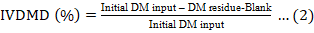The Effects of Monosodium Glutamate by Product Treated Rice Straw in Total Mixed Rations on Rumen Fermentation and Ruminal Microbial Populations Using an In Vitro Gas Technique
Research Article
The Effects of Monosodium Glutamate by Product Treated Rice Straw in Total Mixed Rations on Rumen Fermentation and Ruminal Microbial Populations Using an In Vitro Gas Technique
Suparada Saphaphan, K. Teepalak Rangubhet, Phongthorn Kongmun*
Department of Animal Science, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand 10900.
Abstract | This study aimed to investigate the effects of monosodium glutamate by-product-treated rice straw (MSGBTRS) as a roughage source in total mixed rations (TMR) on rumen fermentation and microbial ecology using an in vitro gas production technique. Four treatments, Pangola grass hay (PH), rice straw (RS), MSGBTRS, and 3.5% urea-treated rice straw (UTRS), were mixed thoroughly with the concentrated feed ingredients to make TMR at a ratio of 60:40. Treatments were assigned in a completely randomized design. Results showed that cumulative gas production at 72 h was significantly higher (P<0.05) in the MSGBTRS treatment, while PH exhibited the lowest value (P<0.05). Additionally, in vitro dry matter digestibility (IVDMD) at 24 and 48 h was highest in the 3.5% UTRS treatment (P<0.001), while that in the MSGBTRS treatment was comparable to PH. The concentrations of ammonia nitrogen (NH3-N) and total volatile fatty acids (VFA) showed no significant differences across the treatments. The populations of total ruminal bacteria and cellulolytic bacteria did not show significant differences among treatments. In conclusion, the utilization of MSGBTRS as a roughage source in TMR can effectively replace 3.5% UTRS as well as PH, as shown by the lack of significant differences in the results of rumen fermentation, dry matter digestibility, and ruminal microbial population analysis.
Keywords | Total mixed rations, Rice straw, MSGB-treated rice straw, In vitro gas production technique, Goats
Received | January 07, 2024; Accepted | March 13, 2024; Published | May 07, 2024
*Correspondence | Phongthorn Kongmun, Assistant Professor, Department of Animal Science, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand 10900; Email: fagrptk@ku.ac.th
Citation | Saphaphan S, Rangubhet KT, Kongmun P (2024). The effects of monosodium glutamate by product treated rice straw in total mixed rations on rumen fermentation and ruminal microbial populations using an in vitro gas technique. Adv. Anim. Vet. Sci., 12(6):1157-1165.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.6.1157.1165
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Goat farming in Thailand has experienced a significant increase in recent years, supported by the Department of Livestock Development’s concerted efforts to promote this livestock sector. Therefore, the goat population in the country has significantly increased by approximately 56% (Megan et al., 2019). While this development was promising for the agricultural landscape, it has been becoming a new challenge, including the need for a stock of roughage, both in terms of quantity and quality (Seo, 2019). Roughage, as one of the important components of goat diets, plays a key role in ensuring their overall health and productivity (Woolsoncroft et al., 2018; Ahmed et al., 2019; Mulisa-Faji, 2021).
Rice straw, which is readily available as a major by-product in East and Southeast Asia, has traditionally served as a primary source of roughage for ruminants in Thailand (Foiklang et al., 2016; Wahyono et al., 2021). However, rice straw is characterized by its inherent limitations, notably its low nutritive values, as indicated by a low crude protein and metabolizable energy contents (Nader et al., 2012; Zaghloul et al., 2018; Khonkhaeng and Cherdthong, 2020). Furthermore, it exhibits poor digestibility by rumen microbes, thus necessitating interventions to enhance its nutritional profile (Van Soest, 2006).
In response to the challenge of improving the usability of rice straw for ruminants, previous studies have focused on employing various methods, including physical techniques, such as soaking and grinding, biological approaches with the addition of enzymes and white rot fungi, and chemical methods, such as the application of sodium hydroxide and urea (Ibrahim and Pearce, 1984; Zhang et al., 2018; Zayed, 2018). Despite these efforts, the relatively low nutritional content of rice straw, averaging 3–5% protein in dry matter, prompts continuous attempts to enhance its quality. The primary objective is to boost protein content, which is crucial for ruminal microbial fermentation in the rumen, supporting overall productivity. Traditional protein sources are often expensive, scarce, or limited; new alternatives that are useful for solving this problem include industrial by-products (Sheikh et al., 2018). Utilizing such by-products can enhance the nutritional content of rice straw, rendering it a more efficient roughage source. This approach involves cost-effective feed components, control over forage to concentrate ratios, reduced metabolic and digestive issues, and decreased feeding labor (Owen, 1984). Among these industrial by-products, monosodium glutamate by product (MSGB) has emerged as a promising candidate because it serves as a rich source of energy, essential amino acids, nitrogen content, and minerals, making it a high-value component (Rukboon et al., 2019).
Previous research explored the application of MSGB in animal diets, with studies such as that by Keaokliang et al. (2018) indicating its potential as a protein source for non-ruminants and nonprotein nitrogen for ruminants. Moreover, glutamic acid is one of the main components in MSGB (Padunglerk et al., 2016), which is a precursor for essential amino acids, and stimulates bacterial growth in ruminal bacteria incubations from dairy cows, which is crucial for maximal ruminal bacterial growth (Kajikawa et al., 2002). However, Nombekela et al. (1994) found no improvements in dry matter intake with monosodium L-glutamate as a flavor supplement in early lactation cows. Particularly promising is its positive impact on goat concentrate diets, improving feed intake, crude protein digestibility, volatile fatty acid concentrations, and overall growth performance (Rukboon et al., 2019). Additionally, MSGB proved effective in enhancing rice straw quality, leading to increased protein content and digestibility compared to traditional urea fermentation methods (Kongsil, 2017). In summary, MSGB exhibits diverse benefits in animal nutrition, ranging from improved ruminal bacterial growth to enhanced livestock performance and feed quality.
