Human Cytomegalovirus Tegument Protein pUL23 Interacts with Capsid Protein pUL85
Human Cytomegalovirus Tegument Protein pUL23 Interacts with Capsid Protein pUL85
Shaoling Lin and Jiamiao Hu
Identification of the interaction between pUL23 and pUL85. A) pUL23 interacts with pUL85 in a yeast two-hybrid assay. Yeast clones grown on selective plates and formation of blue colour in filter paper containing X-gal indicate protein-protein interaction between full-length pUL23 and pUL85; B) GST protein or GST-fusion proteins of pUL85 bound to glutathion-Sepharose bead were incubated with lysates prepared from cos7 cells transfected pcDNA3.1(+)-UL23-Flag. Bound proteins were analyzed by western blot (WB) with anti-Flag Antibody; C) Identificaiton of the interaction between pUL23 with pUL85 by co-immunoprecipitation (Co-IP) analysis. Cos-7 cell were transiently transfected with vectors expressing both Flag-tagged-pUL23 and HA-tagged-pUL85 fusion proteins (co-transfected) or with either vector alone, lysed, and subjected to immunoprecipitation with anti-Flag antibody. The immunoprecipitated material was electrophoresed and detected with anti-Flag and anti-HA antibody, respectively
Map the interaction domains between pUL85 and pUL23. A) Schematic illustration of pUL85 structures and indicated region truncated. The cDNA fragments encoding region 1-111aa, 104-202aa and 196-307aa of pUL85 were obtained by PCR and cloned into vector pGADT7 and pGEX4T-1, respectively, for yeast two-hybrid assay and GST Pull-down assay; B) Mapping the pUL85 binding domain to pUL23 by yeast two hybrid. Yeasts transformants were streaked on SD/-Trp/-Leu and SD/-Trp/-Leu/-His/-Ade selective plates and cultured (Left). Yeast colonies were analyzed for expression of the reporter gene β-galactosidase by filter lift assays (Right); C) Mapping the pUL85 binding domain to pUL23 by GST Pull-down. GST protein or GST-fusion proteins of pUL85-N / M / C bound to glutathion-Sepharose bead were incubated with lysates prepared from cos-7 cells transfected with pcDNA3.1(+)-UL23-Flag.Bound proteins were analyzed by western blot (WB) with anti-Flag Antibody
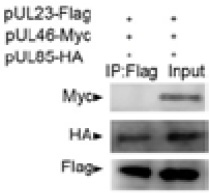
No interaction between HCMV pUL23 and pUL46 was detected in over-expressed Cos-7 cells. Cos-7 cell were transiently transfected with vectors expressing both Flag-tagged-pUL23, HA-tagged-pUL85 fusion proteins and Myc-tagged-pUL46 (co-transfected), lysed, and subjected to immunoprecipitation with anti-Flag antibody. The immunoprecipitated material was electrophoresed and detected with anti-Flag, anti-HA antibody and anti-Myc antibody, respectively
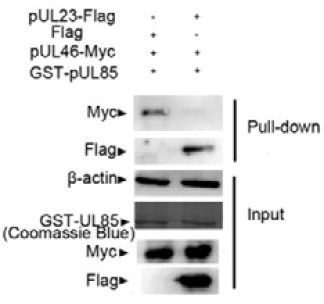
Existence of pUL23 impairs the interaction between pUL85 and pUL46. GST-Fusion proteins of pUL85 bound to glutathion-Sepharose bead were incubated with Myc-tagged pUL46 together with Flag-tagged pUL23. Bound proteins were analyzed by western blot (WB) with anti-Myc and anti-Flag antibody, respectively