |
71.2-100 |
χ2=0.03NS |
|
Sheep |
8/9 |
88.8 |
56.1-99.4 |
||
|
Type of farming |
Single species |
10/167 |
5.98 |
3.08-10.4 |
χ2=10.55** |
|
Mixed |
143/833 |
17.2 |
14.7-19.8 |
||
|
Region |
Western |
117/536 |
21.8 |
18.4-25.4 |
χ2=28.26** |
|
Southern |
7/89 |
7.9 |
3.5-14.9 |
||
|
Central |
29/375 |
7.7 |
5.3-10.7 |
||
|
Presence of ticks |
Yes |
98/163 |
60.1 |
52.2-67.7 |
χ2=301.914** |
|
No |
55/837 |
6.6 |
5.0-8.5 |
||
|
Animal health status |
Emaciated |
74/172 |
43.0 |
35.7-50.5 |
χ2=76.80** |
|
Healthy |
79/828 |
9.5 |
7.6-11.6 |
||
|
Season of sampling |
Wet |
51/240 |
21.2 |
16.4-26.7 |
χ2=6.13* |
|
Dry |
102/760 |
13.4 |
11.1-15.9 |
*, means significant (P<0.05); **, means highly significant (P<0.01); NS, non-significant.
have significant association with increased C. burnetii seropositivity. Also farms belonging to different production systems and geographical locations results in significant differences in disease prevalence (Anasta´cio et al., 2013; Klaasen et al., 2014).
Flock-level sero-prevalence (92.3%) of coxiellosis in small ruminants was found to be 88% in sheep and 100% in goats. A similar high sero-prevalence was reported by Zahid et al. (2016), with flock-level prevalence of 48.8-94.4% in Pakistan and Carbonero et al. (2015), with 8-100% flock-level prevalence in Ecuador. However, a low flock-level sero-prevalence (14.5%) of coxiellosis in small ruminants was reported by Rizzo et al. (2016). In case of small ruminants, variations in intrinsic vulnerability to C. burnetii have not been studied precisely (Klaasen et al., 2014). The higher flock density at livestock farms could contribute to higher sero-prevalence of the disease (Zahid et al., 2016; Khaled et al., 2016).
Based on types of farming i.e. single species and mixed, mixed farming had relatively higher sero-prevalence recorded and were significantly associated (P<0.01) with seropositivity of the infection. Anasta´cio et al. (2013) and Rizzo et al. (2016) also found a significantly higher seropositivity against C. burnetii antibodies in animals kept in the mixed flocks, while Khaled et al. (2016) reported lower sero-prevalence of the disease in mixed flocks (46.4%) compared to flocks with single species (71.4%). Since, goats are more susceptible to Q fever infection than any other livestock species, therefore inter-species contact, common watering points and shared seasonal grazing may increase the odds of acquiring and transmitting the infection (Schimmer et al., 2014; Rizzo et al., 2016).
Sero-prevalence of C. burnetii antibodies was higher during Wet Season than in samples collected during Dry season. Carbonero et al. (2015) also reported higher prevalence of coxiellosis during wet season (15.8%) compared to dry season (9.7%). However, Paul et al. (2012) found higher prevalence of the disease in summer season than in winter and autumn. In pregnant animals, substantial growth of this microorganism occurs within trophoblastic cells of placenta. Higher prevalence of the disease during wet season might be attributed to massive bacterial shedding that usually occurs during lambing and kidding season (December to April) (Schimmer et al., 2011; Jung et al., 2014; Saglam and Sahin, 2016).
Many previous studies have considered ticks as an important reservoir and vector for transmission of C. burnetii infection. Even the highly virulent Nile Mile reference strain of C. burnetii was isolated from ticks in United States (Duron et al., 2015). In the current study, animals with ticks infestation (60.1%) showed significantly (P<0.05) higher seropositivity for C. burnetii than those without ticks infestation (6.6%). These results are consistent with the findings of Duron et al. (2015) and Zahid et al. (2016) who recorded significant association between ticks infestation and Coxiellosis. Result of current present study also revealed a strong association between seropositivity for C. burnetii and ticks infestation which reflects the importance of ticks as natural reservoir for C. burnetii.
The sero-prevalence of C. burnetii infection varied significantly (P<0.05) among various regions of Punjab province. Highest prevalence was recorded in Western region, followed by Southern and Central regions. In Iran, Ezatkhah et al. (2015) revealed an overall prevalence of 26.4% in the studied locales with highest prevalence in Iranshahr region (39.2%) and lowest in Sarbaz region (5%). The higher sero-prevalence of C. burnetii antibodies in an area might be attributed to area-wise tropism or endemic nature of infection as well as to the district-wise changes in weather, moisture in soil, vegetation and presence of diseased animals in the vicinity (Shabbir et al., 2015; Rizzo et al., 2016).