MSGB demonstrates its potential in various livestock species, including swine, beef cattle, dairy cows, and goats (Padunglerk et al., 2016). The volume of MSGB produced is up to 6,200 tons/year (Katsumata et al., 2020). In Thailand, MSGB plays a key role in improving the roughage quality and solving the problem of shortage of roughage for goats.
In the past, research focused on using MSGB in goat concentrate diets and increasing the rice straw quality by using MSGB fermentation methods. However, research into the benefits of MSGB for improving rice straw as a roughage source in TMR in Thailand remains scarce. Therefore, this study seeks to investigate the feasibility of using MSGB-treated rice straw as a high-quality roughage source in TMR for fattening goats. The primary objectives are to reduce the cost of feed while simultaneously enhancing animal productivity without compromising the health and well-being of the animals. This research aimed to shed light on an innovative and sustainable approach to address the pressing issue of roughage scarcity in the context of expanding goat farming in Thailand.
Materials and Methods
The in vitro fermentation study was carried out at the feed laboratories of the Faculty of Agriculture, Kasetsart University, Bangkok, Thailand.
Feed preparation and treatments
In the present study, the nutritive potentials of four roughage sources were examined: Pangola grass hay, rice straw, MSGB-treated rice straw, and urea-treated rice straw.
Pangola grass hay was prepared following the Thai Agricultural Standard (2011) guidelines. Briefly, the grass was cut at a regrowth age of 30-d, 5 cm aboveground, sun-dried in the field for a 3-d period, baled, and kept in the shade.
Rice straw was obtained directly from rice fields after sun-drying over 3 consecutive days. The straw was chopped into 2–5 cm lengths using a straw-cutting machine. The chopped rice straw was either untreated or treated with MSGB or urea.
For the MSGB-treated rice straw, the MSGB obtained from the MSGB factory (Padunglerk et al., 2016) was mixed with chopped rice straw at a ratio of 8.8:1.2 (w/w). The MSGB was sprayed evenly over the rice straw and subsequently allowed to dry in a hot air oven at 60 °C for 72 h.
The urea-treated rice straw was prepared based on the following basis according to the method of Kongsil (2017). In short, 30 g of urea fertilizer (46-0-0) was mixed thoroughly with 1L of clean water. The solution was then poured into 1 kg of chopped rice straw. The urea-treated rice straw was stored at 30°C for 21 days and then dried at 60°C in a forced-air oven for 72 h.
These individual roughage sources (i.e., Pangola grass hay, rice straw, MSGB-treated rice straw, and urea-treated rice straw) were mixed thoroughly with the concentrated feed ingredients to make total mixed rations (TMR) at a ratio of 60:40.
Feed components and the chemical composition of individual feed formulas (treatments) are presented in Table 1. Samples of the rations were dried at 60°C in a hot-air oven for 72 h and ground to pass through a 1 mm sieve using a hammer mill before subjecting to chemical analysis.
Table 1: Feed ingredients and chemical compositions of TMRs used in this study.
|
Ingredients |
Treatments |
|||
|
PH |
RS |
MSGBTRS |
UTRS |
|
|
Ingredient (kg of DM) |
||||
|
Pangola grass hay |
60.0 |
- |
- |
- |
|
Rice straw |
- |
60.0 |
- |
- |
|
MSGB-treated rice straw |
- |
- |
60.0 |
- |
|
Urea-treated rice straw |
- |
- |
- |
60.0 |
|
Soybean meal |
20.5 |
24.5 |
19.5 |
20.0 |
|
Corn |
5.5 |
4.5 |
6.0 |
5.0 |
|
Cassava chip |
12.0 |
9.0 |
12.5 |
13.0 |
|
Molasses |
0.5 |
0.5 |
0.5 |
0.5 |
|
Mineral mix |
0.5 |
0.5 |
0.5 |
0.5 |
|
Sulphur |
0.5 |
0.5 |
0.5 |
0.5 |
|
Salt |
0.5 |
0.5 |
0.5 |
0.5 |
|
Chemical composition |
||||
|
Dry matter, % |
92.12 |
90.86 |
89.79 |
81.70 |
|
(On DM basis, %) |
||||
|
Crude protein |
15.82 |
16.08 |
15.67 |
15.77 |
|
Neutral detergent fibre |
55.46 |
52.65 |
51.91 |
53.25 |
|
Acid detergent fibre |
29.82 |
31.17 |
28.64 |
29.65 |
|
Ether extract |
3.09 |
2.41 |
1.83 |
2.09 |
PH, Pangola hay; RS, Rice straw; MSGBTRS, MSGB treated rice straw; UTRS, 3.5% Urea treated rice straw.
Chemical analysis
Dry matter (DM), crude protein (CP), and ether extract (EE) of individual TMRs were analyzed according to AOAC (2016) standards. In addition, neutral detergent fiber (NDF) and acid detergent fiber (ADF) of those individual TMRs were evaluated using the methods described by Van Soest et al. (1991).
Rumen fluid preparation and in vitro studies
Feeding values of TMRs were determined using in vitro fermentation protocols (Paul et al., 2023). Rumen fluid was collected from five freshly slaughtered goats at an abattoir in Bangkok. Approximately 1.5 L of rumen fluid was filtered through four layers of cheesecloth, put in an airtight vacuum flask, and brought immediately to the laboratory.
An in vitro gas production technique was used to measure gas production and its related parameters (Menke and Steingass, 1988). The required buffers were prepared following the procedures outlined by Menke and Steingass (1988). The ratio of buffer to rumen fluid was maintained at 2:1. Approximately 200 g of individual TMRs (substrate) were weighed and placed in a 50 ml serum bottle (10 bottles per treatment). Subsequently, 30 ml mixed rumen solution was added to each bottle; this included 10 bottles of blank, which contained everything except the substrate. Then, all of the bottles were incubated at 39°C in a hot-air oven. The volumes of gas produced were recorded at 2, 4, 6, 8, 10, 12, 18, 24, 36, 48, 60 and 72 h post-incubation. The in vitro dry matter digestibility (IVDMD) of the treatments was also determined following the protocols of Blümmel et al. (1997). In brief, approximately 500 mg of individual TMRs were weighed and placed in a 100 ml serum bottle (5 bottles per treatment). Subsequently, approximately 75 ml of rumen solution was added to each bottle and placed in a hot-air oven for incubation at 39°C; five bottles of blank containing everything except the substrate were run with the samples. At 24 and 48 h post-incubation, measurements were performed to assess in vitro dry matter digestibility.
For the collection of rumen metabolite data, the process involved preparing serum bottles for the mixture using the same method as outlined in the in vitro gas production procedure in section 2. Each treatment was performed with 3 replicates. Samples were collected during fermentation at 1, 4, 8, 12, and 24 h post-incubation. The inoculum in each bottle was emptied and strained through four layers of cheesecloth, which was then divided into two portions. The first 18 ml of rumen fluid inoculum was collected and stored in a plastic bottle to which 2 ml of 1 M H2SO4 was added to halt microbial activity. It was then centrifuged at 10,000 rpm for 15 minutes. After that, 10 ml of cell-free supernatant was collected and analyzed for ammonia nitrogen (NH3-N) following the phenol-hypochlorite reaction method and measured by spectrophotometer, as outlined by Chaney and Marbach (1962) and Mbiriri et al. (2012); the remaining 2 ml of cell-free supernatant was loaded into an HPLC vial and then analyzed for volatile fatty acids (VFAs) using high-performance liquid chromatography (instruments by controller Waters model 600E: Waters model 484 UV detector, Milford, MA; column Bio-Rad HPX 87H ion-exchange column; column size 300×7.8 mm (Bio-Rad Laboratories Ltd, Watford, UK); mobile phase 10 mmol/L H2PO4) according to Rooke et al. (2014).
The second portion, consisting of 1 ml of rumen fluid inoculum was collected and preserved at -20°C for measuring microbial populations by using real-time PCR. The analysis included total bacteria, total anaerobic fungi, and total protozoa. A community of microorganism DNA was extracted from 0.25 g of rumen fluid and digested using the repeated bead beating plus column method (Yu and Morrison, 2002). The quality and quantity of these DNA samples were determined by agarose gel electrophoresis and spectrophotometry.
Data handling and statistical analysis
The cumulative gas production data were fitted to the model of Ørskov and McDonald (1979), as shown in Equation 1.
where y is the volume of gas (mL per 200 mg DM) produced at the time (t), a is the gas production from a soluble fraction (mL/200 mg DM), b is the gas production from the insoluble fraction (mL/200 mg DM), c is the gas production rate constant (mL/h), |a|+b the potential gas production (mL/200 mg DM) and t is the incubation time (h).
In vitro dry matter digestibility (IVDMD) was calculated using Equation 2.
Data were subjected to analysis of variance using the General Linear Model (GLM) procedures (SAS, 2002). Multiple comparisons between treatment means were performed using Duncan’s New Multiple Range Test (Steel and Torrie, 1980). Pair comparisons of (1) PH versus others, (2) RS versus MSGMTRS and UTRS, and (3) MSGMTRS versus UTRS were performed using an orthogonal contrast method (SAS, 2002). Unless otherwise stated, the significance was declared at P<0.05.
RESULTS AND DISCUSSION
In vitro gas production and IVDMD
The result showed that the cumulative gas production did not show a significant difference between the MSGBTRS and UTRS at 72 h post-incubation (P>0.05). However, the MSGBTRS exhibits a higher cumulative gas production compared to that of the PH (Table 2).
The gas produced from soluble fractions (a) and the rate constants of gas production (c) showed no significant differences. Conversely, gas production from the insoluble fraction (b) and the potential extent of gas production (d) displayed non-significant variations between the MSGBTRS and UTRS (P<0.05). Notably, both treated rice straw treatments demonstrated the highest values, surpassing those of the PH (Table 2).
In the IVDMD digestibility investigation conducted at 24 and 48 h post-incubation, a statistically significant difference was noted (P<0.001), with the UTRS demonstrating the highest digestibility. The MSGBTRS and PH, while not exhibiting statistical differences, displayed digestibility levels surpassing that of the RS (Table 2).
The manufacturing of monosodium glutamate (MSG) generates a liquid by-product that has significant amounts of high-quality protein and NPN (non-protein nitrogen), providing valuable resources for the development of rumen bacteria and animals (Keaokliang et al., 2018). In addition, The MSGB was notable for its crude protein content of 40.31% and the fact that it contains essential amino acids such as glutamic acid, alanine, proline, and aspartic acid, among others (Padunglerk et al., 2016).