In the current study, animals with higher prevalence of C. burnetii antibodies showed apparently poor health status. Agerholm (2013) reported that ewes suffering from C. burnetii infection give birth to remarkably weak and emaciated lambs. Similarly, Saegerman et al. (2013) found an association between seropositivity against C. burnetii antibodies and birth of weak offspring. In goats, Ganter (2015) reported that full term kids of C. burnetii seropositive animals were weak with higher mortality rate and reduced body weight. Moreover, most of the apparently healthy seropositive kids also suffered with gastro-intestinal and pulmonary disorders later on.
Conclusion
In conclusion, this study indicates the presence of anti-C. burnetii antibodies in small ruminants kept at government livestock farms. Moreover type and locality of the livestock farm, seasonal variation, and presence of ticks and poor health status of the animal have significant role in seropositivity for C. burnetii. Authors hope publication of this study will draw attention of the concerned authorities. Further population based studies at district level are required to clearly understand the epidemiology of the disease.
Acknowledgement
Authors are thankful to Higher Education Commission (HEC), Islamabad, Pakistan for financial support to Mr. Qudrat Ullah under Indigenous M. Phil~PhD Scholarship Programme to carry out this research work. Diagnostic/lab support from Friedrich Loeffler Institute (FLI), Jena, Germany is highly appreciated. We are also thankful to the Livestock and Dairy Development Department, Punjab and the concerned Veterinary Officials for helping us in sampling process.
Statement of conflict of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
Agerholm, J.S., 2013. Coxiella burnetii associated reproductive disorders in domestic animals: A critical review. Acta Vet. Scand., 55: 13-21. https://doi.org/10.1186/1751-0147-55-13
Ahmad, T., Khan, I., Razzaq, S., Khan, S. and Akhtar, R., 2017. Prevalence of bovine brucellosis in Islamabad and Rawalpindi districts of Pakistan. Pakistan J. Zool., 49: 1123-1126. https://dx.doi.org/10.17582/journal.pjz/2017.49.3.sc5
Anasta´cio, S., Tavares, N., Carolino, N. and Sidi-Boumedine, K., 2013. Serological evidence of exposure to Coxiella burnetii in sheep and goats in central Portugal. Vet. Microbiol., 167: 500-505. https://doi.org/10.1016/j.vetmic.2013.08.004
Anonymous, 2016. Manual of the diagnostic tests and vaccines for terrestrial animals. Office International des Epizootics, Paris, France, pp. 1-15.
Anonymous, 2017. Economic survey of Pakistan (2016-17). Economic Advisors Wing, Finance Division, Government of Pakistan, Islamabad, Pakistan.
Anonymous, 2018. Weather of Pakistan. Pakistan Meteorological Department, Regional Meteorological Center, 46- Jail Road Lahore, Punjab, Pakistan.
Carbonero, A., Guzmán, L.T., Montano, K., Torralbo, A., Arenas-Montes, A. and Saa, L.R., 2015. Coxiella burnetii seroprevalence and associated risk factors in dairy and mixed cattle farms from Ecuador. Prevent. Vet. Med., 118: 427-435. https://doi.org/10.1016/j.prevetmed.2015.01.007
Duron, O., Sidi-Boumedine, K., Rousset, E., Moutailler, S. and Jourdain, E., 2015. The importance of ticks in Q fever transmission: what has (and has hot) been demonstrated? Trends Parasitol., 31: 536-552. https://doi.org/10.1016/j.pt.2015.06.014
Ezatkhah, M., Alimolaei, M., Khalili, M. and Sharifi, H., 2015. Seroepidemiological study of Q fever in small ruminants from Southeast Iran. J. Infect. Publ. Hlth., 8: 170-176. https://doi.org/10.1016/j.jiph.2014.08.009
Ganter, M., 2015. Zoonotic risks from small ruminant. Vet. Microbiol., 181: 53-65. https://doi.org/10.1016/j.vetmic.2015.07.015
Hadush, A., Kandi, V. and Pal, M., 2016. Epidemiology and public health implications of Q fever. Perspect. Med. Res., 4: 42-46.