Also, it should be noted that both ammonia and urea can disrupt the silicified cuticular barrier in leaves as well as in rice straw (Muthia et al., 2021). The increasing digestibility was observed with these effects and the disruption of specific lignin-carbohydrate bonds (Selim et al., 2004). The use of ammonia from urea fertilizer plays a key role in enhancing the quality of urea-treated rice straw, resulting in a 31% increase in digestibility (Van Soest, 2006).
Moreover, Wuisman et al. (2006) observed a significant increase in the rumen degradability of dry matter (DM) and neutral detergent fiber (NDF) in roughage with NPN supplementation. Moreover, Chizzotti et al. (2008) and Khattab et al. (2013) indicate that higher levels of non-protein nitrogen (NPN) in the diet enhanced the digestibility of DM, organic matter (OM), crude protein (CP), and non-fiber carbohydrates (NFC).
Rumen metabolites
In this study, rumen metabolites were observed at 2, 4, 8, 12, and 24 h post-incubation. The results of both NH3-N and VFAs are presented in Table 3.
Table 2: The impact of different roughage sources on enhancing rice straw quality in goat TMR diet on cumulative gas production, the kinetics of gas production and the percentages of IVDMD (%).
|
Items |
Treatments |
SEM |
P-value |
Contrasts |
|||||
|
PH |
RS |
MSGBTRS |
UTRS |
PH vs Others |
RS vs MSGBTRS+UTRS |
MSGBTRS vs UTRS |
|||
|
Cumulative gas production (h/ml) |
|||||||||
|
1 |
3.10 |
4.26 |
3.58 |
3.20 |
0.199 |
0.149 |
0.194 |
0.071 |
0.479 |
|
2 |
4.08b |
6.33a |
5.55ab |
4.65ab |
0.267 |
0.006 |
0.008 |
0.027 |
0.149 |
|
4 |
6.45b |
9.35a |
7.483ab |
6.45b |
0.434 |
0.001 |
0.001 |
0.005 |
0.257 |
|
6 |
7.15b |
12.05a |
9.483ab |
8.38b |
0.531 |
0.003 |
0.006 |
0.005 |
0.344 |
|
8 |
9.42b |
14.37a |
11.63ab |
10.28b |
0.585 |
0.007 |
0.022 |
0.007 |
0.318 |
|
10 |
11.25b |
16.83a |
14.25ab |
12.32b |
0.652 |
0.005 |
0.013 |
0.010 |
0.198 |
|
12 |
12.70b |
18.67a |
16.23ab |
13.97ab |
0.733 |
0.011 |
0.017 |
0.024 |
0.196 |
|
18 |
14.80b |
22.63a |
19.77ab |
17.38ab |
0.947 |
0.014 |
0.010 |
0.047 |
0.013 |
|
24 |
16.82b |
25.73a |
23.40ab |
20.88ab |
1.105 |
0.018 |
0.006 |
0.129 |
0.349 |
|
36 |
20.83 |
29.95 |
29.05 |
26.72 |
1.375 |
0.071 |
0.014 |
0.505 |
0.514 |
|
48 |
24.08 |
35.17 |
34.77 |
32.58 |
1.671 |
0.054 |
0.008 |
0.686 |
0.609 |
|
60 |
26.98 |
39.02 |
39.47 |
35.78 |
1.814 |
0.041 |
0.006 |
0.724 |
0.422 |
|
72 |
29.05b |
42.08ab |
43.21a |
38.20ab |
1.932 |
0.027 |
0.005 |
0.738 |
0.297 |
|
Fermentation kinetic values1 |
|||||||||
|
a |
2.63 |
4.36 |
3.53 |
2.65 |
0.247 |
0.025 |
0.082 |
0.023 |
0.151 |
|
b |
30.20b |
40.97ab |
51.52a |
47.03a |
2.739 |
0.024 |
0.006 |
0.161 |
0.503 |
|
c |
0.031 |
0.035 |
0.021 |
0.023 |
0.002 |
0.074 |
0.283 |
0.017 |
0.767 |
|
d |
32.94b |
45.33ab |
55.06a |
49.68a |
2.801 |
0.024 |
0.006 |
0.243 |
0.434 |
|
24 h |
72.47b |
65.02c |
72.27b |
82.07a |
1.587 |
<0.001 |
0.697 |
<0.001 |
<0.001 |
|
48 h |
83.25b |
78.56c |
82.45b |
89.23a |
0.920 |
<0.001 |
0.819 |
<0.001 |
<.0.001 |
a,b,c Means with different superscripts in row are highly significantly different (P<0.01) and significantly different (P<0.05). 1a = The gas production from soluble fractions (ml), b = The gas production from insoluble fraction (ml), c = The rate constants of gas production (ml) and d = The potential extent of gas production (ml).
The mean concentration of NH3-N showed no statistically significant differences (P>0.05) among the treatments. However, the MSGB exhibited the highest concentration of NH3-N when compared to other treatments. Nevertheless, significant variations were observed at 4, 8, and 24 h post-incubation; in particular, the UTRS showed the highest values, but the other treatments did not show significant differences. However, the concentration of NH3-N in the MSGB was higher than in the RS and PH (Table 3).
The increase in ruminal NH3-N concentration could be attributed largely to the efficient breakdown of urea into ammonia (Weiner et al., 2015).
The mean concentrations of total VFAs, the proportion of acetate, propionate, and butyrate and the C2:C3 ratio did not show statistically significant differences among the treatments (P>0.05). Nonetheless, at 8 h post-incubation, the results of all parameters for VFAs were significantly different between treatments, except for the proportion of butyrate. The RS and MSGBTRS showed the highest concentrations of total VFAs and proportions of propionate; however, the proportion of acetate and the C2:C3 ratio showed the lowest concentration (P<0.05). These VFA results suggested that the potential of enhancing rice straw with MSGB might be more appropriate for improving rice straw quality than the UTRS. Moreover, MSGB-treated rice straw can increase the rice straw quality by controlling the rumen conditions, which show high rumen metabolites equivalent to those of the PH (Table 3).
Ruminal microorganism populations
As shown in Table 4, the mean populations of total bacteria, total anaerobic fungi, total protozoa, R. albus, R. flavefaciens and F. succinogenes were not significantly different between treatments (P>0.05). Nonetheless, in the case of R. albus, the PH represented the highest value for their population, while the MSGBTRS and UTRS recorded the lowest values (P<0.05).
Table 3: The impact of various roughage sources for enhancing rice straw quality in goat TMR diets on ruminal ammonia-nitrogen and volatile fatty acid concentrations.
|
Items |
Treatments |
SEM |
P-value |
Contrasts |
|||||
|
PH |
RS |
MSGBTRS |
UTRS |
PH vs Others |
RS Vs MSGBTRS + UTRS |
MSGBTRS vs UTRS |
|||
|
Ruminal ammonia-nitrogen concentration, (mg/dl) |
|||||||||
|
2 h |
15.47 |
15.56 |
16.06 |
16.77 |
0.193 |
0.029 |
0.062 |
0.030 |
0.094 |
|
4 h |
15.50b |
16.38ab |
17.38ab |
18.83a |
0.414 |
0.003 |
0.003 |
0.010 |
0.041 |
|
8 h |
17.43b |
17.47b |
17.73b |
18.62a |
0.171 |
0.015 |
0.104 |
0.292 |
0.004 |
|
12 h |
20.45 |
21.43 |
21.94 |
22.71 |
0.423 |
0.316 |
0.129 |
0.390 |
0.514 |
|
24 h |
18.99b |
19.42b |
19.39b |
20.50a |
0.192 |
0.032 |
0.031 |
0.135 |
0.023 |
|
Mean |
17.58 |
18.10 |
18.44 |
19.12 |
0.333 |
0.876 |
0.478 |
0.852 |
0.775 |
|
Total VFAs, mmol/l |
|||||||||
|
1 h |
97.94 |
91.02 |
90.83 |
94.25 |
0.239 |
0.751 |
0.356 |
0.819 |
0.656 |
|
4 h |
100.73 |
108.34 |
98.92 |
103.90 |
0.290 |
0.740 |
0.693 |
0.397 |
0.592 |
|
8 h |
127.96ab |
139.27a |
131.29ab |
120.68b |
0.247 |
0.028 |
0.548 |
0.012 |
0.057 |
|
Mean |
108.88 |
112.88 |
107.01 |
106.28 |
1.488 |
0.449 |
0.965 |
0.126 |
0.866 |
|
Acetate (C2), mol/100 mol total VFAs |
|||||||||
|
1 h |
80.16 |
79.86 |
80.73 |
80.48 |
0.489 |
0.948 |
0.884 |
0.600 |
0.879 |
|
4 h |
79.51 |
77.63 |
79.47 |
79.96 |
0.489 |
0.572 |
0.738 |
0.202 |
0.784 |
|
8 h |
75.49ab |
73.43b |
74.76b |
77.44a |
0.521 |
0.021 |
0.725 |
0.013 |
0.026 |
|
Mean |
78.38 |
76.98 |
78.32 |
79.28 |
0.392 |
0.230 |
0.823 |
0.070 |
0.371 |
|
Propionate (C3), mol/100 mol total VFAs |
|||||||||
|
1 h |
14.96 |
15.33 |
14.19 |
14.31 |
0.510 |
0.880 |
0.798 |
0.467 |
0.941 |
|
4 h |
15.32 |
17.09 |
15.39 |
14.71 |
0.510 |
0.517 |
0.758 |
0.177 |
0.679 |
|
8 h |
19.00ab |
21.35a |
19.76a |
17.16b |
0.547 |
0.020 |
0.619 |
0.011 |
0.033 |
|
Mean |
16.43 |
17.930 |
16.45 |
15.40 |
0.397 |
0.150 |
0.841 |
0.043 |
0.307 |
|
Butyrate (C4), mol/100 mol total VFAs |
|||||||||
|
1 h |
4.87 |
4.80 |
5.07 |
5.2 |
0.122 |
0.716 |
0.631 |
0.341 |
0.745 |
|
4 h |
5.16 |
5.26 |
5.13 |
5.32 |
0.122 |
0.822 |
0.705 |
0.836 |
0.423 |
|
8 h |
5.51 |
5.20 |
5.47 |
5.44 |
0.066 |
0.398 |
0.367 |
0.159 |
0.875 |
|
Mean |
5.18 |
5.09 |
5.22 |
5.31 |
0.071 |
0.805 |
0.883 |
0.678 |
0.678 |
|
C2:C3 ratio |
|||||||||
|
1 h |
5.36 |
5.33 |
5.82 |
5.66 |
0.22 |
0.878 |
0.688 |
0.522 |
0.833 |
|
4 h |
5.26 |
4.54 |
5.37 |
5.44 |
0.24 |
0.613 |
0.825 |
0.211 |
0.926 |
|
8 h |
3.99ab |
3.46b |
3.78b |
4.51a |
0.13 |
0.011 |
0.714 |
0.008 |
0.013 |
|
Mean |
4.87 |
4.44 |
4.99 |
5.20 |
0.13 |
0.278 |
0.965 |
0.072 |
0.581 |
a,b Means with different superscripts in a row are significantly different (P<0.05). PH = Pangola hay (T1), RS = rice straw (T2), MSGBTRS = MSGB-treated rice straw (T3) and UTRS = 3.5% urea-treated rice straw (T4).