Jung, B.Y., Seo, M.G., Lee, S.H., Byun, J.W., Oem, J.K. and Kwak, D., 2014. Molecular and serological detection of Coxiella burnetii in native Korean goats (Capra hircus coreanae). Vet. Microbiol., 173: 152-155. https://doi.org/10.1016/j.vetmic.2014.06.029
Khaled, H., Sidi-Boumedine, K., Merdja, S., Dufour, P., Dahmani, A., Thiéry, R., Rousset, E. and Bouyoucef, A., 2016. Serological and molecular evidence of Q fever among small ruminant flocks in Algeria. Comp. Immunol. Microbiol. Infect. Dis., 47: 19-25. https://doi.org/10.1016/j.cimid.2016.05.002
Klaasen, M., Roest, H.J., Hoek, W., Goossens, B., Secka, A. and Stegeman, A., 2014. Coxiella burnetii seroprevalence in small ruminants in the Gambia. PLoS One, 9: 0085424. https://doi.org/10.1371/journal.pone.0085424
Lambton, S.L., Smith, R.P., Gillard, K., Horigan, M., Farren, C. and Pritchard, G.C., 2016. Serological survey using ELISA to determine the prevalence of Coxiella burnetii infection (Q fever) in sheep and goats in Great Britain. Epidemiol. Infect., 144: 19-24. https://doi.org/10.1017/S0950268815000874
Mori, M., Boarbi, S., Michel, P., Bakinahe, R., Rits, K., Wattiau, P. and Fretin, D., 2013. In vitro and in vivo infectious potential of Coxiella burnetii: A study on Belgian livestock isolates. PLoS One, 8: e67622. https://doi.org/10.1371/journal.pone.0067622
Paul, S., Agger, J.F., Markussen, B., Christoffersen, A.B. and Agerholm, J.S., 2012. Factors associated with Coxiella burnetii antibody positivity in Danish dairy cows. Prevent. Vet. Med., 107: 57-64. https://doi.org/10.1016/j.prevetmed.2012.05.015
Rizzo, F., Vitale, N., Ballardini, M., Borromeo, V., Luzzago, C., Chiavacci, L. and Mandola, M.L., 2016. Q fever seroprevalence and risk factors in sheep and goats in northwest Italy. Prevent. Vet. Med., 130: 10-17. https://doi.org/10.1016/j.prevetmed.2016.05.014
Saegerman, C., Speybroeck, N., Pozzo, F.D. and Czaplicki, G., 2013. Clinical indicators of exposure to Coxiella burnetii in dairy herds. Transbound. Emerg. Dis., 62: 46-54. https://doi.org/10.1111/tbed.12070
Saglam, A.G. and Sahin, M., 2016. Coxiella burnetii in samples from cattle herds and sheep flocks in the Kars region of Turkey. Vet. Med., 61: 17-22. https://doi.org/10.17221/8678-VETMED
Schimmer, B., Luttikholt, S., Hautvast, J.L.A. and Graat, E.A., 2011. Seroprevalence and risk factors of Q fever in goats on commercial dairy goat farms in the Netherlands, 2009–2010. BMC Vet. Res., 7: 81-85. https://doi.org/10.1186/1746-6148-7-81
Schimmer, B., de Lange, M.M.A., Hautvast, J.L.A., Vellema, P. and van Duynhoven, Y., 2014. Coxiella burnetii seroprevalence and risk factors on commercial sheep farms in the Netherlands. Vet. Rec., 175: 1-17. https://doi.org/10.1136/vr.102155
Shabbir, M.Z., Akram, S., Hassan, Z., Hanif, K., Rabbani, M., Muhammad, J., Chaudhary, M.H., Abbas, T., Ghori, M.T., Rashid, H., Jamil, T., Islam, Z., Rasool, H., Bano, A., Ahmad, A., Ali, M.A., Yaqub, T., Vey, W.M. and Jayarao, B.M., 2016. Evidence of Coxiella burnetii in Punjab province, Pakistan. Acta Trop., 163: 61-69. https://doi.org/10.1016/j.actatropica.2016.07.017
Shah, S.Y., Kovacs, C., Tan, C.D., Pettersson, G., Shrestha, N.K., Lutwick, L. and Gordon, S.M., 2015. Delayed diagnosis of Q fever endocarditis in a rheumatoid arthritis patient. IDCases, 2: 94-96. https://doi.org/10.1016/j.idcr.2015.09.002
Thrusfield, M., 2007. Veterinary epidemiology, 3rd ed. Blackwell Science Ltd., New Jersey, USA.
Van den Brom, R., van Engelen, E., Roest, H.I.J., van der Hoek, W. and Vellema, P., 2015. Coxiella burnetii infections in sheep or goats: An opinionated review. Vet. Microbiol., 181: 119-129. https://doi.org/10.1016/j.vetmic.2015.07.011
Zahid, M.U., Hussain, M.H., Saqib, M., Neubauer, H., Abbas, G., Khan, I., Mansoor, M.K., Asi, M.N., Ahmad, T. and Muhammad, G., 2013. Sero-prevalence of Q fever (Coxiellosis) in small ruminant of two districts in Punjab, Pakistan. Vector Borne Zoonot. Dis., 16: 449-454. https://doi.org/10.1089/vbz.2015.1852
To share on other social networks, click on any share button. What are these?