The essential amino acids in the MSGB act as precursors for VFAs and are vital for the proliferation of ruminal microorganisms (Kajikawa et al., 2002; Bhatia and Yang, 2017).
According to the other parameters, the UTRS showed results similar to those of the MSGBTRS. Compared with the UTRS, improving rice straw with MSGB was an easier and less time-consuming process. This can simply be done by spraying MSGB directly onto the rice straw. As the results of MSGBTRS are equivalent to those of UTRS and PH, it is therefore an attractive alternative for improving rice straw quality.
The utilization of MSGBTRS as a roughage source in TMR diets for goats, as demonstrated in the present study, revealed that MSGB is cost-effective, easily accessible, and rich in nutritional value. It presents a promising alternative to traditional roughage sources such as PH and UTRS. The MSGBTRS offers the advantages of high crude protein content and improved digestibility with no adverse effects on rumen ecology (Padunglerk et al., 2016; Kongsil., 2017; Rukboon et al., 2019).
Table 4: The influence of different roughage sources on enhancing rice straw quality in the goat TMR diet on ruminal microorganism populations and the predominance of cellulolytic bacteria.
|
Items |
Treatments |
SEM |
P value |
Contrasts |
|||||
|
PH |
RS |
MSGBTRS |
UTRS |
PH vs others |
RS vs MSGBTRS + UTRS |
MSGBTRS vs UTRS |
|||
|
Total bacteria, ×108 copies/ml |
|||||||||
|
1 h |
10.0 |
1.55 |
1.38 |
1.04 |
0.208 |
0.393 |
0.013 |
0.946 |
0.954 |
|
4 h |
2.19 |
1.93 |
1.57 |
5.19 |
0.585 |
0.079 |
0.524 |
0.232 |
0.023 |
|
Mean |
6.12 |
1.74 |
1.48 |
3.12 |
1.06 |
0.444 |
0.143 |
0.837 |
0.602 |
|
Total anaerobic fungi, ×106 copies/ml |
|||||||||
|
1 h |
3.82 |
6.90 |
6.56 |
5.33 |
0.752 |
0.532 |
0.205 |
0.664 |
0.574 |
|
4 h |
14.61 |
32.9 |
5.60 |
8.16 |
0.719 |
0.580 |
0.956 |
0.199 |
0.914 |
|
Mean |
9.21 |
19.29 |
6.09 |
6.45 |
0.350 |
0.569 |
0.872 |
0.183 |
0.973 |
|
Total protozoa, ×107 copies/ml |
|||||||||
|
1 h |
9.93 |
10.23 |
7.87 |
8.13 |
0.994 |
0.828 |
0.652 |
0.440 |
0.936 |
|
4 h |
9.59 |
10.23 |
7.42 |
9.54 |
0.697 |
0.574 |
0.763 |
0.356 |
0.332 |
|
Mean |
9.78 |
10.23 |
7.65 |
8.86 |
0.812 |
0.747 |
0.684 |
0.388 |
0.643 |
|
Ruminococcus albus, ×107 copies/ml |
|||||||||
|
1 h |
8.96 |
4.90 |
1.98 |
1.27 |
1.359 |
0.167 |
0.051 |
0.289 |
0.837 |
|
4 h |
4.35 |
2.72 |
2.94 |
1.78 |
0.611 |
0.581 |
0.239 |
0.520 |
0.903 |
|
Mean |
6.66a |
3.34ab |
2.46b |
1.99b |
0.736 |
0.076 |
0.015 |
0.045 |
0.780 |
|
Ruminococcus flavefaciens, ×105 copies/ml |
|||||||||
|
1 h |
20.8a |
7.84b |
8.99b |
9.55b |
0.214 |
0.087 |
0.015 |
0.741 |
0.910 |
|
4 h |
17.60 |
28.71 |
29.56 |
15.76 |
0.494 |
0.720 |
0.583 |
0.657 |
0.389 |
|
Mean |
1.92 |
1.83 |
1.93 |
1.26 |
0.269 |
0.841 |
0.735 |
0.760 |
0.458 |
|
Fibrobactor succinogenes, ×106 copies/ml |
|||||||||
|
1 h |
3.09 |
2.66 |
1.35 |
3.52 |
0.436 |
0.353 |
0.600 |
0.825 |
0.120 |
|
4 h |
1.91 |
2.43 |
2.65 |
2.64 |
0.345 |
0.884 |
0.479 |
0.845 |
0.989 |
|
Mean |
2.38 |
2.43 |
2.00 |
3.08 |
0.248 |
0.630 |
0.143 |
0.837 |
0.602 |
a,bMeans with different superscripts in a row are significantly different (P<0.05). PH = Pangola hay (T1), RS = rice straw (T2), MSGBTRS = MSGB-treated rice straw (T3) and UTRS = 3.5% urea-treated rice straw (T4).
CONCLUSIONS and Recommendations
The investigation into the utilization of MSGBTRS as a roughage source in TMR diets for goats revealed the effectiveness of MSGB in enhancing the protein content of rice straw. In addition, MSGB can increase the digestibility of rice straw to be comparable to the commonly used high-quality roughage sources like PH and UTRS. Also, MSGBTRS facilitated normal rumen metabolism and did not adversely affect rumen ecology. Consequently, the utilization of MSGBTRS presents a promising alternative as a roughage component for fattening goats in future practices.
ACKNOWLEDGEMENT
This research is supported in part by the Graduate Program Scholarship from The Graduate School, Kasetsart University.
Novelty Statement
The by-product of Monosodium glutamate serves as an alternative source of non-protein nitrogen and true protein for the diet of ruminants. Specifically, it can be utilized to enhance the crude protein content of lowquality roughages like rice straw and similar materials.
AUTHOR’S CONTRIBUTION
SS: Conceptualization, methodology, data curation and formal analysis, writing original draft. RKT: Writing-review and editing. KP: Project administration and resources, writing-review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Ahmed-Sayed ME, Shaarawy AM (2019). Effect of feeding Moringa oleifera forage on productive performance of growing goat kids. Egypt. J. Sheep Goat Sci., 14(1): 25-37.
AOAC (2016). Official methods of analysis. Association of office analysis chemists international, AOAC International, M.A, USA.
Bhatia SK, Yang YH (2017). Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio/Technol., 16(2): 327-345. https://doi.org/10.1007/s11157-017-9431-4
Blümmel M, Makkar HPS, Becker K (1997). In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr., 77: 24–34. https://doi.org/10.1111/j.1439-0396.1997.tb00734.x
Chaney AL, Marbach EP (1962). Modified reagents for determination of urea and ammonia. Clin. Chem., 8(2): 130-132. https://doi.org/10.1093/clinchem/8.2.130
Chizzotti FHM, Pereira OG, Tedeschi LO, Filho V, Chizzotti ML, Leão MI, Pereira DH (2008). Effects of dietary nonprotein nitrogen on performance, digestibility, ruminal characteristics and microbial efficiency in crossbred steers. J. Anim. Sci., 86(5): 1173-1181. https://doi.org/10.2527/jas.2006-654
Foiklang S, Wanapat M, Norrapoke T (2016). In vitro rumen fermentation and digestibility of buffaloes as influenced by grape pomace powder and urea treated rice straw supplementation. Anim. Sci. J., 87(3): 370-377. https://doi.org/10.1111/asj.12428
Ibrahim MNM, Pearce GR (1984). A soak-and-press method for the alkali treatment of fibrous crop residues. Evaluation of sodium hydroxide-treated rice straw fed to sheep. Agric. Wastes, 9(1): 17-33. https://doi.org/10.1016/0141-4607(84)90073-8
Kajikawa H, Mitsumori M, Ohmomo S (2002). Stimulatory and inhibitory effects of protein amino acids on growth rate and efficiency of mixed ruminal bacteria. J. Dairy Sci., 85(8): 2015-2022. https://doi.org/10.3168/jds.S0022-0302(02)74278-1
Katsumata S, Angthong W, Narmsilee R, Oishi K, Hirooka H, Kumagai H (2020). Effects of feeding mother liquor, by product of monosodium glutamate, on digestibility, energy and nitrogen balances and rumen condition in Thai native bulls. Anim. Sci. J., 91(1): e13421. https://doi.org/10.1111/asj.13421
Keaokliang O, Kawashima T, Angthong W, Suzuki T, Narmseelee R (2018). Chemical composition and nutritional values of cassava pulp for cattle. Anim. Sci. J., 89(8): 1120-1128. https://doi.org/10.1111/asj.13039
Khattab IM, Salem AZM, Abdel-Wahed AM, Kewan KZ (2013). Effects of urea supplementation on nutrient digestibility, nitrogen utilisation and rumen fermentation in sheep fed diets containing dates. Livest. Sci., 155: 223–229. https://doi.org/10.1016/j.livsci.2013.05.024
Khonkhaeng B, Cherdthong A (2020). Improving nutritive value of purple field corn residue and rice straw by culturing with white-rot fungi. J. Fungi, 6(2): 69. https://doi.org/10.3390/jof6020069
Kongsil S (2017). Improving rice straw quality by treated with monosodium glutamate by product for use as roughage source in ruminant diets., In: Kasetsart University, Bangkok
Mbiriri DT, Oh SJ, Lee A, Chae JI, Chae CW, Choi NJ (2012). In vitro rumen fermentation patterns of environment friendly whole crop barley, Italian ryegrass and rice straw silages. Korean J. Organ. Agric., 20(2): 221-230.
Megan EP, Jenpanich C, Amonsin A, Bunpapong N, Chanachai K, Somrongthong R, Alexander BH, Bender JB (2019). Knowledge, attitudes and practices associated with Brucellosis among small-scale goat farmers in Thailand. J. Agromed., 24(1): 56-63. https://doi.org/10.1080/1059924X.2018.1538916
Menke KH, Steingass H (1988). Estimation of the energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim. Res. Dev., 28: 7–55.
Mulisa-Faji D (2021). Strategies for goat feeding and management during drought. In: Goat science-environment, health and economy.
Muthia D, Ridla M, Laconi EB, Ridwan R, Fidriyanto R, Abdelbagi MP, Harahap R, Jayanegara A (2021). Effects of ensiling, urea treatment and autoclaving on nutritive value and in vitro rumen fermentation of rice straw. Adv. Anim. Vet. Sci., 9(5): 655-661. https://doi.org/10.17582/journal.aavs/2021/9.5.655.661
Nader GA, Cunb GS, Robinson PH (2012). Impacts of silica levels and location in the detergent fibre matrix, on in vitro gas production of rice straw. Anim. Feed Sci. Technol., 174: 140-147. https://doi.org/10.1016/j.anifeedsci.2012.03.009
Nombekela SW, Murphy MR, Gouyou HW, Marden JI (1994). Dietary preferences in early lactation cows as affected by primary tastes and some common feed flavor. J. Dairy Sci., 77: 2393–2399. https://doi.org/10.3168/jds.S0022-0302(94)77182-4
Ørskov ER, McDonald I (1979). The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci., 92: 499-503. https://doi.org/10.1017/S0021859600063048
Owen JB (1984). Complete diet feeding for cattle. Livest. Prod. Sci., 11(3): 269-285. https://doi.org/10.1016/0301-6226(84)90019-8
Padunglerk A, Prasanpanich S, Kongmun P (2016). Use of monosodium glutamate by-product in cow diet on performance of lactating dairy cows. Anim. Sci. J., 88(1): 86-93. https://doi.org/10.1111/asj.12572
Paul OB, Urmi SS, Biswas MAA (2023). Effect of TMR and fermented TMR on ruminal in vitro digestion and gas production. Adv. Anim. Vet. Sci., 11(4): 586-594. https://doi.org/10.17582/journal.aavs/2023/11.4.586.594
Rooke JA, Wallace RJ, Duthie CA, McKain N, de Souza SM, Hyslop JJ, Rose DW, Waterhouse T, Roehe R (2014). Hydrogen and methane emissions from beef cattle and their rumen microbial community vary with diet, time after feeding and genotype. Br. J. Nutr., 112(3): 398-407. https://doi.org/10.1017/S0007114514000932
Rukboon P, Prasanpanich S, Kongmun P (2019). Effects of cassava pulp mixed with monosodium glutamate by-product (CPMSG) as a protein source in goat concentrate diet. Indian J. Anim. Res., 53: 774-779. https://doi.org/10.18805/ijar.B-958
SAS (2002). SAS STAT programme. Version 9 Edition. SAS Institute, Inc., Cary, NC Seo, S. Will farmers fully adapt to monsoonal climate change through technological developments? An analysis of rice and livestock production in Thailand. J. Agric. Sci., 157(2): 97–108. https://doi.org/10.1017/S0021859619000418
Seo S (2019). Will farmers fully adapt to monosoonal climate change through technological developments? An analysis of rice and livestock production in Thailand. J. Agric. Sci., 157(2):97-108. https://doi.org/10.1017/S0021859619000418
Selim ASM, Pan J, Takano T, Suzuki T, Koike S, Kobayashi Y, Tanaka K. (2004). Effect of ammonia treatment on physical strenge of rice straw, distribution of straw particles and particle-associated bacteria in sheep rumen. Anim. Feed. Sci. Technol., 115(1): 117-128. https://doi.org/10.1016/j.anifeedsci.2004.01.011
Sheikh GG, Ganai AM, Reshi PA, Bilal S, Mir S, Masood D (2018). Improved paddy straw as ruminant feed: A review. Indian J. Anim. Res., 39: 137-143. https://doi.org/10.18805/ag.R-1667
Steel RGD, Torrie JT (1980). Principles and procedures of statistics. New York: Mc Graw-Hill Book Co.
Thai Agricultural Standard (2011). Good agricultural practices for Pangola grass, Thailand.
Van Soest PJ (2006). Rice straw, the role of silica and treatments to improve quality. Anim. Feed Sci. Technol., 130(3): 137-171. https://doi.org/10.1016/j.anifeedsci.2006.01.023
Van Soest PJ, Robertson JB, Lewis BA (1991). Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J. Anim. Sci., 74: 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Wahyono T, Sasongko WT, Maharani Y, Ansori D, Handayani T, Priyoatmojo D, Trinugraha AC (2021). Investigation of eighteen Indonesian mutant rice straw varieties as ruminant roughage. Adv. Anim. Vet. Sci., 9(11): 1757-1764. https://doi.org/10.17582/journal.aavs/2021/9.11.1757.1764
Weiner ID, Mitch WE, Sands JM (2015). Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol., 10(8): 1444. https://doi.org/10.2215/CJN.10311013
Woolsoncroft MA, Youngers ME, McPhillips LJ, Lockard CG, Haviland CL, DeSocio ES, Ryan WR, Richards CJ, Wilson BK (2018). Effects of exercise and roughage source on the health and performance of receiving beef calves. Prof. Anim. Sci., 34(2): 183-191. https://doi.org/10.15232/pas.2017-01673
Wuisman Y, Hiraoka H, Yahaya MS, Takeda M, Kim W, Takahashi T, Karita S, Horiguchi K, Takahashi T, Goto M (2006). Effects of phenylalanine fermentation byproduct and sugarcane molasses on fermentation quality and rumen degradation of whole crop barley (Hordeum vulgare L.) silage in situ. Grassl. Sci., 52(2): 73-79. https://doi.org/10.1111/j.1744-697X.2006.00050.x
Yu Z, Morrison M (2004). Improved extraction of PCR-quality community DNA from digesta and faecal samples. BioTechniques, 36(5): 808–812. https://doi.org/10.2144/04365ST04
Zaghloul A, El-Dewany C, Awad F (2018). Utilization of rice straw as a low-cost natural by-product in agriculture. Int. J. Environ. Pollut. Environ. Model., 1(4): 91-102.
Zayed MS (2018). Enhancement the feeding value of rice straw as animal fodder through microbial inoculants and physical treatments. Int. J. Recycl. Organ. Waste Agric., 7(2): 117-124. https://doi.org/10.1007/s40093-018-0197-7
Zhang W, Wu S, Cai L, Liu X, Wu H, Xin F, Zhang M, Jiang M (2018). Improved treatment and utilization of rice straw by Coprinopsis cinerea. Appl. Biochem. Biotechnol., 184(2): 616-629. https://doi.org/10.1007/s12010-017-2579-0
To share on other social networks, click on any share button. What are these?